Abstract
Introduction
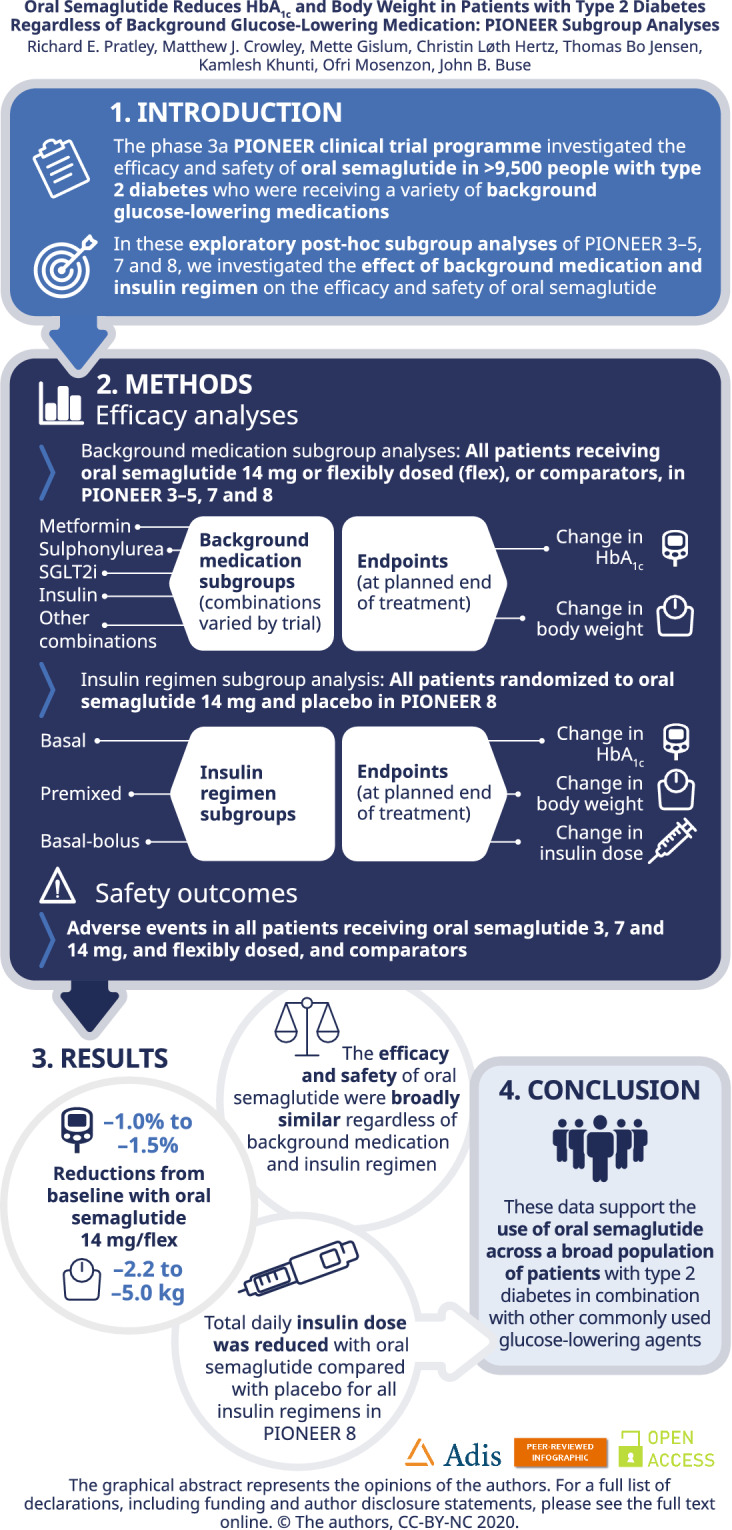
The efficacy and safety of oral semaglutide, the first oral glucagon-like peptide-1 receptor agonist, were investigated in patients with type 2 diabetes (T2D) in the Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) programme. The current post-hoc exploratory subgroup analyses evaluated outcomes by background medication and insulin regimen subgroups.
Methods
Data from patients in the PIONEER 3–5, 7 and 8 trials receiving once-daily oral semaglutide (14 mg/flexibly dosed) or a comparator (placebo, sitagliptin 100 mg or liraglutide 1.8 mg) were analysed for efficacy (glycated haemoglobin [HbA1c] and body weight changes from baseline to planned end of treatment) and safety outcomes. Patients were grouped according to background medication (metformin, sulphonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, insulin, or combinations thereof). Efficacy outcomes were analysed using the trial product estimand (which assumes that patients remained on the trial product without rescue medication use). A separate analysis by background insulin regimen (basal, premixed or basal-bolus) was done for PIONEER 8 using the treatment policy estimand (regardless of trial product discontinuation or rescue medication use). Safety outcomes were analysed descriptively for all patients.
Results
In total, 2836 patients receiving oral semaglutide 14 mg/flexibly dosed or comparators were included. Baseline characteristics were generally similar across background medication subgroups within each trial. Diabetes duration tended to be longer in patients receiving more background medications. Greater HbA1c and body weight reductions were seen across background medication subgroups with oral semaglutide (changes from baseline: − 1.0 to − 1.5% and − 2.2 to − 5.0 kg, respectively) than with comparators (except for similar HbA1c reductions vs liraglutide). There were no statistically significant interactions by treatment and background medication subgroup for change in HbA1c or body weight except for change in HbA1c (background insulin vs insulin plus metformin) in PIONEER 8 (p = 0.0408). Changes in HbA1c and body weight were generally similar across insulin regimen subgroups, without significant treatment interactions by subgroup, and the total daily insulin dose was decreased for patients receiving oral semaglutide. The incidence of adverse events was generally similar in background medication subgroups.
Conclusion
Oral semaglutide was effective at lowering HbA1c and body weight, regardless of background medications, and appears suitable for a broad range of patients with T2D in combination with other glucose-lowering agents.
Trial Registration
Clinicaltrials.gov: NCT02607865 (PIONEER 3), NCT02863419 (PIONEER 4), NCT02827708 (PIONEER 5), NCT02849080 (PIONEER 7) and NCT03021187 (PIONEER 8).
