Abstract
Objective: Physical activity is a major challenge to glycemic control for people with type 1 diabetes. Moderate-intensity exercise often leads to steep decreases in blood glucose and hypoglycemia that closed-loop control systems have so far failed to protect against, despite improving glycemic control overall.
Research Design and Methods: Fifteen adults with type 1 diabetes (42 ± 13.5 years old; hemoglobin A1c 6.6% ± 1.0%; 10F/5M) participated in a randomized crossover clinical trial comparing two hybrid closed-loop (HCL) systems, a state-of-the-art hybrid model predictive controller and a modified system designed to anticipate and detect unannounced exercise (APEX), during two 32-h supervised admissions with 45 min of planned moderate activity, following 4 weeks of data collection. Primary outcome was the number of hypoglycemic episodes during exercise. Continuous glucose monitor (CGM)-based metrics and hypoglycemia are also reported across the entire admissions.
Results: The APEX system reduced hypoglycemic episodes overall (9 vs. 33; P = 0.02), during exercise (5 vs. 13; P = 0.04), and in the 4 h following (2 vs. 11; P = 0.02). Overall CGM median percent time <70 mg/dL decreased as well (0.3% vs. 1.6%; P = 0.004). This protection was obtained with no significant increase in time >180 mg/dL (18.5% vs. 16.6%, P = 0.15). Overnight control was notable for both systems with no hypoglycemia, median percent in time 70–180 mg/dL at 100% and median percent time 70–140 mg/dL at ∼96% for both.
Conclusions: A new closed-loop system capable of anticipating and detecting exercise was proven to be safe and feasible and outperformed a state-of-the-art HCL, reducing participants' exposure to hypoglycemia during and after moderate-intensity physical activity. ClinicalTrials.gov NCT03859401.
Keywords: Artificial pancreas, Disturbance anticipation, Moderate-intensity exercise, Closed-loop system
Background
People with diabetes are encouraged to engage in 150 min of accumulated physical activity every week, with no more than two consecutive days without physical activity.1 However, this recommendation is challenging for people with type 1 diabetes, in part, because of fear of hypoglycemia.2 Among the different types of exercise, moderate-intensity aerobic exercise poses a major challenge for glycemic control in this population as it is often associated with sharp declines in blood glucose (BG) concentration.2 Guidelines for avoiding hypoglycemia suggest either using a reduced temporary basal insulin rate (e.g., 20%–50%) for up to 90 min before the commencement of physical activity and/or consuming fast acting carbohydrates (e.g., 30–60 g/h) before and during exercise.2,3 Although effective, these actions contribute to the already high burden of managing glycemia for people with diabetes, assume foreknowledge of the activity, and can run contrary to some common purpose of exercise (e.g., weight loss).
Closed-loop control (CLC), commonly known as the artificial pancreas (AP), offers a reliable technological solution to suboptimal glycemic control. CLC systems typically involve the pairing of a continuous glucose monitor (CGM) and a continuous subcutaneous insulin infusion pump with dedicated software (referred to as the control system or controller) embedded in the pump, a handheld computer, or a smartphone. The controller automatically adjusts the insulin infusion rate frequently (e.g., every 5 min) based on past CGM values, insulin infusions, and announced meals if available.4 Within the last few years, two hybrid closed-loop (HCL) (CLC with meal announcements) systems have been approved in the United States by the Food and Drug Administration (FDA): the Medtronic 670 G (September 2016)5 and more recently the t:slim X2 with Control-IQ (December 2019).6
Despite providing rapid insulin dose modulation to prevailing glycemia, CLC systems remain unable to adequately protect against physical activity-induced hypoglycemia without user input.7–9 Currently, investigational exercise-informed CLC systems rely on CGM and activity trackers to react as soon as possible to movement and/or steep glucose declines, but do not automate prospective actions (e.g., insulin reduction in the hour leading to the activity) such as advised by Zaharieva et al.10 Therefore, we sought to develop and test a new advanced CLC system that can anticipate historical exercise patterns, using methods from artificial intelligence to identify existing exercise behaviors, and prospectively adjust insulin delivery to improve glycemic control, without the need to announce the activity hours ahead. We conducted a randomized crossover clinical trial to compare, in a supervised environment, the hypoglycemia protection and glycemic control achieved by such a system (APEX) and contrasted its performance with a state-of-the-art HCL system with no exercise-related information.
