Abstract
The development of Lateral Flow Immunochromatography Assay can be divided into two levels; standardizing membrane characteristics and optimizing molecular level immunoassay reaction between analyte and detector molecules. In the preliminary phase the reaction specificity of capture and detector antibodies with the analyte has to be checked with other techniques like ELISA. Molarity and pH of conjugation buffer have prime importance in the immunoreaction among analyte and antibodies. Epitope mapping of the capture and detector antibodies is also recommended to confirm the specificity of the assay. Standardization of membrane characteristics directly relates to the sensitivity of the assay through its porosity, hydrophobicity, protein holding/releasing capacity and wicking rate. Under optimised condition a perfect Lateral Flow Immunochromatography Assay should have high on-rate (target binding efficiency), low off-rate (target releasing efficiency) and low Cross-reactivity. In this manuscript, we share our experience, especially on developmental strategies and troubleshooting, that we have experienced during Lateral Flow Immunochromatography Assay kit development.
Keywords: Lateral flow immunochromatography, Colloidal gold, Sensitivity, Trisodium citrate
Introduction
Lateral flow assays based on Immunochromatography principles exhibit a wide range of applications as diagnostic as well as prognostic tool. The target analytes of lateral flow assays include infectious disease agents, drugs of abuse, hormones, cancer markers, food contaminants and agricultural contaminants [1, 2]. The color formation due to antigen and antibody reaction in test line and control line of nitrocellulose membrane offers qualitative assay of the analytes. The qualitative assays can be transformed into quantitative with customized equipments which can quantify color intensity or fluorescence. Lateral flow Immunochromatography Assay (LFIA) is an affordable technology aiming at rapid detection of analyte within a short period of 5–30 min [3, 4].
Sandwich immunoassay is the principle used for the detection of protein/peptide antigens, while competitive assays are used for steroid based small sized antigens and drugs of abuse detection [5, 6]. Multiplexing of the assay is widely used nowadays, which allows the simultaneous detection of multiple analytes in one single test. Based on the nature of analyte, LFIA can be classified as rapid test for antibody detection or rapid test for antigen detection. Rapid test for pregnancy (HCG), Malaria P.f/Pan, Malaria P.f/P.v, Dengue NS1 are examples for antigen detection rapid diagnostic tests (RDTs) whereas rapid test for Dengue IgG/IgM, Treponema pallidum antibody test comes under antibody detection RDTs. In case of antigen detection test, two different monoclonal antibodies having distant epitopes have to be selected and subjected to sandwich immunoassay for color development. Primary antibody or capture antibody is coated on the nitrocellulose membrane and secondary antibody or detector antibody is conjugated with gold nanoparticles or latex nanoparticles which will be coated on the conjugate pad. For antibody detection tests, anti-antibody of the analyte is considered as the capture antibody and antigen coated with nanoparticles will be used as detector molecule. In the case of competitive assay for antigen detection, only one monoclonal antibody will be available and is considered as the capture antibody. Standard antigen will be subjected to conjugation with nanoparticles and then allowed to compete with the antigen from sample. For competitive assay for antibody test, competition occurs between the conjugated standard antibodies and antibodies from blood to bind with the antigen coated on nitrocellulose membrane. Due to the competition among the conjugated and unconjugated analyte, absence of color in the test line will be a positive test and color formation gives a negative result. In all the cases control line consist of goat anti mouse antibody as capture antibody and corresponding mouse antibody conjugated with nanoparticles will be smeared on conjugated pad. The test will considered invalid if the control line is not giving color [6–10].
LFIA—Developmental Strategies
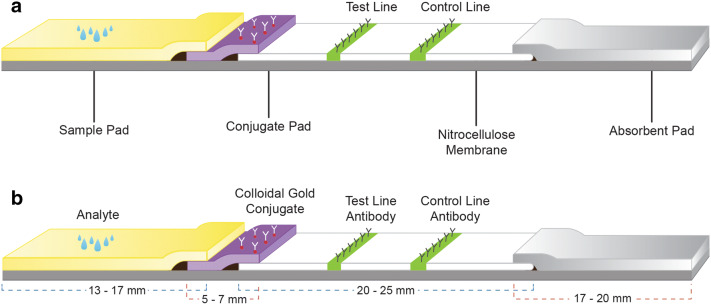
The development of LFIAs primarily relies on standardization of membrane characteristics on which the antigen antibody interaction occurs. Capillary flow rate mainly depends on the physical and chemical characteristics of the membranes. Specificity and sensitivity of the assay depends on epitope specificity of capture and detector antibodies and bio conjugation efficiency of detector molecules with the colloidal gold nanoparticles. The strip design comprises the overlapping arrangement of membranes in sequential order of sample pad, conjugate pad, nitrocellulose membrane and absorbent pad. In the case of whole blood sample as analyte, additional membranes for blood cell separation and background clearing are used. A typical LFIA strip consist of aforementioned membranes sequentially arranged as a strip in plastic backing and is placed in specially designed cassette for proper flow rate and stability(Fig. 1a, b).