Graphic Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-020-00994-9.
Keywords: Diabetes mellitus, type 2; Glucagon-like peptides; Hypoglycaemic agents; Insulin; Metformin; Oral semaglutide; Sodium-glucose cotransporter 2 inhibitors; Sulphonylurea compounds
Key Summary Points
| Why carry out this study? |
| To help manage blood glucose, people with type 2 diabetes have glucose-lowering medications added in a stepwise manner as their disease progresses; early use of combination therapy has recently been advocated in international guidelines. |
| Oral semaglutide is the first oral glucagon-like peptide-1 receptor agonist and is likely to be used in combination with other glucose-lowering agents. |
| In the current post-hoc subgroup analyses, data from the PIONEER clinical trial programme were used to explore the efficacy and safety of oral semaglutide versus comparators in patients receiving various combinations of background medications and background insulin regimens. |
| What was learned from the study? |
| Oral semaglutide had similar efficacy and tolerability regardless of the background medication in PIONEER 3–5, 7 and 8, and the insulin regimen in PIONEER 8. Oral semaglutide generally improved glycated haemoglobin and body weight parameters to a greater extent than comparator treatments across background medication and insulin regimen subgroups and trials. |
| Oral semaglutide appears suitable for a broad population of patients with type 2 diabetes in combination with other glucose-lowering agents. |
Digital Features
This article is published with digital features, including a summary slide and graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13477029.
Introduction
Semaglutide is a glucagon-like peptide-1 (GLP-1) analogue that is the first and, thus far, only GLP-1 receptor agonist (GLP-1RA) to be formulated as a once-daily oral tablet for people with type 2 diabetes (T2D) [1–4]. GLP-1RAs are generally recommended for patients with T2D whose glycated haemoglobin (HbA1c) is above target despite diet, exercise and existing glucose-lowering medication, and GLP-1RAs with proven cardiovascular benefit are also recommended in patients with T2D at elevated cardiovascular risk, regardless of HbA1c [5, 6]. Consequently, patients receiving oral semaglutide will typically already be receiving treatment with other glucose-lowering agents. It is therefore important to understand how the efficacy and safety of oral semaglutide may vary based on the presence of concomitant treatments.
The efficacy and safety of oral semaglutide was investigated in the global phase 3a Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) programme [2]. The PIONEER 3, 4, 5, 7 and 8 trials included patients with T2D who were using more than one background medication [7–11]. In PIONEER 3 and 7, oral semaglutide 7 mg, 14 mg, and flexibly dosed significantly reduced HbA1c and body weight compared with sitagliptin 100 mg (up to week 52 in PIONEER 7 and week 78 in PIONEER 3) [7, 10]. In PIONEER 4, oral semaglutide 14 mg was noninferior to liraglutide 1.8 mg and superior to placebo in decreasing HbA1c, and superior to both for reducing body weight, at week 26 [8]. In PIONEER 5 in patients with moderate renal impairment, and PIONEER 8 in patients receiving insulin, oral semaglutide was superior to placebo for reducing HbA1c and body weight at week 26 [9, 11]. The safety profile of oral semaglutide in these trials was similar to that expected of other GLP-1RAs, with tolerability issues mainly comprising mostly transient, mild-to-moderate gastrointestinal events [7–11].
The aim of the current exploratory analyses was to investigate whether different combinations of background medications and insulin regimens affected the efficacy and safety of oral semaglutide compared with active comparators and placebo in the PIONEER 3, 4, 5, 7 and 8 trials.
Methods
Trial Designs
The designs of the PIONEER 3–5, 7 and 8 trials have been reported previously and are summarised in Table 1 [7–11]. In brief, patients were adults who had been diagnosed with T2D ≥ 90 days before screening, had baseline HbA1c above target (7.0–10.5% [53–91 mmol/mol] in PIONEER 3 and 7.0–9.5% [53–80 mmol/mol] in PIONEER 4, 5, 7 and 8) and were receiving stable doses of glucose-lowering background medication(s). Patients in PIONEER 5 had moderate renal impairment (estimated glomerular filtration rate [eGFR] 30–59 ml/min/1.73 m2).
Table 1.
Trial designs for PIONEER 3, 4, 5, 7 and 8
| PIONEER 3 | PIONEER 4 | PIONEER 5 | PIONEER 7 | PIONEER 8 | |
|---|---|---|---|---|---|
| FAS, N | 1864 | 711 | 324 | 504 | 731 |
| Oral semaglutide dose |
3 mg OD 7 mg OD 14 mg OD |
14 mg OD | 14 mg OD | Flexiblea |
3 mg OD 7 mg OD 14 mg OD |
| Comparator(s) | Sitagliptin 100 mg OD |
Liraglutide 1.8 mg OD Placebo |
Placebo | Sitagliptin 100 mg OD | Placebo |
| Randomisation | 1:1:1:1 | 2:2:1 | 1:1 | 1:1 | 1:1:1:1 |
| Duration | 78 weeks | 52 weeks | 26 weeks | 52 weeks | 52 weeks |
| Trial design | Double-blind, double-dummy, noninferiority and superiority | Double-blind, double-dummy, noninferiority and superiority | Double-blind, superiority | Open-label, superiority | Double-blind, superiority |
| Stratification by permitted background medication |
Met SU + met |
Met SGLT2i + metb |
Met SU ± met Ins ± met |
1–2 of: Met, SU, SGLT2i, TZD |
Basal, premixed or basal-bolus insulin ± met |
FAS full analysis set, ins insulin, met metformin, N number of patients included in analyses, OD once daily, RCT randomised controlled trial, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea, TZD thiazolidinedione
aDose adjustable according to efficacy and safety criteria and investigator’s clinical judgement
bShort-term insulin (≤ 14 days) was also permitted but is not part of these subgroup analyses
The PIONEER trial protocols were approved by institutional review boards or independent ethics committees at the participating trial sites, and the studies were conducted in accordance with ICH Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent before undertaking trial-related activities.