Research Design and Methods
Study design
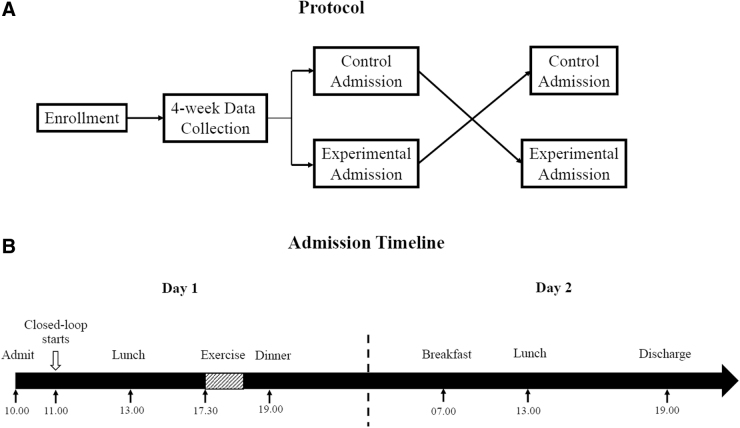
A novel CLC algorithm developed at the University of Virginia and capable of anticipating and detecting habitual exercise was tested in an open-labeled randomized crossover study and compared with an uninformed and exercise-naive HCL (Figure 1). The research protocol was approved by the FDA (IDE G190016) and the University of Virginia Institutional Review Board (IRB–HSR 180039). Study participants (18–65 years old) were recruited by phone, advertisement, and using a database of people who expressed an interest in such clinical studies. Main inclusion criteria were an existing diagnosis of type 1 diabetes (>1 year), use of an insulin pump (>6 months), and hemoglobin A1c (HbA1c) levels ≤8.6% (70 mmol/mol); exclusion criteria included a history of diabetic ketoacidosis in the previous 12 months, pregnancy, and clinically significant cardiac conditions. After signing informed consent forms, participants proceeded to a 4-week at-home data collection, during which they were asked to use a CGM, an insulin pump, and an activity tracker consistently and to record carbohydrate intake using their pumps or dedicated smartphone app.
FIG. 1.
Study protocol. (A) Participants were admitted twice to test one of the artificial pancreas systems in a randomized order. (B) Both admissions followed the same time line.
Participants were asked to exercise (moderate-intensity, target heart rate of 110–140 beats per minute) between 4 and 7 PM, for at least 30 min per day and at least four times per week (generating an habitual pattern), and were free to follow any other routines otherwise. CGM, insulin pump, and activity tracker data were assessed weekly to ensure data quality. At the end of the data collection period, participants engaged in two 32-h admissions under supervised conditions at a local hotel, using either the APEX or standard HCL algorithms, in random order. The admissions started at around 11 AM on day 1 and concluded around 7 PM on day 2; meals (selected by the participants from a local chain restaurant) were provided at 1 and 7 PM on day 1, and at 7 AM and 1 PM on day 2. Meals were consistent across days and admissions.
At around 5:30 PM on day 1 and after being transported from the hotel to the University of Virginia research facility, participants engaged in three 15-min aerobic, moderate-intensity, exercise bouts on an elliptical or treadmill machine, separated by 5-min resting periods. The target heart rate during exercise was 110–140 beats per minute. Upon completion, participants were asked to perform quiet activities until dinner. After dinner, participants were transported back to the hotel to rest until the next day. On day 2, participants had their meals and engaged in sedentary activities until discharge (Fig. 1). This design was chosen to test the additional protection of anticipating a habitual pattern (day 1 exercise occurring between 4 and 7 PM, mirroring the data collection period), and the risk of such anticipation in the absence of exercise (day 2).
Devices/systems
Participants were asked to wear a CGM (Dexcom G6; Dexcom, San Diego, CA), activity tracker (Fitbit Charge 2; Fitbit, San Francisco, CA), and their personal insulin pump during data collection. The participants were asked to insert a new sensor 24–48 h before each admission. No CGM calibration was required as per the glucose sensor manufacturer's guidelines. Upon arrival for each admission, the study staff ensured that glucose levels were between 70 and 300 mg/dL and ketone concentration was ≤0.6 mM using the study ketone meter (Abbott Precision Xtra, Abbott Diabetes Care, Witney, United Kingdom); a new infusion set compatible with the study pump was inserted. Each participant's CGM system, admission day activity tracker (SmartBand2; Sony, Tokyo, Japan), and admission day pump (t:AP2; Tandem Diabetes Care, San Diego, CA) were connected to the Diabetes Assistant (DiAs) mobile platform, which consists in a collection of smartphone applications suited for the implementation and clinical testing of open-loop and closed-loop glucose management strategies.11–13 A study glucometer (Contour Next One; Ascencia Diabetes Care, Parsippany, NJ) was used to assure patient safety, as required by the study protocol's glycemic guidelines (Supplementary Data).
All participant-specific controller parameters (e.g., average basal rate) were reviewed by a study physician before every admission and parameters were kept consistent for both admissions. Standard intensive insulin therapy meal boluses were computed in DiAs using a correction target of 120 mg/dL and the previously reviewed pump parameters irrespective of the CLC algorithm used with the exception of the postexercise dinner where APEX further modulated the meal insulin dose based on prior detected exercise.14,15
Remote monitoring and safety protocols
Participants were monitored during the entire admission by the study team using the remote Diabetes Web Monitoring (DWM) system with a study physician available as needed.13 As per protocol, the study team intervened if (1) CGM values were <60 mg/dL when not exercising or <80 mg/dL during exercise (any such intervention counted as a hypoglycemia irrespective of the prevailing glucose value), (2) when CGM values were >300 mg/dL for more than 1 h or >400 mg/dL at any time, or (3) when a ketone test was >0.6 mM. All interventions consisted of a BG or ketone measurement followed, if necessary, by the application of the safety protocol (see protocol in Supplementary Data). In addition, interventions could be requested by participants experiencing symptoms with treatment at the discretion of the study physician. BG measurements were performed at the end of every 15-min exercise bout. If CGM <80 mg/dL and/or BG <80 mg/dL, the physical activity was stopped and a hypoglycemia treatment of ∼15 g of fast-acting rescue carbohydrates was provided. The study team continued to monitor CGM rise and considered treating again if CGM remained <80 mg/dL after ∼20 min. Exercise resumed once CGM ≥80 mg/dL. Participants were discharged at the end of the admission if CGM was between 80 and 250 mg/dL and ketone concentration was ≤0.6 mM.
