Abstract
Microvascular and macrovascular diseases are the main causes of morbidity in type 2 diabetes patients through chronic hyperglycaemic condition via oxidative stress and inflammation. Reactive oxygen species (ROS) activate p38 MAPK phosphorylation and inflammation which enhances protein modification by carbonylation. The use of metformin and a p38 MAPK inhibitor is hypothesised to reduce ROS production and inflammation but effects of metformin and p38 MAPK inhibitor (SB203580) on ROS production and inflammation in vascular type 2 diabetes mellitus non-obese (T2DM) have not been investigated. The Goto-Kakizaki rat T2DM model was divided into three groups as T2DM, T2DM treated with 15 mg/kg bw metformin and T2DM treated with 2 mg/kg bw SB203580 for 4 weeks. Rat aortas were isolated and protein carbonyl (PC) contents were measured by spectrophotometric DNPH assay. Aortic IL-1ß level was determined by ELISA. Results showed that aortic PC contents in the T2DM group were significantly higher than in non-diabetic rats. Treatment with metformin or SB203580 significantly reduced PC contents while only metformin significantly reduced IL-1ß levels. Findings indicated that metformin reduced ROS production and inflammation in diabetic vessels and possibly reduce vascular complications in non-obese T2DM.
Keywords: Type 2 diabetes mellitus, Vascular complications, Protein carbonyl, Inflammation, Metformin, SB203580
Introduction
Type 2 diabetes mellitus (T2DM) is positively associated with obesity. However, T2DM is also prevalent in non-obese communities, especially Asian countries [1]. Non-obese type 2 diabetes has similar risk complications to those in obese diabetic patients and should be given greater attention.
Microvascular and macrovascular diseases are major causes of morbidity and mortality in patients with T2DM; especially for ischemic heart disease, which is a major cause of death worldwide [2]. Uncontrollable production of blood glucose results in hyperglycaemia which triggers the generation of reactive oxygen species (ROS) and oxidative stress in various internal organ tissues [3]. Massive ROS enhanced redox imbalance activates stress-sensitive intracellular signalling pathways such as p38 mitogen-activated protein kinase (p38 MAPK) which regulates ROS production to generate a “feed-forward loop” that consequently enhances vascular complications via inflammation, endothelial dysfunction and vascular damage [4]. In addition, obesity and T2DM activated inflammation- and stress-induced kinases IκB kinase-ß (IKKß) and JUN N-terminal kinases (JNKs) pathway via elevated circulating levels of inflammatory cytokine such as interleukin-1ß (IL-1ß) [5]. This also related to the findings that glucotoxicity may promote inflammatory response by activating the NLR pyrin domain containing 3 (NLRP3) inflammasome to release proinflammatory IL-1β that further amplifies inflammation [6]. Therefore, controlling blood sugar by anti-diabetic drugs and p38 MAPK inhibitor is hypothesized to reduce ROS generation and inflammation and consequently reduce diabetic complications.
Treatment of type 2 diabetic patients can be primarily performed using metformin, a biguanide first-line oral medication recommended for glycaemic control and prevention of diabetic complications. Current studies are tempting to assessing the non-metabolic effects of metformin in modulating inflammation and oxidation [6]. Metformin reduces ROS generation and inflammation [7] thereby ameliorating diabetic complications. Diabetes can also cause an increase in basal p38 MAPK activation which aggravates disease conditions. Thus, inhibition of p38 MAPK using an inhibitor offers therapeutic potential for diabetes treatment [8].
Excessive ROS lead to biomolecular damage, especially proteins. The oxidative damage imparts on protein can lead to loss of cell function and structural integrity [4].
Protein carbonylation is an irreversible oxidative modification of protein on lysine, arginine, proline and threonine residues with reactive carbonyl compounds. Determination of protein carbonyl (PC) content in samples can be used as a potential oxidative stress biomarker [9]. Here, level of oxidative stress generation in blood vessels was determined by vascular PC content in lean T2DM animal models. Effects of the antidiabetic drug metformin as well as p38 MAPK inhibitor (SB203580) treatment on vascular PC content were also investigated.
Materials and Methods
Experimental Animals
Male Wistar rats (control group) and non-obese type 2 diabetic Goto-Kakizaki (GK) rats were obtained from Nomura Siam International Co., Ltd. Bangkok, Thailand. The animals were maintained under controlled temperature (22 ± 1 °C) with 12 h light–12 h dark cycles at the Centre for Animal Research, Naresuan University, Phitsanulok, Thailand. All protocols used were approved by the Committee of the Centre for Animal Research, Naresuan University (NU-AE581023).