In PIONEER 3, patients were randomised to oral semaglutide 3, 7 or 14 mg once daily or sitagliptin 100 mg once daily added to background metformin with or without a sulphonylurea [7]. In PIONEER 4, oral semaglutide 14 mg once daily was compared with both liraglutide 1.8 mg and placebo in patients receiving metformin with or without a sodium-glucose cotransporter-2 inhibitor (SGLT2i) [8]. PIONEER 5 was a placebo-controlled trial of oral semaglutide 14 mg once daily in patients with moderate renal impairment receiving metformin or sulphonylurea or both or basal insulin with or without metformin [9]. In PIONEER 7, flexibly dosed oral semaglutide 3, 7 and 14 mg once daily were compared with sitagliptin 100 mg once daily in patients receiving 1–2 of the following oral glucose-lowering medications: metformin, thiazolidinedione, sulphonylurea and SGLT2i [10]. Finally, in PIONEER 8, patients were randomised to oral semaglutide 3, 7 or 14 mg once daily or placebo as an add-on to basal, premixed or basal-bolus insulin with or without metformin [11].
Randomisation in these trials was stratified by baseline background medication. In the current post-hoc subgroup analyses, data from the PIONEER clinical trial programme were used to explore the efficacy and safety of oral semaglutide versus comparators in patients receiving various combinations of background medications and background insulin regimens. Data from PIONEER 1 were not included because oral semaglutide was given as monotherapy [12], PIONEER 2 was excluded because all patients randomised to oral semaglutide or comparator were receiving background metformin (and no other background glucose-lowering medication) [13], and PIONEER 6 was excluded because it was a cardiovascular outcomes trial [14].
Subgroup Analyses
All randomised patients in PIONEER 3–5, 7 and 8 were included in the main background medication subgroup analyses, which were done for each trial separately. Subgroup data were not pooled across trials because of substantial differences in study design and patient population. Randomisation was stratified according to patients’ background medications: metformin with or without sulphonylurea in PIONEER 3; metformin with or without SGLT2i in PIONEER 4; metformin alone, sulphonylurea with or without metformin, or basal insulin with or without metformin in PIONEER 5; metformin, SGLT2i or thiazolidinedione with or without sulphonylurea in PIONEER 7; and insulin with or without metformin in PIONEER 8.
In addition, a separate subgroup analysis was performed for PIONEER 8 to explore the effect of the insulin regimen on the efficacy and safety of oral semaglutide compared with placebo.
Efficacy endpoints were change from baseline in HbA1c and body weight (and, for the separate PIONEER 8 subgroup analysis, change from baseline in insulin dose) at the planned end of treatment (weeks 26, 52 or 78, according to the trial). Efficacy data are presented for oral semaglutide 14 mg or flexibly dosed, and for comparators. Safety outcomes were analysed by subgroup across all trials for all patients (including those receiving oral semaglutide 3 and 7 mg).
Statistical Methods for Study-Level Analyses
The PIONEER programme employed the use of two estimands [15]. The trial product estimand evaluates the treatment effect for all randomised patients under the assumption that all patients remained on the trial product for the entire planned duration of the trial and did not use rescue medication. The treatment policy estimand evaluates the treatment effect for all randomised patients, regardless of trial product discontinuation or the use of rescue medication.
For the main background medication subgroup analyses, the trial product estimand was used to reflect the effect of oral semaglutide versus each comparator without the confounding effect of rescue medication. For the efficacy outcomes (HbA1c and body weight), treatment differences were estimated by a mixed model for repeated measurements (MMRM) using data collected prior to premature trial product discontinuation or initiation of rescue medication from all randomised patients. A restricted maximum likelihood was used. The independent effects included in the model were treatment, region, strata (renal function for PIONEER 5 and insulin regimen for PIONEER 8), background medication and interaction between treatment and background medication as categorical fixed effects and baseline value as a covariate, all nested within visit, and using an unstructured residual covariance matrix.
The MMRM is a well-established method that accounts for the uncertainty pertaining to missing data. This analysis assumes that the missing data mechanism is missing at random.
In PIONEER 8, patients whose insulin dose was increased by ≥ 20% during treatment were considered to have required rescue medication. Therefore, for the insulin regimen subgroup analysis, the treatment policy estimand was used to reflect the effect of initiating treatment with oral semaglutide compared with initiating treatment with placebo, both potentially followed by either discontinuation of the trial product and/or initiation of rescue medication. Treatment differences by insulin regimen were estimated by a pattern mixture model using multiple imputation to handle missing data. All data collected at the planned end of treatment (week 52), irrespective of discontinuation of the trial product or initiation of rescue medication, were included in the statistical analysis. Missing data were imputed within groups defined by trial product and treatment status at week 26. Both the imputation and the analysis were based on analysis of covariance models with treatment, region, stratum (with or without metformin), insulin regimen and interaction between treatment and insulin regimen as categorical fixed effects and baseline value as a covariate. The results were combined using Rubin’s rule [16].
p values were calculated for the unadjusted two-sided test of the treatment by background medication subgroup interaction. Estimated treatment differences (ETDs) with 95% confidence intervals were calculated, but p values are not presented because these comparisons were exploratory in nature and not controlled for multiplicity.
Safety data were analysed descriptively by treatment group (oral semaglutide 3 mg, 7 mg, 14 mg, flexibly dosed, and comparators) and background medication/insulin regimen subgroup for all patients in the trials for the incidence of adverse events (AEs), including serious AEs, gastrointestinal AEs and genitourinary AEs, as well as events leading to trial product discontinuation and hypoglycaemic episodes.