Exercise anticipating controller (APEX)
This study compared two CLC systems, a state-of-the-art HCL (control condition) and a new, exercise-aware, controller (APEX, experimental condition), for their ability to avoid hypoglycemic episodes during and after habitual moderate-intensity exercise bouts. Both controllers are hybrid implementations (in terms of meal announcement) based on the model predictive control (MPC) technology, aimed at commanding an optimized basal insulin injection through the pump every 5 min.15 MPC, rather than a single control strategy, encompasses a general control paradigm. The main elements of an MPC structure are (1) an explicit mathematical model to predict the variable of interest and (2) an online optimization problem that aims to find the best control action subject to possible constraints over the model variables.
Following the ideas above, the control systems use model predictions from a personalized model15,16 and CGM measurements to determine the basal insulin injection that minimizes a cost function that includes (1) a term to correct the participant's glucose concentration to the target, (2) a term penalizing low-glucose values, and (3) a regularization term to weight the difference between two consecutive basal injections. Both control systems were implemented with the University of Virginia Unified Safety System (USS Virginia), enabling a safety supervision module that limits basal injections based on the perceived risk of hypoglycemia.17
The APEX controller builds upon multiple (five) parallel MPCs (a method often referred to as multistage MPC) and implements three key innovations: (1) the incorporation of participant-specific exercise behavior patterns (exercise input) into the model prediction and controller design (each parallel MPC receives a different possible pattern to account for),15,18,19 (2) an online exercise detection that imposes a known (vs. anticipated) exercise-driven glycemic disturbance, and (3) an exercise-aware premeal bolus. Following the findings from McMahon et al.,20 we designed a method to capture participant-specific daily exercise profiles from activity tracker information gathered during the data collection period.15 Then, the daily profiles were clustered into five distinct groups using the k-medoids method as a way to summarize the participant's exercise behaviors. The system had the ability to switch between anticipatory and reactive modes upon exercise detection. The anticipatory mode utilized a probabilistic framework to account for exercise scenarios that the user was more likely to engage in, while the reactive mode was used with certainty once exercise was detected. Finally, the exercise-informed premeal bolus was used only in the dinner meal following exercise to adjust the patient's insulin bolus based on the anticipated increase in glucose uptake during the hours following exercise.14
Glycemic outcomes and statistical analysis
The primary outcome of the study was the number of hypoglycemic episodes during and immediately after exercise (∼5 to 7 PM) as defined by more than one consecutive CGM value below 70 mg/dL or hypoglycemic treatment as per protocol guidelines. Secondary outcomes included hypoglycemic events and treatments at other times during the admission as well as CGM-based outcomes: percent time in hypoglycemia <54 and <70 mg/dL, in tight range 70–140 mg/dL, in range 70–180 mg/dL (TIR), hyperglycemia >180 and >250 mg/dL, average CGM, CGM coefficient of variation, and CGM-based low and high BG indexes, LBGI and HBGI, respectively.21 Finally, system operation (time with CGM, time in closed loop), as well as carbohydrate intake and insulin delivery metrics, is reported.
No power analysis was computed for the design of the clinical trial given the safety and feasibility nature of the study. The target enrollment was 15 based on previous sample sizes used in our pilot clinical trials, leading to a detectable effect size of 0.8 at 80% power and 0.05 significance. Paired t-tests or nonparametric Wilcoxon signed-rank tests were used to compare the control and experimental admissions in terms of glycemic outcomes in the case of normally or non-normally distributed samples, respectively (Shapiro–Wilk test, Q-Q plot). The significance level was set at a P-value <0.05. No correction for multiple analysis was performed. Data are reported as mean ± SD if normally distributed and median [IQR] if the distribution is non-normal. Data formatting and preparation, as well as the statistical analysis, were carried out with MATLAB R2019b (MATLAB; MathWorks, Natick, MA).
Results
Eighteen adults with type 1 diabetes were enrolled and 15 completed the study. Participants were very well controlled with a low HbA1c of 6.7% [6.4%–7.0%] (50 [46.5–53] mmol/mol) and all were experienced pump users. Gender distribution was 10 women and 5 men. Additional baseline characteristics of the study participants are outlined in Supplementary Table S1. Overall, the data collection confirmed the baseline glycemic control with TIR and time in hypoglycemic range <70 mg/dL at 69.2% ± 12% and 2.6% ± 1.7%, respectively; and 67.7% ± 16% and 2.1% ± 2.1% overnight. Additional baseline CGM outcomes can be found in Supplementary Table S2.
Only one adverse event occurred during the study (infection at pump site) and was deemed unrelated to the study devices. No severe hypoglycemia (requiring third-party assistance) or diabetes ketoacidosis occurred during the study.