Study Groups and Drug Treatment
All rats were maintained for 4 weeks after purchase and glycaemic parameters including fasting blood glucose, haemoglobin A1C level and oral glucose tolerance test (OGTT) were performed to confirm their diabetic status. The rats were divided into two major groups as a control group (Wistar rats; n = 6) and a diabetic group (GK rats; n = 18). Diabetic GK rats were then divided into three subgroups as a diabetic group (GK rats; n = 6) receiving deionised water as control, a diabetic group (n = 6) that received metformin at 15 mg/kg bw twice daily by oral gavage and a diabetic group (n = 6) that received 2 mg/kg bw of SB203580 by intraperitoneal (IP) injection every 3 days. Rats were treated with drugs for 4 weeks before sacrifice and aortic tissue collection.
Determination of Glycaemic Parameters
Rats were fasted for 12–14 h before collecting a tail vein blood sample to measure blood glucose level using a glucometer (SD GlucoNavii® G DH, SB Biosensor, Korea). The glycated haemoglobin test (HbA1c) was performed using a Clover A1C™ Self-analyser. For the oral glucose tolerance test (OGTT), rats were fed with 2 g/kg bw of 40% (w/v) glucose solution by oral gavage. Blood was collected from the tail vein at 30, 60, 90 and 120 min after glucose treatment and analysed for blood glucose with a glucometer.
Isolation of Descending Aorta to Abdominal Aorta in Rats and Tissue Homogenisation
Rats were anaesthetised by intraperitoneal injection (IP) with 100 mg/kg pentobarbital and heparin 150 units. The aortas were isolated from the iliac bifurcation [10] and maintained at − 20 °C in a freezer before protein collection.
Aortic Protein Extraction
Approximately 50 mg of aortic tissue was weighed and then homogenised in 500 µl of homogenisation buffer. Homogenates were centrifuged at 14,000 rpm, 4 °C for 10 min and supernatants were collected for further analysis.
Pre-analytical Quality Control
Linearity tests were measured by control serum (HUMATROL P) in 6 concentrations with deionized water (DI) at 0, 2, 4, 6, 8 and 10 mg/ml. Protein carbonyl content levels were measured by spectrophotometric DNPH assay. Within-run precision assays were determined at a single concentration of control serum in 15 reactions. Between-day precision assays were performed by determining the concentration of control serum every day for 5 consecutive days and coefficients of variance (%CV) were analysed.
Spectrophotometric Determination of Vascular Protein Carbonyl Levels
Vascular protein carbonyl content was spectrophotometrically determined by colorimetric DNPH assay as described previously [11]. Briefly, extracted vascular protein was diluted 1:10 with phosphate-buffered saline (PBS). Two hundred microliters of diluted vascular protein were added into 800 µl of 10 mM DNPH in 2.5 M HCl. One millilitre of 20% (w/v) trichloroacetic acid (TCA) was then added and centrifuged at 10,000×g for 10 min at 4 °C to precipitate protein. The protein pellet was washed 3 times with 1 ml of 1:1 (v/v) ethanol: ethyl acetate and centrifuged at 10,000×g for 10 min at 4 °C. After final washing, the protein pellet was resuspended in 500 µl of 6 M guanidine hydrochloride and centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was collected and absorbance measured at 370 nm using 6 M guanidine hydrochloride as a blank. Protein carbonyl content (nmol/mg) was calculated following the procedure used in a previous study [11].
Determination of Inflammatory Cytokine Levels by Enzyme-Linked Immunosorbent Assay (ELISA)
Determination of inflammatory cytokines level by ELISA was performed using ABTS ELISA Buffer Kit, Prepotech®. ELISA reagents were prepared at room temperature by gentle mixing. First, a pre-coated 96-well plate was prepared by adding 100 μl of 1 μg/ml capture antibody to each well and incubating overnight at room temperature. On the next day, the plate was inverted to remove blocking reagent and blotted on a paper towel to remove excess liquid. The plate was then washed 4 times with 200 μl of washing buffer solution. Blocking solution was added into each well at 200 μl and incubated for 1 h. Samples were added into the wells following the volume calculated by Bradford assay to control a total protein mass as 100 μg and incubated at room temperature for at least 2 h. Then, 100 μl of detection antibody was added and incubated at room temperature for 2 h. Finally, horseradish peroxidase (HRP) conjugated Avidin was added before incubating with ABTS liquid substrate. Reaction colour was measured by a spectrophotometer at 405 nm with wavelength correction set at 650 nm.