Results
Patient Disposition and Baseline Characteristics
In total, 2836 patients receiving oral semaglutide 14 mg/flexibly dosed or comparators were included in the background medication subgroup analyses, and 365 patients receiving oral semaglutide 14 mg or placebo in PIONEER 8 were included in the insulin regimen subgroup analysis. As may be expected, mean diabetes duration tended to be longer in patients receiving a greater number of background medications, ranging from 7.2 to 10.6 years in patients on metformin monotherapy to 8.4 to 17.3 years in patients receiving more than one background medication. Otherwise, baseline characteristics were generally similar across subgroups within each trial (Table 2(i)). As expected, based on the design and the inclusion criteria, patients in PIONEER 5 tended to be older (mean age ≥ 70 years) and had reduced renal function (mean eGFR 46–50 ml/min/1.73 m2) compared with those in the other trials (55–62 years and ≥ 90 ml/min/1.73 m2, respectively, across other trials and subgroups). In PIONEER 8, compared with patients receiving basal or premixed insulin, patients receiving basal-bolus insulin appeared to have a greater duration of diabetes (13 and 14 years vs 16 years, respectively), greater body weight (85 and 78 kg vs 89 kg) and a higher total daily insulin dose (35 and 48 U vs 79 U) (Table 2(ii)). The proportion of patients who completed treatment with rescue medication varied across trials, but was consistently lower with oral semaglutide 7 mg, 14 mg, and flexibly dosed (0–26% across background medication subgroups) than comparator treatments (7–34%), whereas the proportion of patients who discontinued the trial product was often somewhat higher (12–25% vs 5–15% except when sitagliptin was added to multiple background medications in PIONEER 7 [50% in a very small subgroup of 8 patients]) (see Table S1 in the “Supplementary Information”). There was no clear pattern to these intercurrent events by background medication subgroup.
Table 2.
Baseline characteristics for the background medication subgroup analysis (i) and the insulin regimen subgroup analysis (ii)
| (i) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Background medication | Patients, N | Female, n (%) | Age, years | HbA1c, % | Duration of diabetes, years | Body weight, kg | BMI, kg/m2 | eGFR, ml/min/1.73 m2 |
| PIONEER 3 | Met | 493 | 221 (44.8) | 57 ± 10 | 8.1 ± 0.8 | 7.7 ± 5.7 | 92.0 ± 22.1 | 32.4 ± 6.4 | 97 ± 16 |
| SU + met | 439 | 226 (51.5) | 59 ± 9 | 8.4 ± 0.9 | 10.0 ± 6.2 | 90.0 ± 20.5 | 32.4 ± 6.1 | 94 ± 16 | |
| PIONEER 4 | Met | 528 | 265 (50.2) | 57 ± 10 | 7.9 ± 0.7 | 7.3 ± 5.7 | 93.1 ± 20.3 | 32.9 ± 6.1 | 95 ± 14 |
| SGLT2i + met | 183 | 76 (41.5) | 55 ± 9 | 8.0 ± 0.6 | 8.4 ± 5.1 | 96.7 ± 22.9 | 33.1 ± 6.7 | 97 ± 15 | |
| PIONEER 5 | Met | 77 | 34 (44.2) | 70 ± 8 | 7.8 ± 0.7 | 10.6 ± 6.7 | 89.2 ± 15.4 | 31.7 ± 4.6 | 50 ± 10 |
| SU ± met | 132 | 75 (56.8) | 71 ± 8 | 8.0 ± 0.7 | 13.1 ± 6.8 | 89.1 ± 17.6 | 32.0 ± 5.7 | 48 ± 9 | |
| Ins ± met | 115 | 59 (51.3) | 70 ± 8 | 8.0 ± 0.7 | 17.3 ± 8.9 | 94.0 ± 18.7 | 33.3 ± 5.6 | 46 ± 10 | |
| PIONEER 7 | Met | 189 | 79 (41.8) | 57 ± 10 | 8.2 ± 0.6 | 7.2 ± 4.9 | 92.1 ± 20.2 | 32.2 ± 6.3 | 97 ± 16 |
| SU ± met | 244 | 110 (45.1) | 58 ± 9 | 8.4 ± 0.6 | 9.8 ± 6.8 | 85.8 ± 19.8 | 31.1 ± 6.5 | 96 ± 14 | |
| SGLT2i ± met | 51 | 21 (41.2) | 58 ± 10 | 8.3 ± 0.6 | 8.5 ± 5.1 | 89.1 ± 15.9 | 30.8 ± 4.7 | 94 ± 13 | |
| Other | 20 | 9 (45.0) | 59 ± 13 | 8.2 ± 0.6 | 11.8 ± 8.1 | 89.4 ± 22.0 | 32.0 ± 6.7 | 96 ± 14 | |
| PIONEER 8 | Ins | 118 | 45 (38.1) | 62 ± 11 | 8.2 ± 0.7 | 16.0 ± 8.7 | 75.4 ± 19.2 | 27.6 ± 5.5 | 90 ± 14 |
| Ins + met | 247 | 130 (52.6) | 60 ± 9 | 8.2 ± 0.7 | 13.6 ± 7.4 | 90.0 ± 20.4 | 32.5 ± 6.2 | 92 ± 15 | |