Fig. 1.
a Typical structure of LFIA strip. b Strip alignment pattern with approximate length of membranes
Vital Components for LFIA
Nitrocellulose Membrane
Nitrocellulose (NC) membranes are considered as the back bone of rapid test strip where capture antibodies for test line and control line are coated. The size of the analyte and sample type (whole blood, Serum, Plasma, Urine) has to be considered while fixing the pore size of the NC membrane. As the pore size increase the flow rate of the membrane also increases. Sensitivity of the assay and pore size are inversely proportional. NC membranes are neutral in nature and can electrostatically bind to proteins through dipole–dipole interaction between the nitrate esters with peptide bond of proteins. The pH of buffer for capture reagents has to be optimized based on the isoelectric point of protein used in order to enhance the electrostatic interaction.
The pore size of NC membrane ranges from 1–20 µm are selected based on the analyte characteristics. NC membranes are rated either by pore size or by the wicking time. As the pore size decreases the wicking time increases which offers adequate interaction time for antigen–antibody and thereby enhances sensitivity of the assay. Based on our experience, it is suggested to use 10 µm membrane for HCG, 15 µm for Malaria and Dengue rapid tests. Normally 20–25 mm is the length for NC membrane with 3–4 mm width. The test line coating should be minimum 7 mm distance from sample pad and the distance between test lines and control line should have minimum of 5 mm. The NC membrane should always be kept in a dry condition with relative humidity (RH) below 40% as it is vulnerable to moisture. During development of an assay, it is better to maintain the controlled environment of 25 °C—RH < 40% for dealing NC membrane for consistent results. Once the capture reagents are coated on the membrane, drying has to be done by using vacuum drying or 37 °C hot air oven for 1 h or even with an air dryer. Vacuum drying is not preferred as it is difficult for bulk batch drying. 37 °C drying for 1 h in a hot air oven is suitable as most of the proteins can withstand the temperature of 37 °C.
Sample Pad
Cellulose or glass fibres are considered as suitable sample pads which act as the platform for sample analyte. Preferably glass fibre is used as sample pad because of its low protein affinity and high absorption capacity which offers steady and uniform flow of sample to conjugate pad. The sample pad is the main site of pre-treatment in LFIA for reducing background noise and non-specific interactions. Pre-treatment includes addition of blockers like BSA (1%), casein (0.1–0.5%), gelatin (0.05–0.1%) and surfactants like Tween-20 (< 0.05%), Triton X-100 (< 0.05%). Sample pad pre-treated with aforementioned blockers and surfactants (preferably in carbonate buffer or tris buffer) is then dried at 60–70 °C in hot air oven for 1 h. In case of whole blood samples, cell separating membranes are using in support of sample pad in continuation and overlap with the conjugate pad.
Conjugate Pad
Glass fibers, cellulose filters and polyester are used as conjugate pad material. The conjugate pad is the seat for nanoparticle/microparticle conjugated detector antibody/antigen. It should have low protein binding property and high releasing capacity. Dipping and spraying are the methods used to coat detector reagent on to the conjugate pad. Care should take for the uniform spreading of detector particles on conjugate pad. Drying of the dipped pad can be done at 37 °C for 1 h or with a handheld air dryer. Conjugate pad is a critical component of LFIA as important as the NC membrane. The stability of conjugated detector molecules and the release kinetics are directly related to the sensitivity of the assay.
Absorbent Pad
The commonly used material for absorbent pad is cellulose filters which are highly polar in nature. It is placed at the lower end of strip and absorbs the complete solution from membrane, provides maximum background clearing. To a certain extent, it is possible to improve the sensitivity of the assay by adjusting the absorbent pad thickness and length. Increasing thickness of absorbent pad will resolve the issue of backflow in test strip.