Statistical Analysis
Data were analysed by GraphPad Prism version 5.00 and expressed as mean ± standard error of mean (SEM). Differences of PC content level between the three diabetic groups were analysed by one-way ANOVA with comparisons between groups analysed by unpaired t test. A p value ≤ 0.05 was considered to be statistically significant.
Results
Confirmation of Lean Type 2 Diabetic-Like Animal Model
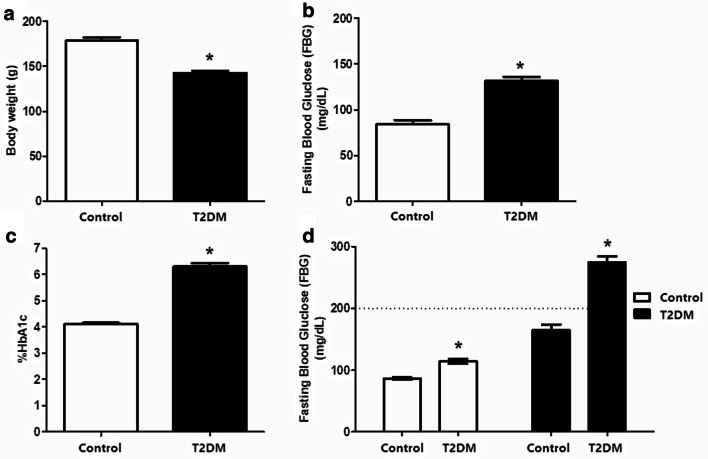
To ensure validity of the lean type 2 diabetic animal model several glycaemic parameters were explored. First, the non-obese or lean animal model was confirmed by measuring body weight as well as glycaemic parameters. Results showed that body weights of GK rats at the same age were significantly lower than control Wistar rats (142.5 ± 2.622 g vs. 178.2 ± 4.048 g, p value < 0. 05) (Fig. 1a). Glycaemic measurements showed that mean fasting blood glucose (FBG) levels of GK rats were significantly higher than Wistar rats (131.5 ± 4.507 mg/dL vs. 84.00 ± 4.507 mg/dL, p value < 0.05) (Fig. 1b). Mean percentages of HbA1c levels of GK rats were also higher than control group (6.310 ± 0.1251% vs. 4.120 ± 0.03887%, p value < 0.05) (Fig. 1c). Results from the oral glucose tolerance test (OGTT) showed impaired glucose tolerance in GK rats 2 h after glucose uptake with blood glucose greater than 200 mg/dl (Fig. 1d).
Fig. 1.
Confirmation of lean type 2 diabetic-like animal model. The characteristics of GK rat as T2DM like—model consisted of body weight (a) and glucose parameters including fasting blood glucose (FBS) (b), Glycated hemoglobin (HbA1c) (c), and oral glucose tolerance test (OGTT) (d)
Pre-analytical Quality Control Assessment for Protein Carbonyl Content Measurement
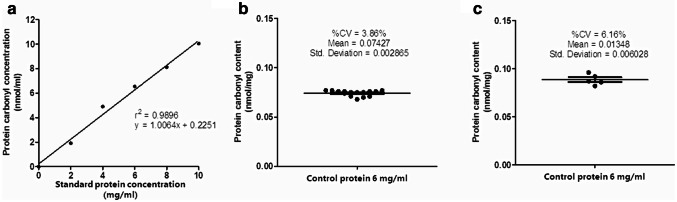
Linearity regression of the test using control serum was 0.9927 (r2 = 0.9896, y = 1.0064x + 0.2251 (Fig. 2a). Within-run coefficient of variation (CV) was 3.86% (Fig. 2b) and between-day variation (CV) was 6.16% (Fig. 2c).
Fig. 2.