| (ii) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Insulin regimen in PIONEER 8 | Patients, N | Female, n (%) | Age, years | HbA1c, % | Duration of diabetes, years | Body weight, kg | BMI, kg/m2 | eGFR, ml/min/1.73 m2 | Total daily insulin dose, U |
| Basal | 156 | 70 (44.9) | 60.2 ± 9.6 | 8.2 ± 0.7 | 13.0 ± 7.0 | 85.0 ± 20.2 | 30.4 ± 5.6 | 92 ± 14 | 35 ± 29 |
| Premixed | 67 | 31 (46.3) | 59.5 ± 11.7 | 8.2 ± 0.6 | 14.4 ± 9.2 | 78.4 ± 20.0 | 29.2 ± 6.6 | 91 ± 17 | 48 ± 31 |
| Basal-bolus | 142 | 74 (52.1) | 61.6 ± 8.9 | 8.2 ± 0.7 | 16.0 ± 8.0 | 88.8 ± 22.0 | 32.2 ± 6.9 | 90 ± 13 | 79 ± 53 |
All baseline characteristic values shown are the mean ± standard deviation unless otherwise specified. (i) data are for patients receiving oral semaglutide 14 mg or flexibly dosed. (ii) data are for patients receiving oral semaglutide 14 mg or placebo. Other includes thiazolidinediones and other oral glucose-lowering medications
BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated haemoglobin, ins insulin, met metformin, N number of patients included in analyses, n number of patients with at least one event, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea
Efficacy Outcomes by Background Medication Subgroup
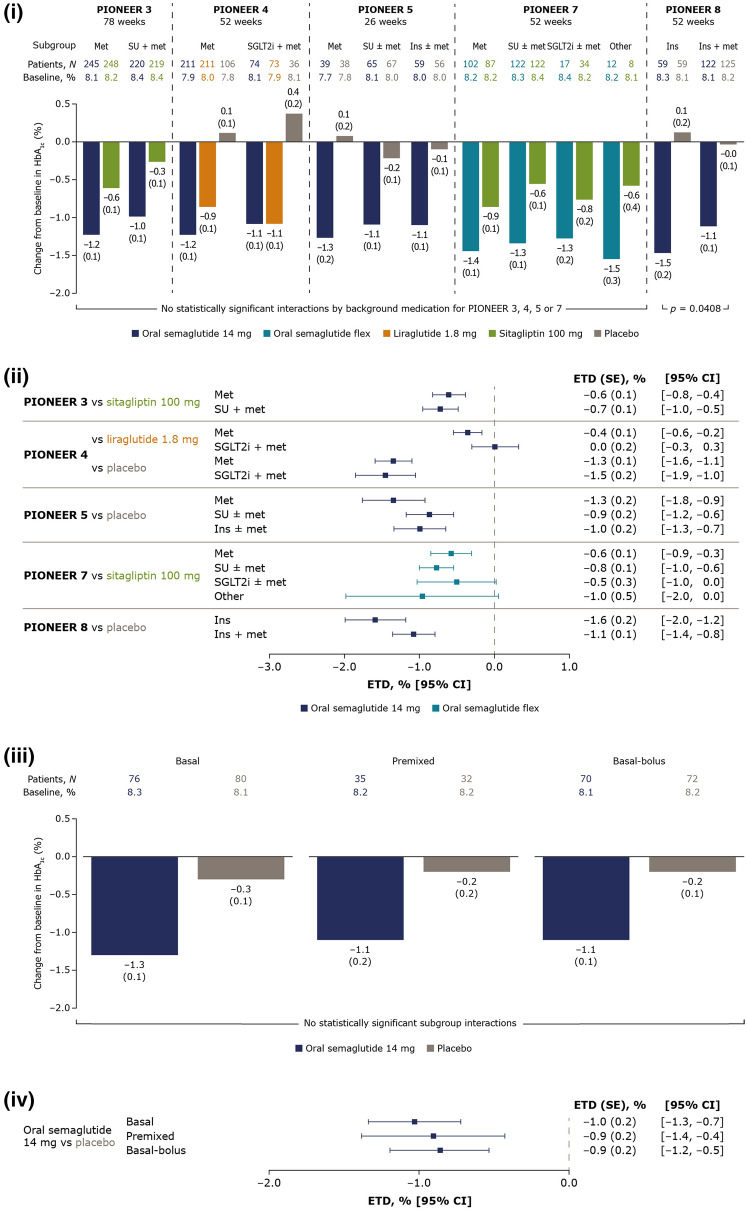
HbA1c and body weight reductions were generally greater with oral semaglutide 14 mg and flexibly dosed than with comparators, although glycaemic efficacy was similar to liraglutide 1.8 mg. HbA1c reductions with oral semaglutide 14 mg and flexibly dosed were broadly similar across background medication subgroups within each trial; change from baseline ranged from − 1.0 to − 1.5% at the end of treatment (Fig. 1(i)). In comparison, changes from baseline were − 0.3 to − 0.9% with sitagliptin 100 mg and − 0.9 to − 1.1% with liraglutide 1.8 mg. ETDs between oral semaglutide and comparators were generally consistent across background medication subgroups within each trial. Background medication only had a significant effect on the change in HbA1c in PIONEER 8, where the treatment difference between oral semaglutide and placebo was smaller in patients taking insulin and metformin compared with insulin alone (Fig. 1(ii)).
Fig. 1.
Change from baseline in HbA1c and estimated treatment differences for oral semaglutide 14 mg and flexibly dosed versus comparators by background medication (i and ii) and by insulin regimen (iii and iv). (i) Data are estimated changes from baseline (means with SEs in brackets) for the trial product estimand (on trial product without rescue medication). The p value is for the unadjusted two-sided test of the treatment by subgroup interaction. N number of patients contributing to the analysis, Other includes thiazolidinediones and other oral glucose-lowering medications. (ii) Data are for the trial product estimand (assumes patients remained on the trial product without rescue medication use). (iii) Data are estimated changes from baseline (means with SEs in brackets) for the treatment policy estimand (regardless of trial product discontinuation or rescue medication use in all randomised patients). N total number of patients in each subgroup (full analysis set). (iv) Data are for the treatment policy estimand. CI confidence interval, ETD estimated treatment difference, flex flexible dose adjustment, HbA1c glycated haemoglobin, ins insulin, met metformin, SE standard error of the mean, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea
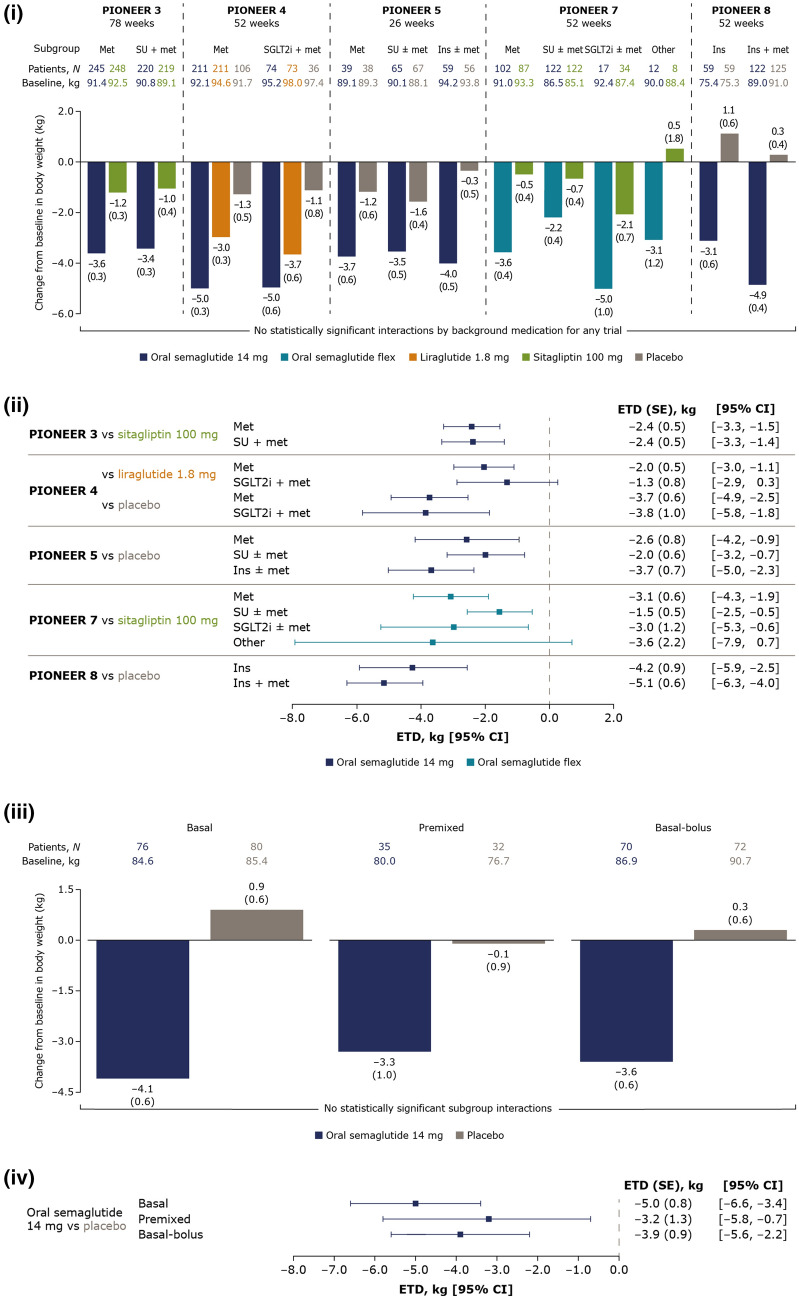