Capture Reagent
Most of the LFIA are based on sandwich immunoassay for antibody/antigen analyte detection in body fluids. For an antigen test capture antibody and detector antibody have to be selected in such a way that the epitope binding sites are far distant from each other. Once selected the capture antibody has to be coated on the NC membrane. LFIA demands a little more concentration of capture antibodies as compared to other immunoassays and it ranges from 50 to 500 ng per strip (Strip width 3–4 mm). Molarity and pH of the buffer have tremendous effect on proper coating and stability of the capture antibody on the dried membrane. Phosphate buffer or bicarbonate buffer are commonly used and the pH has to be optimized based on the isoelectric point of the protein. Monoclonal antibody is preferred as capture antibody and the molarity of the buffer should keep a little high for the proper analyte interaction. In order to increase the capture antibody binding on NC membrane 1–3% of methanol can be used in capture reagent.
Detector Reagent
The detector reagent is responsible for the color formation in LFIA due to aggregation of nanoparticles taking place during sandwich assay reaction. Gold nanoparticles are the widely using material for conjugation with antibodies because of it inert nature and perfect spherical structure. Coloured latex microparticles, silver nanoparticles, graphene nanoparticles are also possible to use for detector reagent preparation in LFIA. Trisodium citrate reduction is the established method for colloidal gold nanoparticle preparation and the size of the nanoparticles can be customized by altering the concentration of trisodium citrate. Normally 20–80 nm nanoparticles are used for the conjugation purpose and 40 nm is the best option. The change in particle size can be analysed by absorbance scan in spectrophotometer. Colour of the nanoparticles will change upon alteration in the size (Table 1).
Gold Nanoparticle–Antibody Conjugation
Conjugation efficiency of detector antibody to gold nanoparticles can be considered as the rate limiting process in the successful development of LFIA. The pH of the conjugation buffer has to be optimized in such a way that the antibody molecules completely bind to the colloidal gold particles. The pH optimization is performed by checking the aggregation reaction while mixing conjugation buffer with antibody and colloidal gold nanoparticles at different pH gradients in presence of 2 M sodium chloride. At optimum pH all the colloidal gold particles are equally bind by detector antibodies and no free gold particles are available in the buffer. If the pH is not optimum, the free gold nanoparticles available in the buffer will react will 2 M sodium chloride which results in black colour aggregation reaction (Table 2).
After the conjugation reaction, the antibody coated colloidal gold should be blocked with blocking agents like bovine serum albumin (1%) or Gelatin (0.5%). The final storage buffer contains stabilizers like trehalose (1–5%) or sucrose (5–20%). Once prepared the antibody conjugated colloidal gold solution has to be diluted in storage buffer based on the absorbance OD and coated on the conjugate pad and dried at 37 °C. The commonly used absorbance OD for coating of colloidal gold conjugate in conjugate pad is OD 10. The conjugated antibody should be stored at temperature < 25 °C and RH < 20%. The dried detector reagent on the conjugate pad under controlled humidity condition will be stable up to 3 years. The conjugated detector reagent in liquid form will be stable maximum up to 1 month at 4 °C.
Points for Consideration
-
Absorbance peak shift for colloidal nanoparticles upon conjugation
The colloidal gold nanoparticle solution prepared by trisodium citrate reduction method will give an absorption peak at 520–524 nm. Upon conjugation with the detector antibody molecules the absorption peak will have shifted to 528–530 nm. Using an antibody conjugated gold nanoparticle solution having absorption peak > 531 nm is not recommended, because of the chance of aggregation and settling.
-
Blocking on membrane
There are two options for blocking on membranes; either on sample pad or on conjugate pad.
-
Covalent conjugation of detector molecule on gold nanoparticles
Ionic attraction and hydrophobic interaction between the antibody and colloidal gold surface makes the bonding in detector reagent preparation for LFIA. It is possible to use the linkers like EDAC and NHS or adapters like streptavidin and biotin for covalent conjugation of detector antibody with gold nanoparticles. Of course covalent conjugation will give better conjugation stability but have no effect on sensitivity of the assay. It is recommended using covalent conjugation for low adsorption capacity molecules. Covalent conjugation will increase the cost of the total assay.
-
Accelerated stability study for LFIA
In order to evaluate the long term stability of developed biochemical or immunoassay reagents, the Arrhenius equation is normally used. But the Arrhenius equation calculations are not applicable for LFIA tests. Accelerated stability of 30 days at 37 °C and 45 °C are not sufficient to claim 2 year stability for the developed assay. Performing real time stability study is the only way to claim the shelf life of LFIA products.