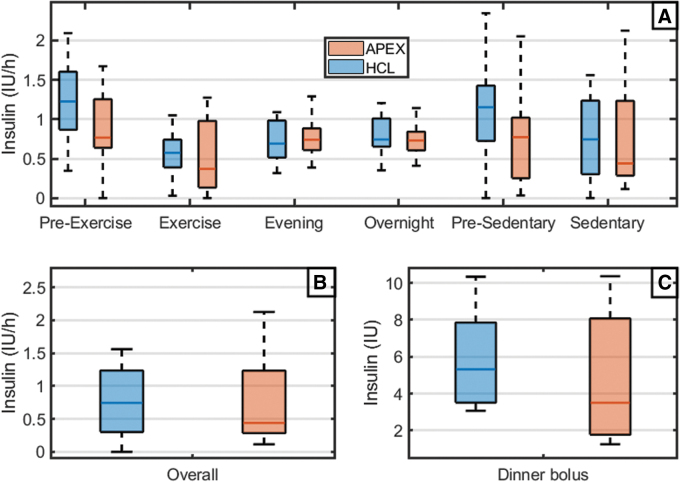
For the following results, statistical comparisons are set as APEX versus HCL unless stated otherwise. Hypoglycemic episodes during the exercise period (primary outcome) were significantly fewer with APEX than with HCL (0 [0–1] vs. 1 [0–1.7], P = 0.04). Significant fewer hypoglycemic episodes were also found in the hours following the exercise (0 [0–0] vs. 0 [0–1.7], P = 0.02) and overall (0 [0–1] vs. 1 [1–3], P = 0.02), see Figure 2. This followed a reduction of infused insulin in the hours before exercise (1.3 ± 0.9 vs. 1.8 ± 0.7 IU, P = 0.004) and in the meal bolus after exercise (5.0 ± 2.9 vs. 5.4 ± 3.2 IU, P < 0.001); overall less insulin was delivered during the APEX admission (46.2 ± 13.0 vs. 50.6 ± 15.2 IU, P = 0.003), see Figure 3. A summary of glycemic outcomes is presented in Table 1 following standard guidelines22; we summarize the main findings below:
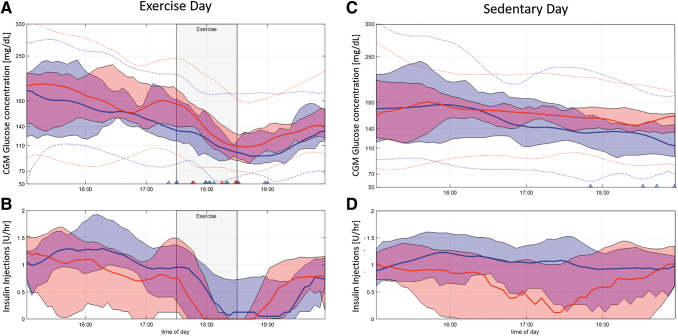
FIG. 2.
CGM and insulin in the hours before and after exercise are expected for APEX (red) and HCL (blue). (A, B) Correspond to exercise day (gray banner) and (C, D) to sedentary day. (A, C) CGM glucose values median (filled line), quartiles (envelopes), and minimum/maximum (dotted lines). Triangles are hypoglycemia treatments. (B, D) Insulin injections median (filled line) and quartiles (envelopes). APEX; CGM, continuous glucose monitor; HCL, hybrid closed-loop.
FIG. 3.
Insulin infusion during different periods of interest (box plots). (A) Pre-exercise period [15:30–17:00], exercise [17:00–19:00], evening [19:00–23:00], and overnight [23:00–7:00] for day 1. Presedentary [15:30–17:00], sedentary [17:00–19:00] for day 2. (B) Overall. (C) Dinner bolus.
Table 1.
Continuous Glucose Monitor-Measured Glucose Metrics and Insulin Metrics
| Time | HCL | APEX | P | |
|---|---|---|---|---|
| Primary outcome | ||||
| Episodes of hypoglycemiac | [17:00–19:00] day 1 | 1[0–1.7] | 0 [0–1] | 0.04b |
| Exploratory outcomes | ||||
| Episodes of hypoglycemiac | Overall | 1[1–3] | 0 [0–1] | 0.01b |
| [17:00–19:00] day 2 | 0 [0–0] | 0 [0–0] | 0.25b | |
| [19:00–23:00] | 0[0–1.7] | 0 [0–0] | 0.02b | |
| Overnight | 0 [0–0] | 0 [0–0] | 1b | |
| Mean BG (mg/dL) | Overall | 136.9 (19.9) | 141.9 (20.3) | 0.03a |
| [17:00–19:00] day 1 | 125.0 (27.2) | 140.3 (27.1) | 0.02a | |
| [17:00–19:00] day 2 | 130.4 (34.4) | 152.6 (28.8) | 0.007a | |
| [19:00–23:00] | 113.6 (23.2) | 131.3 (33.3) | 0.01a | |
| Overnight | 115.8 (10.9) | 116.2 (9.0) | 0.5a | |
| % time <54 mg/dL | Overall | 0 [0–0.3] | 0 [0–0] | 0.06 |
| [17:00–19:00] day 1 | 0 [0–0] | 0 [0–0] | 0.5b | |
| [17:00–19:00] day 2 | 0 [0–0] | 0 [0–0] | 1b | |
| [19:00–23:00] | 0 [0–2.0] | 0 [0–0] | 0.06b | |
| Overnight | 0 [0–0] | 0 [0–0] | 1b | |
| % time <70 mg/dL | Overall | 1.3 [0.0–2.7] | 0 [0–0.7] | 0.02b |
| [17:00–19:00] day 1 | 0 [0–0] | 0 [0–4.0] | 0.5b | |
| [17:00–19:00] day 2 | 0 [0–0] | 0 [0–0] | 0.1b | |
| [19:00–23:00] | 0 [0–10.2] | 0 [0–0] | 0.01b | |
| Overnight | 0 [0–0] | 0 [0–0] | 0.6b | |