Body weight reductions with oral semaglutide 14 mg and flexibly dosed varied slightly across background medication subgroups within each trial, but with no significant treatment interactions by background medication (Fig. 2(i)). Changes from baseline in body weight were greater with oral semaglutide (− 2.2 to − 5.0 kg) than comparators (0.5 to − 2.1 kg with sitagliptin and − 3.0 to − 3.7 kg with liraglutide) across background medication subgroups. ETDs between oral semaglutide and comparators were generally consistent across background medication subgroups within each trial (Fig. 2(ii)).
Fig. 2.
Change from baseline in body weight and estimated treatment differences for oral semaglutide 14 mg and flexibly dosed versus comparators by background medication (i and ii) and by insulin regimen (iii and iv). (i) Data are estimated changes from baseline (means with SEs in brackets) for the trial product estimand (on trial product without rescue medication). N number of patients contributing to the analysis, Other includes thiazolidinediones and other oral glucose-lowering medications. (ii) Data are for the trial product estimand (assumes patients remained on the trial product without rescue medication use). (iii) Data are estimated changes from baseline (means with SEs in brackets) for the treatment policy estimand (regardless of trial product discontinuation or rescue medication use in all randomised patients). N total number of patients in each subgroup (full analysis set). (iv) Data are for the treatment policy estimand. CI confidence interval, ETD estimated treatment difference, flex flexible dose adjustment, ins insulin, met metformin, SE standard error of the mean, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea
Efficacy Outcomes by Insulin Regimen
At week 52, reductions in HbA1c and body weight with oral semaglutide 14 mg were greater than those with placebo when added to insulin (Figs. 1(iii), 2(iii)). ETDs for changes from baseline in HbA1c and body weight were generally similar across all insulin regimen subgroups, and no significant treatment interactions by insulin regimen were found (Figs. 1(iv), 2(iv)). At week 52, the total daily insulin dose was decreased from baseline with oral semaglutide 14 mg for patients on basal, premixed and basal-bolus insulin, and were generally consistent regardless of background insulin regimen (see Fig. S1 in the “Supplementary Information”).
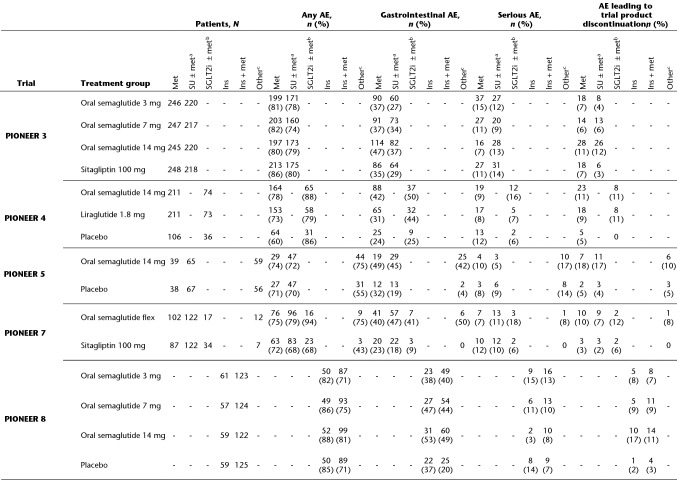
Safety Outcomes
The incidences of any on-treatment AEs, gastrointestinal AEs, serious AEs and AEs leading to trial product discontinuation by treatment and background medication subgroup are shown in Table 3. The overall incidence of AEs was similar between oral semaglutide and comparators (approximately 60–80% of patients in any subgroup had an AE) and, as expected, gastrointestinal AEs (primarily nausea, vomiting and diarrhoea) made up a substantial proportion of the overall events for oral semaglutide. However, the incidence of gastrointestinal AEs was generally similar regardless of the background medication subgroup. Serious AEs occurred in 3–17% of patients, and up to 17% of patients permanently discontinued their assigned medication across background medication subgroups. There was no discernible pattern in the incidence of serious AEs and AEs leading to trial product discontinuation by treatment group or background medication subgroup. In the insulin regimen analysis, the proportion of patients with any on-treatment AE was somewhat higher in the patients receiving oral semaglutide added to basal-bolus insulin (82–87%) than among those receiving oral semaglutide plus basal (65–82%) or premixed (69–80%) insulin, but no such trend was evident in the incidence of gastrointestinal or serious AEs, or in those leading to trial product discontinuation (see Table S2 in the “Supplementary Information”).