Pre-analytical quality control assessment for protein carbonyl content measurement. The quality control of spectrophotometric DNPH assay including linearity test (r2 = 0. 9896) (a), the within-run coefficient of variation (CV) was 3.86% (b) and the between-day of variation (CV) was 6.16% (c)
Metformin and SB203580 Reduced Plasma Glucose
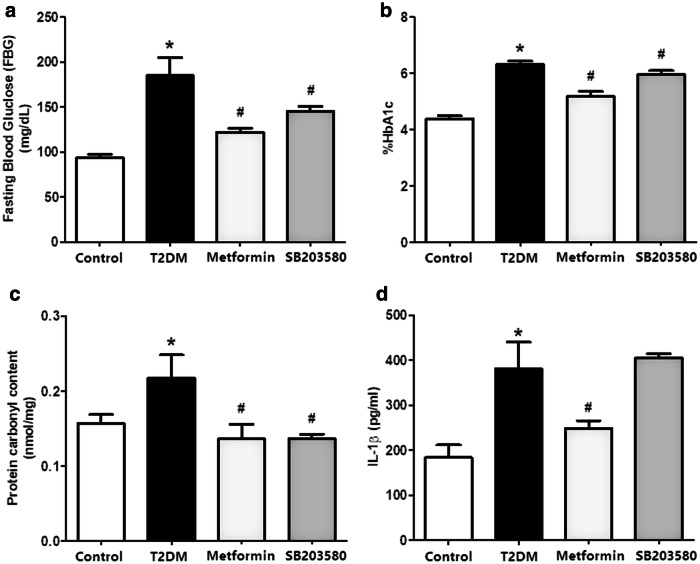
After 4 weeks of drug treatment, blood was collected to measure FBS and HbA1c levels. Results indicated that FBG levels of the diabetic group were significantly higher than the control group (185.1 ± 19.92 mg/dl vs. 93.78 ± 3.593 mg/dl, p value < 0.05), while treatment with metformin or SB203580 significantly decreased FBS levels compared to the T2DM group (122.3 ± 4.412 mg/dl and 145.5 ± 5.106 mg/dl vs. 185.1 ± 19.92 mg/dl, p value < 0.05, respectively) (Fig. 3a). Glycated haemoglobin (%HbA1c) demonstrated a significant increase in the T2DM group compared to control (6.310 ± 0.1251% vs. 4.378 ± 0.1128%, p value < 0.05). Treatment with metformin or SB203580 significantly reduced %HbA1c compared to the T2DM group (5.189 ± 0.1620% and 5.957 ± 0.1378% vs. 6.310 ± 0.1251%, p value < 0.05, respectively) (Fig. 3b).
Fig. 3.
Metformin and SB203580 reduced aortic protein carbonyl content level and only metformin reduced aortic IL-1ß level. After 4 weeks of drugs treatment, blood was collected for measuring FBS (a) and HbA1c (b) level. After that, aortic PC content were determined by spectrophotometric DNPH assay (c) and determine IL-1ß in vascular tissues (d)
Metformin and SB203580 Reduced Aortic Protein Carbonyl Content Levels
Results showed that aortic PC contents in the T2DM group were significantly higher than control (0.2174 ± 0.03115 nmol/mg vs. 0.1568 ± 0.01201 nmol/mg, p value < 0.05). Treatment with metformin or SB203580 significantly reduced PC contents in aortic tissue (0.1364 ± 0.01944 nmol/mg and 0.1364 ± 0.006114 nmol/mg vs. 0.2174 ± 0.03115 nmol/mg, p value < 0.05, respectively (Fig. 3c).
Metformin Reduced Aortic IL-1ß Levels
To determine vascular inflammation, IL-1ß levels were measured in the aortic protein extract. Results showed that aortic IL-1ß levels were significantly higher in the T2DM group than control (381.1 ± 59.56 pg/ml vs. 183.8 ± 28.20 pg/ml, p value < 0.05). Metformin treatment alone significantly reduced IL-1ß levels (249.1 ± 16.98 pg/ml, 381.1 ± 59.56 pg/ml, 406.2 ± 8.713 pg/ml p value < 0.05) (Fig. 3d).
Discussion
Treatment with metformin or selective p38 MAPK inhibitor (SB203580) demonstrated a significant reduction in FBG and HbA1c levels compared to the T2DM group. Moreover, high protein carbonyl contents in the diabetic group reduced when treated with metformin or p38 MAPK inhibitor. Metformin not only reduced vascular oxidative protein but also lowered inflammatory cytokine IL-1β. However, SB203580 failed to reduce inflammatory cytokine in isolated aortas of diabetic rats.