| % time [70–180] mg/dL | Overall | 81.0 (12.4) | 81 (15.7) | 0.5a |
| [17:00–19:00] day 1 | 96.0 [64.0–100] | 84.0 [66.0–100] | 0.31b | |
| [17:00–19:00] day 2 | 92.0 [81.6–100] | 100 [96.0–100] | 0.2b | |
| [19:00–23:00] | 89.9 [87.8–100] | 100 [90.8–100] | 0.3b | |
| Overnight | 100 [100–100] | 100 [100–100] | 0.9b | |
| % time [70–140] mg/dL | Overall | 62.0 (14.1) | 58.4 (15.3) | 0.08a |
| [17:00–19:00] day 1 | 68.0 [46.0–92.0] | 48.0 [39.1–74.0] | 0.08b | |
| [17:00–19:00] day 2 | 55.6 [30.4–89.3] | 3.2 [0.0–50.0] | 0.02b | |
| [19:00–23:00] | 75.5 [51.0–88.8] | 77.5 [30.6–93.0] | 0.3b | |
| Overnight | 96.9 [85.0–100] | 95.8 [77.3–100] | 0.3b | |
| % time >180 mg/dL | Overall | 16.6 (12.6) | 18.5 (15.9) | 0.1a |
| [17:00–19:00] day 1 | 0.0 [0.0–34.0] | 12.0 [0.0–32.0] | 0.8b | |
| [17:00–19:00] day 2 | 0.0 [0.0–10.2] | 0.0 [0.0–4.0] | 0.4b | |
| [19:00–23:00] | 0 [0–5.1] | 0 [0–5.1] | 0.9b | |
| Overnight | 0 [0–0] | 0 [0–0] | 1b | |
| % time >250 mg/dL | Overall | 0 [0–2.0] | 0 [0–4.4] | 0.8b |
| [17:00–19:00] day 1 | 0 [0–0] | 0 [0–0] | 1b | |
| [17:00–19:00] day 2 | 0 [0–0] | 0 [0–0] | 1b | |
| [19:00–23:00] | 0 [0–0] | 0 [0–0] | 1b | |
| Overnight | 0 [0–0] | 0 [0–0] | 1b | |
| CV—glucose (%) | Overall | 30.5 (6.1) | 29.1 (6.3) | 0.2a |
| [17:00–19:00] day 1 | 24.3 (8.9) | 21.1 (12.6) | 0.2a | |
| [17:00–19:00] day 2 | 14.7 (9.2) | 6.0 (3.5) | 0.002a | |
| [19:00–23:00] | 22.0 (7.4) | 17.2 (6.5) | 0.02a | |
| Overnight | 11.8 (4.0) | 15.1 (7.2) | 0.04a | |
| SD—glucose (mg/dL) | Overall | 42.0 (11.8) | 42.0 (12.2) | 0.5a |
| [17:00–19:00] day 1 | 30.8 (14.0) | 29.5 (18.0) | 0.4a | |
| [17:00–19:00] day 2 | 18.4 (10.3) | 8.8 (5.1) | 0.002a | |
| [19:00–23:00] | 24.7 (8.7) | 22.0 (8.7) | 0.2a | |
| Overnight | 13.7 (5.2) | 17.7 (9.3) | 0.05a | |
| LBGI | Overall | 0.6 [0.4–0.9] | 0.3 [0.3–0.7] | 0.04b |
| [17:00–19:00] day 1 | 0.9 [0.4–1.8] | 0.5 [0.2–1.4] | 0.07b | |
| [17:00–19:00] day 2 | 0.3 [0.1–1.5] | 0.0 [0.0–0.0] | 0.002b | |
| [19:00–23:00] | 1.3 [0.5–2.9] | 0.2 [0–1.9] | 0.01b | |
| Overnight | 0.2 [0.1–0.5] | 0.2 [0.1–0.7] | 0.5b | |
| Infused insulin (U) | Overall | 50.6 (18.2) | 46.2 (13.0) | 0.002a |
| [15:30–17:00] day 1 | 1.8 (0.7) | 1.3 (0.9) | 0.004a | |
| [17:00–19:00] day 1 | 1.1 [0.8–1.5] | 0.7 [0.2–2] | 0.21 | |
| [15:30–17:00] day 2 | 1.6 (1.0) | 1.1 (0.8) | 0.02a | |
| [17:00–19:00] day 2 | 1.5 [0.6–2.4] | 0.9 [0.6–2.5] | 0.4 | |
| [19:00–23:00] | 9.3 (5.5) | 8.2 (2.7) | 0.12 | |
| Overnight | 6.9 (3.0) | 6.1 (2.0) | 0.05a | |
| Meal information | ||||
| Lunch (1st day) | Dinner (1st day) | Breakfast (2nd day) | Lunch (2nd day) | |
| Carbohydrate content (g) | 48 (5.5) | 44 (15.8) | 59 (25.4) | 48 (5.5) |
| Meal estimation error (%) | 2 [0–4.7] | 0 [0–0] | 6.5 [0–18.5] | 2 [0–4.8] |
| System operation | ||||
| Time in closed-loop (%) | HCL: 100 [96.7–100] | APEX: 100 [100–100] | ||
Values are shown as mean (SD) for normally distributed samples and median [Q1–Q3] for non-normally distributed samples. Significance levels <0.05 are presented in bold font.