Table 3.
On-treatment adverse events
Gastrointestinal AEs were defined by MedDRA version 20.1 and comprised events of nausea, diarrhoea, vomiting, abdominal pain, constipation, gastroesophageal reflux disease, abdominal discomfort, dyspepsia, gastritis, abdominal pain upper, abdominal distention, dry mouth, flatulence, large intestine polyp, chronic gastritis, umbilical hernia, Barrett’s oesophagus, and dental caries
AE adverse event, flex flexible dose adjustment, ins insulin, met metformin, N number of patients included in analyses, n number of patients with at least one event, SGLT2i sodium-glucose cotransporter-2 inhibitor, SU sulphonylurea, TZD thiazolidinedione
aSU + met in PIONEER 3, SU ± met in PIONEER 5 and 7
bSGLT2i + met in PIONEER 4, SGLT2i ± met in PIONEER 7
cMet, SU ± met, or ins ± met in PIONEER 5, 1–2 of met, SU, SGLT2i or TZD in PIONEER 7
There was generally a low incidence of severe and symptomatic blood glucose-confirmed hypoglycaemia, but the rate was higher in background medication subgroups containing sulphonylurea or insulin, regardless of the trial product received (see Table S3 in the “Supplementary Information”). As expected, there was a higher incidence of hypoglycaemia in PIONEER 8, in which all patients were receiving background insulin, but this incidence was similar for the oral semaglutide and placebo groups. In the insulin regimen analysis, most hypoglycaemic episodes generally occurred in patients on basal-bolus insulin. There were few on-treatment severe hypoglycaemic episodes, regardless of the background medication or insulin regimen.
There were few events of genital infection and increased urination in PIONEER 4 and 7, and no indication that the risk in patients treated with an SGLT2i was aggravated by concurrent treatment with oral semaglutide (see Table S4 in the “Supplementary Information”).
Discussion
In the primary analyses of the global PIONEER trials, oral semaglutide consistently demonstrated clinically meaningful reductions in HbA1c and body weight across a broad spectrum of patients with T2D [7–14]. In the current post-hoc exploratory subgroup analyses, the effects of oral semaglutide on HbA1c and body weight were broadly similar regardless of background medication and insulin regimen, and these effects were generally greater with oral semaglutide (14 mg and flexibly dosed) than with comparators. An exception was HbA1c reductions with liraglutide, which were similar to those seen with oral semaglutide. These observations are consistent with those from similar post-hoc analyses of patients receiving glucose-lowering background medication in the clinical trial programme for once-weekly subcutaneous semaglutide (SUSTAIN 2–4 and 10) [17].
In PIONEER 8, duration of diabetes, body weight and total daily insulin dose appeared to be higher in patients receiving basal-bolus insulin than in those on the other insulin regimens. This could be explained by patients requiring more comprehensive treatment as their disease progresses, therefore moving from a basal to a basal-bolus regimen. Patients receiving basal-bolus insulin had higher baseline body weight than other insulin regimens, which may relate to the fact that they were receiving higher doses and prandial doses of insulin, which are associated with weight gain. The treatment difference for change in HbA1c between oral semaglutide and placebo was smaller in patients taking insulin and metformin than in those taking insulin alone, although the treatment difference was still clinically relevant. It is unclear whether the efficacy of oral semaglutide or other GLP-1RAs is impacted by metformin in the presence of another insulin and other glucose-lowering background medications.
The current European Association for the Study of Diabetes (EASD) and American Diabetes Association (ADA) consensus report recommends stepwise addition of glucose-lowering medications, including the newer classes, i.e. GLP-1RAs, SGLT2is and dipeptidylpeptidase-4 inhibitors (DPP4is), with early combination therapy advocated when the patient’s HbA1c is above target despite diet, exercise and metformin [5, 6]. The preference for the medications to add after metformin depends on individual patient factors as well as other practical and economic considerations [5]. It is therefore important to understand the relative advantages and disadvantages of each treatment and how they might be used with other glucose-lowering agents in order to make informed decisions.
In these subgroup analyses, oral semaglutide 14 mg or flexibly dosed was more effective at reducing HbA1c and body weight than the DPP4i sitagliptin when added to a variety of representative background regimens. This is consistent with head-to-head clinical trials and meta-analyses which indicate that GLP-1RAs are more efficacious than DPP4is [18–22]. Furthermore, GLP-1RAs (and SGLT2is) lead to additional benefits that are not evident with DPP4is; for instance, they reduce cardiorenal risk [23–30].
There is considerable interest in the potential to combine GLP-1RAs with SGLT2is because their mechanisms of action are complementary with regard to target organs, disease processes [31], and the effects seen in cardiovascular/cardiorenal outcomes trials [23–29, 32–36]. There are relatively few prospective studies on the combined use of GLP-1RAs and SGLT2is, although a meta-analysis has suggested a benefit of the combination in terms of improved reductions in HbA1c and body weight [37]. In the current subgroup analysis of PIONEER 4, which included relatively few patients receiving both treatments, reductions in HbA1c (of 1.1–1.2%) and body weight (5 kg) were similar after 1 year in patients with T2D treated with oral semaglutide with or without background SGLT2i. The overall tolerability of the combination was similar to that of oral semaglutide alone.
In the PIONEER programme, oral semaglutide had a safety profile similar to that characterised for other GLP-1RAs [2]. In the current subgroup analyses, background medication had a limited effect on the tolerability of oral semaglutide (including on gastrointestinal AEs). The incidence of AEs tended to be higher for patients receiving insulin than other background medications, which may reflect the safety profile and the fact that insulin is often used later in the treatment course of T2D, when patients are more vulnerable. As might be expected, hypoglycaemic episodes were more common in patients receiving background insulin and sulphonylurea.