Goto-Kakizaki rats (GK rats) were used as spontaneous non-obese type 2 diabetic rats to model T2DM. No diagnostic criteria for diabetes in animals, in particular rodents, have been previously identified. Here, we confirmed diabetes status in GK rats using the normal range of blood glucose in rats [12] together with human diabetic criteria based on the American Diabetes Association (ADA) guideline 2017 [13]. Our data indicated that GK rats exhibited a diabetic-like model. In addition, confirmation of a non-obese model was performed using body weight. Results indicated growth rate of GK rats less than Wistar rats and suggested that GK rats had a non-obese type 2 diabetic-like phenotype.
High glucose levels induced ROS production by increasing superoxide production from mitochondrial respiration, glucose auto-oxidation, xanthine oxidation and protein kinase C (PKC) activated NAD(P)H oxidase [14]. In addition, oxidative stress also induced inflammation resulting in endothelial dysfunction, apoptosis and fibrosis that consequently led to diabetic vascular complications [15]. Previous studies demonstrated that protein carbonyl compounds positively correlated with HOMA-IR as the best predictor of insulin resistance [16] and also increased in diabetes patients with microvascular and macrovascular complications [17]. Type 2 diabetes-induced inflammation was also reported [18] but levels of protein carbonyl and inflammation were observed in serum and not in vascular tissue which might provide a better explanation of diabetic-induced vascular complications. Here, we demonstrated that controlling blood sugar levels by metformin also reduced ROS production and inflammation in vascular tissue, thereby preventing both microvascular and macrovascular complications and protecting damage to vital organs such as the heart, pancreas and kidney.
It has been reported that hyperglycaemic condition could increase basal p38 MAPK activation, which is one of the signaling pathway play role in cellular injury and apoptosis [8]. Therefore, inhibition of p38 MAPK activation by inhibitor could have more therapeutic potential to reduce cellular injury and death.
ROS could activate p38 MAPK activation during insulin resistance or T2DM. Meanwhile, p38 MAPK has been known to regulate ROS production, which could consequently induce complications. Using p38 MAPK inhibitor could have therapeutic potential to reduce ROS generation in diabetes, which will prevent diabetic complications in vital internal organs. In this study, we aim to test if p38 MAPK inhibitor could be able to reduce risk for vascular complications by reducing oxidative damage of Aortic tissue. Therefore, the effect of p38 MAPK inhibitor was hypothesized to reduce Aortic protein carbonyl level. If so, the combination of metformin and p38 MAPK inhibitor will be hypothesize in further study to determine the synergistic effect of both drug on oxidative damage of Aortic tissue, as well as other internal organs.
It has been reported that p38 MAPK is strongly activated by high glucose condition in rat aortic smooth muscle cells [19]. In addition, a diabetic mice model using streptozotocin injection showed a significant increase in p38 phosphorylation and proinflammatory cytokines including TNF-α and IL-1ß production in cardiac tissues [20]. Treatment with p38 MAPK inhibitor SB203580 reduced p38 phosphorylation and inflammatory cytokines. Our results showed that SB203580 significantly decreased ROS production in aortic tissue by reducing protein carbonyl content. However, SB203580 failed to reduce IL-1ß levels in vessels of non-obese T2DM. Failure of SB203580 to reduce inflammatory cytokine and lipopolysaccharide-induced IL-6 production in mouse resident peritoneal macrophage was reported [21]. Therefore, the effect of SB203580 on vascular inflammatory marker such as TNF-α and IL-6 requires further investigation. Our results indicated that both metformin and SB203580 can significantly reduce ROS production in vascular tissue. However, the effects of these two drugs, singularly as well as in combination, require further investigation in other vital organs such as the heart, brain, liver, pancreas and kidney.
In this study we determined the protein carbonyl level, which is the product of oxidative modification of protein and reflecting degree of oxidative damage occurred in the Aortic tissue. However, the degree of oxidative stress by ROS generation in hyperglycaemic condition as well as the effect of drugs on ROS generation in aortic tissue should also be determined and could be considered as limitations of this study.
Conclusions
Effects of metformin and p38 MAPK inhibitor (SB203580) on reduction of diabetic-induced oxidative stress and inflammation in vascular tissues of non-obese T2DM were investigated. Results will provide useful information regarding therapeutic or preventive activity of metformin and p38 MAPK inhibition on diabetic complications in vital internal organs.