One-sided paired t-test.
Wilcoxon signed-rank test.
No severe hypoglycemia episodes occurred during the clinical trial.
APEX, treatment controller; BG, blood glucose; CV, coefficient of variation; HCL, hybrid closed-loop (baseline); SD, standard deviation.
The APEX system significantly reduced exposure to hypoglycemia with a lower median percent time <70 mg/dL, both overall (0.0% [0%–0.7%] vs. 1.3% [0.1%–2.7%], P = 0.02) and during the evening after exercise (0% [0%–0%] vs. 0% [0%–10.2%], P = 0.01). Median LBGI was also reduced overall (0.3 [0.3–0.7] vs. 0.6 [0.4–0.9], P = 0.04), and in the evening after exercise (0.2 [0–1.9] vs. 1.3 [0.5–2.9], P = 0.01).
The use of HCL resulted in lower average glycemia overall (141.9 ± 20.3 vs. 136.8 ± 19.9 mg/dL, P = 0.03), during the exercise (140.3 ± 27.1 vs. 124.9 ± 27.2 mg/dL; P = 0.02), and during the evening after exercise (131.3 ± 33.3 vs. 113.6 ± 23.2 mg/dL, P = 0.01). However, no significant difference in TIR was observed between the two control systems. Exposure to hyperglycemia, that is, times >180 and 250 mg/dL, was similarly low for both controllers. It is worth noting that remarkably tight control was achieved by both systems overnight: time in a tight range 70–140 mg/dL was 95.8% [77.3%–100%] versus 96.9% [85.0%–100%], while time in 70–180 mg/dL was 100% [100%–100%] versus 100% [100%–100%], for APEX and HCL respectively, see Supplementary Figure S1. In addition, no hypoglycemia episode was reported with any of the systems overnight.
Finally, the anticipatory behavior of APEX during the day 2 sedentary test (day 2 from 5:00 to 7:00 PM) did not increase the exposure to hyperglycemia (percent time >180 mg/dL) or to significant hyperglycemia (percent time >250 mg/dL): time >180 mg/dL (0% [0%–4.0%] vs. 0% [0%–10.2%]; P = 0.4) and time >250 mg/dL (0% [0%–0%] vs. 0% [0%–0%]; P = 1.0) despite less insulin being again delivered by APEX in the 3:30–5:00 PM time period (1.1 ± 0.8 vs. 1.6 ± 1.0 IU; P = 0.02). However, average glycemia was temporarily higher for APEX during these 120 min (152.6 ± 29.0 vs. 130.4 ± 34.4, P = 0.007). Individual clinical results for both APEX and HCL admissions are presented for all study participants in the Supplementary Data. During both admissions, the DiAs system platform was able to maintain connectivity and remain in CLC mode nearly 100% of the time, 100% [100%–100%] versus 100% [96.7%–100%] of the time for APEX and HCL, respectively.
Discussion
This outpatient, supervised, randomized crossover study demonstrated the safety of anticipatory MPC, and its superiority to a state-of-the-art control system in terms of reduction of hypoglycemic events during and after moderate-intensity physical activity. This system, unlike others, did not rely on preventive carbohydrate consumption23 or glucagon injection,8 but on a prospective manipulation of insulin infusion to decrease the incidence of exercise-induced hypoglycemia. The anticipatory nature of the system gradually decreased the insulin infusion when there was predicted exercise-related glucose uptake within the 2-h prediction horizon. A notable effect of this anticipatory action was a temporary (<2 h) change in the achieved glucose concentration (measured by CGM) from 120 to ∼150 mg/dL, which, combined with lower pre-exercise insulin-on-board, protected the participants from exercise-induced hypoglycemia.
Both systems demonstrated remarkable performance overnight with median time in range of 100% and median time in tight range (70–140 mg/dL) above 95%. No hypoglycemic episodes were reported for any of the systems overnight despite the well-documented prolonged insulin sensitivity after exercising.20 Regarding the performance overnight, no significant correlation was found between the participant's previous glucose control (as per HbA1c) and CLC performance (in both control and experimental). Despite the system's anticipatory actions, glycemic control only marginally deteriorated during the sedentary afternoon, with no additional exposure to hyperglycemia or significant hyperglycemia (>180 and >250 mg/dL).
The success of this intervention can be attributed to three elements: (1) the ability to translate gathered activity information as a behavioral pattern to provide ongoing timely information to the control system, (2) the probabilistic framework of the multistage controller allowing the prioritization and use of specific exercise signals based on their likelihood, and (3) the adjustment of postexercise meal boluses to account for estimated future exercise-related glucose uptake.