Efficacy and tolerability outcomes with oral semaglutide were generally consistent regardless of background insulin regimen in PIONEER 8, although AEs (including severe or blood glucose-confirmed hypoglycaemia) were more common with background basal insulin than with premixed or basal-bolus regimens. The total daily insulin dose requirement was reduced with oral semaglutide compared with placebo, without a clear link between insulin regimen subgroup and the change in total daily insulin dose. Given the increased risk of body weight gain and hypoglycaemia associated with long-term insulin use, the potential for reducing insulin doses with the addition of oral semaglutide (as well as its weight-decreasing potential) may be of clinical importance. In line with these results, in the randomised, placebo-controlled SUSTAIN 5 trial of once-weekly subcutaneous semaglutide as an add-on to basal insulin, the insulin dose was significantly decreased from baseline with both the semaglutide 0.5 and 1.0 mg doses compared with placebo [38].
Randomisation in these trials was prospectively stratified by baseline background medication, which can be considered a strength of the current analyses. Nevertheless, the subgroup analyses presented here were conducted post hoc and thus have limitations compared with prospective studies. Furthermore, the statistical analyses of efficacy outcomes were not controlled for multiplicity. As such, the outcomes should be considered exploratory in nature and warrant further prospective investigation. The PIONEER programme included a large number of patients, and data from more than 2800 were included in these analyses. Despite this, it should be noted that patient numbers in certain background medication subgroups were relatively low. Nevertheless, these analyses help to further characterise the profile of oral semaglutide in the context of guideline-recommended management of patients with T2D.
Conclusions
These exploratory subgroup analyses support the results of the primary analyses of the PIONEER trials and suggest that oral semaglutide can be used across a broad range of patients with T2D in combination with other commonly used glucose-lowering background medications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients who participated, the investigators, all trial site staff and all Novo Nordisk employees involved in these trials.
Funding
These trials and costs relating to the development and publication of this manuscript were funded by Novo Nordisk A/S, Søborg, Denmark. K.K. is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). J.B.B’s effort on this project is supported by grants from the NIH (UL1TR002489, P30DK124723).
Medical Writing and/or Editorial Assistance
The authors would like to thank Barrie Chubb of Novo Nordisk Pharma Ltd. for reviewing the manuscript, and Stephen Purver of Axis, a division of Spirit Medical Communications Group Limited, for assistance with medical writing and editorial support (funded by Novo Nordisk A/S).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
These data were presented at the American Diabetes Association 80th Scientific Sessions Virtual Meeting, 12–16 June 2020.
Disclosures
Richard E. Pratley reports grants from Hanmi Pharmaceutical Co., Janssen, Sanofi, Poxel SA and Novo Nordisk; consulting fees from Merck, Pfizer, Sanofi, Scohia Pharma Inc, Sun Pharmaceutical Industries and Novo Nordisk; and speaker fees from Novo Nordisk. Dr. Richard Pratley's services were paid for directly to AdventHealth, a nonprofit organisation. Matthew J. Crowley reports no conflicts of interest. Mette Gislum, Christin Løth Hertz and Thomas Bo Jensen are employees of Novo Nordisk A/S. Kamlesh Khunti has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme; received grants in support of investigator and investigator-initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Pfizer and Boehringer Ingelheim; and has served on advisory boards for Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme. Ofri Mosenzon has served on advisory boards for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca and BOL Pharma; reports research grant support through Hadassah Hebrew University Hospital, Novo Nordisk and AstraZeneca; and serves on a speaker's bureau for AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim and Janssen. In the past 3 years, John B. Buse has received fees for consultation and travel support for contracted activities that have been paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen. John B. Buse reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; is a consultant to Cirius Therapeutics Inc, CSL Behring, Fortress Biotech, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, Stability Health and Zealand Pharma; and holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio and Stability Health.
Compliance with Ethics Guidelines
The PIONEER trial protocols were approved by institutional review boards or independent ethics committees at each participating trial site, and were undertaken in accordance with ICH Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent before undertaking trial-related activities.
Data Availability
Data will be shared with researchers who submit a research proposal approved by an independent review board. Access request proposals can be found at novonordisk-trials.com. Data will be made available after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in datasets in a de-identified and anonymised format. There will not be any limitations on how these data can be used.
References
- 1.Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10:eaar7047. doi: 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- 2.Thethi TK, Meier JJ, Pratley R. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22:1263–1277. doi: 10.1111/dom.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novo Nordisk A/S. Rybelsus®: US prescribing information 2020. https://www.novo-pi.com/rybelsus.pdf. Accessed 30 June 2020.
- 4.Novo Nordisk A/S. Rybelsus®: EU summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf. Accessed 21 Aug 2020.
- 5.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buse JB, Wexler DJ, Tsapas A, et al. Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulphonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466–80. [DOI] [PMC free article] [PubMed]
- 8.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 9.Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–527. doi: 10.1016/S2213-8587(19)30192-5. [DOI] [PubMed] [Google Scholar]
- 10.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262–2271. doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 13.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 14.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 15.Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21:2203–2210. doi: 10.1111/dom.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: John Wiley & Sons; 1987.
- 17.Capehorn M, Ghani Y, Hindsberger C. Once-weekly semaglutide reduces HbA1c and body weight in patients with type 2 diabetes regardless of background common OAD: a subgroup analysis from SUSTAIN 2–4 and 10. Diabetes Ther. 2020;11:1061–1075. doi: 10.1007/s13300-020-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergenstal R, Wysham C, Macconnell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 19.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 20.Weinstock RS, Guerci B, Umpierrez G, et al. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–858. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 22.Tran A, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(Suppl 1):68–76. doi: 10.1111/dom.13137. [DOI] [PubMed] [Google Scholar]
- 23.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC, Colhoun HM, Dagenaid GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 27.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 28.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 29.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauck MA, Meier JJ. GLP-1 receptor agonists and SGLT2 inhibitors: a couple at last? Lancet Diabetes Endocrinol. 2016;4:963–964. doi: 10.1016/S2213-8587(16)30263-7. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 33.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 34.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 35.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 36.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 37.Castellana M, Cignarelli A, Brescia F, et al. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: a meta-analysis. Sci Rep. 2019;9:1935. doi: 10.1038/s41598-018-37821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. Clin Endocrinol Metab. 2018;103:2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared with researchers who submit a research proposal approved by an independent review board. Access request proposals can be found at novonordisk-trials.com. Data will be made available after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in datasets in a de-identified and anonymised format. There will not be any limitations on how these data can be used.