Acknowledgements
This thesis is sponsored and supported by National Research Council of Thailand. We would like to thank Naresuan University Research endowment fund Grant I.D. Numbers R2559A017, R2560C138. We would like to thank PhD scholarship from Naresuan University for Nuttikarn Nokkaew, Royal Golden Jubilee Ph.D. Program-Thailand Research Fund (TRF) (No. PHD/0087/2556) for Jantira Sanit and (No. PHD/0125/2558) for Kantapitch Kongpol. We are grateful to the Center for Animal Research, Naresuan University for their excellent technical assistance. We would like to thanks ProofRead4Sure service to English proof reading and editing.
Author Contributions
NN, PM and SK conceived and designed the experiments; NN, RJ, SJ, NT, NM, NS, MI, JS, PA, and KK performed the experiments; NN, PM and SK analyzed the data; SK contributed reagents/materials/analysis tools; NN, NN and SK wrote and prepared the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boffetta P, McLerran D, Chen Y, Inoue M, Sinha R, He J, et al. Body mass index and diabetes in Asia: a cross-sectional pooled analysis of 900,000 individuals in the Asia cohort consortium. PLoS ONE. 2011;6(6):e19930. doi: 10.1371/journal.pone.0019930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MK, Narayan KMV, Tandon N. Diabetes and coronary heart disease: current perspectives. Indian J Med Res. 2010;132(5):584–597. [PMC free article] [PubMed] [Google Scholar]
- 3.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34(31):2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 5.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 6.Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–S252. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- 7.Araújo AA, Pereira ASBF, Medeiros CACX, Brito GAC, Leitão RFC, Araújo LS, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE. 2017;12(8):e0183506. doi: 10.1371/journal.pone.0183506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumphune S, Chattipakorn S, Chattipakorn N. Roles of p38-MAPK in insulin resistant heart: evidence from bench to future bedside application. Curr Pharm Des. 2013;19(32):5742–5754. doi: 10.2174/1381612811319320009. [DOI] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9(4):169–176. doi: 10.1016/S1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 10.Robbins N, Thompson A, Mann A, Blomkalns AL. Isolation and excision of murine aorta; a versatile technique in the study of cardiovascular disease. J Vis Exp. 2014;93:52172. doi: 10.3791/52172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maneewong K, Mekrungruangwong T, Luangaram S, Thongsri T, Kumphune S. Combinatorial determination of ischemia modified albumin and protein carbonyl in the diagnosis of NonST-elevation myocardial infarction. Indian J Clin Biochem. 2011;26(4):389–395. doi: 10.1007/s12291-011-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. Estimation of the normal range of blood glucose in rats. Wei sheng yan jiu J Hyg Res. 2010;39(2):133–137. [PubMed] [Google Scholar]
- 13.Association AD. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Supplement 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 14.Son SM. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab. 2012;36(3):190–198. doi: 10.4093/dmj.2012.36.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki S, Inoguchi T. The role of oxidative stress in the pathogenesis of diabetic vascular complications. Diabetes Metab. 2012;36(4):255–261. doi: 10.4093/dmj.2012.36.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar P, Kar K, Mondal MC, Chakraborty I, Kar M. Elevated level of carbonyl compounds correlates with insulin resistance in type 2 diabetes. Ann Acad Med Singap. 2010;39(12):909. [PubMed] [Google Scholar]
- 17.Bigagli E, Raimondi L, Mannucci E, Colombi C, Bardini G, Rotella C, et al. Lipid and protein oxidation products, antioxidant status and vascular complications in poorly controlled type 2 diabetes. Br J Diabetes Vasc Dis. 2012;12(1):33–39. doi: 10.1177/1474651411435588. [DOI] [Google Scholar]
- 18.Herder C, Illig T, Rathmann W, Martin S, Haastert B, Muller-Scholze S, et al. Inflammation and type 2 diabetes: results from KORA Augsburg. Gesundheitswesen. 2005;67(Suppl 1):S115–S121. doi: 10.1055/s-2005-858252. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang Z-Y, Yamauchi T, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Investig. 1999;103(2):185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49(10):2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 21.Shi Q, Cheng L, Liu Z, Hu K, Ran J, Ge D, et al. The p38 MAPK inhibitor SB203580 differentially modulates LPS-induced interleukin 6 expression in macrophages. Cent Eur J Immunol. 2015;40(3):276–282. doi: 10.5114/ceji.2015.54586. [DOI] [PMC free article] [PubMed] [Google Scholar]