Fear of hypoglycemia remains one of the main hurdles preventing people with type 1 diabetes from exercising regularly.9 In this regard, several closed-loop systems have already studied this concern.3,7,8,24–29 Although the literature has shown multiple approaches that achieved some protection during and after moderate-intensity physical exercise, to the best of the authors' knowledge there has not been previous clinical evidence on using behavioral information to automatically anticipate an exercise-related disturbance. Manual anticipation (the user must announce his/her desire to exercise in the future) was proposed by Forlenza et al.24 In this contribution, the authors explored either increasing the controller's set point (from 130 to 150 mg/dL) or decreasing the basal rate to a 50%, 90 min before the exercise commencement. Similar TIR in the 12 h following the exercise bout was found for both interventions, 88.9% ± 17.6% versus 89.1% ± 11.3%, respectively.
Other approaches mainly rely on real-time reactions upon exercise detection but not on prospective actions.25–29 Our group evaluated adding heart rate (HR) signal to a control-to-range controller in 12 participants during exercise during two 26-h admissions.26 That study found a significant reduction of interstitial glucose decline during exercise in the experimental arm. The Ex-Snacks trial studied the effect of adding snacks (15–30 g carbohydrate) at the commencement and in the middle of a 60-min moderate-intensity exercise period (brisk walk, 65%–75% HRmax) while using a Proportional-Integral-Derivative (PID)-like control system in 12 participants.27 The authors found a significant difference in the glucose decrease (53 ± 10 vs. 10 ± 13 mg/dL) and in hypoglycemic treatments (0 vs. 3) for snacking versus nonsnacking admissions, respectively.
The Tandem Control-IQ AP system, using a manually triggered exercise mode, was also challenged in a ski camp trial and compared with the sensor-augmented pump (SAP) in a 48-h study including 24 adolescents in three different sites across the United States.29 Finally, the Control-IQ AP system outperformed SAP with respect to TIR 66.4% ± 16.4% versus 53.9% ± 24.8% and was associated with a lower average CGM glucose level of 161.0 ± 29.9 versus 176.8 ± 36.5 mg/dL. No significant difference was found overall in terms of protection to hypoglycemia 2% [0.5%–3.8%] versus 0.8% [0.4%–3%].
As an early clinical trial, the current study exhibited several limitations. First and foremost, the study had a relatively small number of participants and was performed under supervised conditions (constant supervision by nurses and technicians). Participants were asked to perform moderate physical exercise within a given period of time on 4 out of 7 days a week for 30 days to generate a consistent exercise pattern; this design may also have increased the training status of some of the participants, possibly protecting them from hypoglycemia during both admissions. Finally, to determine the exercise impact on glucose, we used a fixed, population-based, glucose uptake pattern from observations reported in a previous clinical study (changes in glucose uptake following a similar physical activity around the same time of the day). This design focused on moderate-intensity physical activity and it is unclear how this system would have performed with other types of activities. It is well documented that different types of exercise may lead to different, even opposite, metabolic responses; for example, moderate-intensity exercise is known to cause a rapid decrease in glucose levels, while high-intensity and anaerobic exercise has been associated with possible increase initially.2 It is reasonable to expect that the ideal fully automated AP would be able to differentiate between exercise types and plan for their impact on glycemic levels.
Conclusion
In conclusion, results from this randomized, crossover, pilot clinical trial showed that this newly developed robust advanced control system is safe and feasible in a group of 15 participants with type 1 diabetes undergoing moderate-intensity physical activity and meal challenges. The experimental intervention (APEX) was able to reduce the number of hypoglycemic events during and after exercise in comparison with a state-of-the-art HCL system not informed by exercise. Our results suggest that the appropriate use of behavioral information can improve the glycemic control in participants with type 1 diabetes with identifiable patterns. Future studies involving this system would be of longer duration, with less supervision, and leverage natural exercise patterns to address the daily challenges that every person with type 1 diabetes encounters in his/her real life.
Supplementary Material
Acknowledgments
The authors thank the study volunteers and the research staff at the UVA Center for Diabetes Technology, particularly Stacey Anderson, Laura Kollar, Omar Khurshid, and Christian Wakeman.
Authors' Contributions
J.G.-T. designed the core of both baseline and experimental CLC systems, was involved in the study design, provided technical support remotely and on-site in all the admissions, analyzed data, performed statistical analyses, and was involved in writing and editing the article.
S.A.B. was the study physician responsible for all participant activities, contributed to the study design, answered queries from the regulatory boards including the Food and Drug Administration, and was involved in writing and editing the article.
N.L. contributed to the study design, provided on-site support during screenings and admissions, and was involved in writing and editing the article.
P.C. was involved in the design and preclinical testing of the control algorithms, and reviewed and edited the article.
J.P.C. designed the exercise pattern recognition logic for the APEX controller and the exercised detection scheme, he also reviewed and edited the article.
B.O. designed the exercise detector and exercise-informed premeal bolus calculator, and reviewed and edited the article.
C.L.B. was responsible for the technical team during the clinical trial and reviewed and edited the article.
M.C.O. assisted with the development of the protocol, coordinated IRB submission and Investigational Device Exemption (IDE) submission to the Food and Drug Administration (FDA), and contributed to the study planning and preparation. She also reviewed and edited the article.
H.M. was the main research coordinator for the study and responsible for all participant interaction and operations during the trial. She also reviewed and edited the article.
C.L.K.K. maintained and checked the functionality of the DiAs system, the functionality of the closed-loop systems in the smartphone, conducted the testing of the systems before the study, provided technical support remotely and on-site in all the admissions, and reviewed and edited the article.
M.D.B. was the University of Virginia site principal investigator, contributed to both algorithm and study design, supervised its implementation, performed statistical analyses, and was involved in writing and editing the article. He is also the guarantor of this work and, as such, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
S.A.B. reports receiving grant support and supplies, paid to her institution from Tandem Diabetes Care, Insulet, and Tolerion, and supplies, provided to her institution, from Dexcom and Roche Diagnostics outside the submitted work. M.D.B. has received honoraria and travel reimbursement from Dexcom and Tandem, and research support from Dexcom, Novo Nordisk, and Tandem. No other potential conflicts of interest relevant to this article were reported.
Funding Information
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by the National Institutes of Health (NIH) under Grant 1DP3DK106826-01.
Supplementary Material
References
- 1. Colberg SR, Sigal RJ, Yardley JE, et al. : Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riddell MC, Gallen IW, Smart CE, et al. : Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017;5:377–390 [DOI] [PubMed] [Google Scholar]
- 3. Franc S, Daoudi A, Pochat A, et al. : Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: the DIABRASPORT randomized study. Diabetes Obes Metab 2015;17:1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doyle FJ, Huyett LM, Lee JB, et al. : Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Garg S, Weinzimer S, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 6. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breton M, Cherñavvsky DR, Forlenza GP, et al. : Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the Artificial Pancreas Ski Study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs PG, El Youssef J, Reddy R, et al. : Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab 2016;18:1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riddell MC, Zaharieva DP, Yavelberg L, et al. : Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol 2015;9:1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaharieva DP, Mcgaugh S, Pooni R, et al. : Improved open-loop glucose control with basal insulin reduction 90 minutes before aerobic exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care 2019;42:824–831 [DOI] [PubMed] [Google Scholar]
- 11. Kovatchev BP, Renard E, Cobelli C, et al. : Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keith-Hynes P, Mize B, Robert A, Place J.: The Diabetes Assistant: a smartphone-based system for real-time control of blood glucose. Electronics 2014;3:609–623 [Google Scholar]
- 13. Place J, Robert A, Ben Brahim N, et al. : DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozaslan B, Patek SD, Breton MD: Impact of daily physical activity as measured by commonly available wearables on meal time glucose control in type 1 diabetes. Diabetes Technol Ther 2020;22:742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Tirado J, Colmegna P, Corbett JP, et al. : In silico analysis of an exercise-safe artificial pancreas with multistage model predictive control and insulin safety system. J Diabetes Sci Technol 2019;13:1054–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Tirado J, Zuluaga-Bedoya C, Breton MD: Identifiability analysis of three control-oriented models for use in artificial pancreas systems. J Diabetes Sci Technol. 2018;12: 937–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes CS, Patek SD, Breton MD, Kovatchev BP: Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Technol 2010;4:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corbett JP, Colmegna P, Garcia-Tirado J, Breton MD: Anticipating meals with behavioral profiles in an artificial pancreas system—an informed multistage model predictive control approach. In: XXI World IFAC Congress. 2020 [Google Scholar]
- 19. Garcia-Tirado J, Colmegna P, Corbett J, et al. : “Ensemble Model Predictive Control Strategies Can Reduce Exercise Hypoglycemia in Type 1 Diabetes: In Silico Studies,” 2019 American Control Conference (ACC), Philadelphia, PA, USA, 2019, pp. 4752–4758, doi: 10.23919/ACC.2019.8814728 [DOI] [Google Scholar]
- 20. McMahon SK, Ferreira LD, Ratnam N, et al. : Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab 2007;92:963–968 [DOI] [PubMed] [Google Scholar]
- 21. Kovatchev BP: Metrics for glycaemic control—from HbA 1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425. [DOI] [PubMed] [Google Scholar]
- 22. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hajizadeh I, Hobbs N, Samadi S, et al. : Controlling the AP controller: controller performance assessment and modification. J Diabetes Sci Technol 2019;13:1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forlenza GP, Buckingham BA, Christiansen MP, et al. : Performance of omnipod personalized model predictive control algorithm with moderate intensity exercise in adults with type 1 diabetes. Diabetes Technol Ther 2019;21:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherr JL, Cengiz E, Palerm CC, et al. : Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nightswith or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care 2013;36:2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Breton MD, Brown SA, Karvetski CH, et al. : Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther 2014;16:506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel NS, Van Name MA, Cengiz E, et al. : Mitigating reductions in glucose during exercise on closed-loop insulin delivery: the Ex-Snacks study. Diabetes Technol Ther 2016;18:794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huyett LM, Ly TT, Reuschel-DiVirgilio S, et al. : Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther 2016;18:A24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekhlaspour L, Forlenza GP, Chernavvsky D, et al. : Closed loop control in adolescents and children during winter sports: use of the Tandem Control-IQ AP system. Pediatr Diabetes 2019;20:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.