-
Enhancing sensitivity of the assay
The sensitivity of the assay depends on various factors. The sensitivity can be enhanced with decreasing the pore size and thereby increasing the wicking time. Surfactants or detergents used in sample pad and conjugate pad can affect the sensitivity of the assay. Optimizing the concentration of those surfactants are very important. It is possible to increase the sensitivity by altering the air side to belt side interaction among the membranes in unbacked strips.
-
Is monoclonal antibody or polyclonal antibody recommended for LFIA?
Normally sandwich immunoassays prefer monoclonal capture antibody in combination with polyclonal detector antibody. But LFIA prefers monoclonal antibodies which are having far distantly placed epitopes as capture and detector antibodies. Polyclonal antibodies in detector reagent can create large structures resulting in improper migration through membranes and retained unspecifically.
Latex beads application on LFIA
Coloured latex microparticles can be used in LFIA as the detector reagent. Carboxylated latex microparticles can form strong covalent linkage with proteins and are stable for years at 4 °C.
Troubleshooting—LFIA Development
| Issues | Solutions |
|---|---|
| Aggregation of colloidal gold conjugated detector antibody reagent |
1. Readjust the pH of the buffer close to pI of detector antibody 2. Do not freeze the conjugate 3. Do not ever use PBS or Nacl for conjugation |
| Background on NC membrane |
1. Sample pad pre-treatment with surfactants like tween-20 or triton-100 2. Alter the air side to belt side orientation between NC membrane and absorbent pad 3. Increase wick thickness to avoid backflow |
| Retention of conjugated particles on pad |
1. Increase the conjugate blocking 2. If using polyclonal antibody replace it with monoclonal |
| Hydrophilic interactions | 1. Increase salt concentration in pads |
| Hydrophobic interactions | 1. Use non-ionic surfactants |
| Low on-rate of capture and detector antibodies | 1. Use membranes having high wicking time |
| Too quick release of conjugate and run in front of target | 1. Optimize the conjugate releasing buffer |
| Low stability |
1. Use carbohydrate stabilizers like trehalose and sucrose 2. Use five layered aluminium pouches for packing with activated silica |
| Spreading of capture antibody while coating | 1. Use 1–3% of methanol or ethanol with the capture antibody buffer |
| False positive result in whole blood samples | 1. Use 1% casein as the blocker with sample pad pre-treatment buffer |
Table 1.
Size and colour formation by gold nanoparticles
| Size of gold nanoparticles (nm) | Colour |
|---|---|
| 70–80 | Black |
| 60–70 | Dark purple |
| 40–60 | Pinkish purple |
| 20–40 | Reddish pink |
| 10–20 | Intense red |
Table 2.
Example of pH optimization for detector antibody conjugation
| Vial no. | Conjugation buffer pH | Antibody volume µL (concentration 2 µg/mL)a | 40 nm colloidal gold solution pHb | Aggregation/colour |
|---|---|---|---|---|
| 1 | 6.0 | 5 | 6.0 | Yes/black |
| 2 | 6.5 | 5 | 6.5 | Yes/black |
| 3 | 7.0 | 5 | 7.0 | Yes/black |
| 4 | 7.5 | 5 | 7.5 | Yes/black |
| 5 | 8.0 | 5 | 8.0 | No/pinkish purple |
| 6 | 8.5 | 5 | 8.5 | Yes/black |
| 7 | 9.0 | 5 | 9.0 | Yes/black |
Optimum pH for the aforementioned reaction is 8.0, at which there is no aggregation of nanoparticles
aAntibody concentration has to be fixed based on activity
bOnly use potassium carbonate for pH adjustment of colloidal gold solution. Do not use Hcl or NaOH
Compliance with Ethical Standards
Conflict of interest
All authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C. Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors. 2018;9(1):2. doi: 10.3390/bios9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sajid M, Kawde A, Daud M. Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc. 2015;19(6):689–705. doi: 10.1016/j.jscs.2014.09.001. [DOI] [Google Scholar]
- 3.Koczula K, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zherdev AV, Dzantiev BB. Ways to reach lower detection limits of lateral flow immunoassays. Moscow: IntechOpen; 2018. [Google Scholar]
- 5.Posthuma-Trumpie G, Korf J, van Amerongen A. Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2008;393(2):569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 6.Murray C, Gasser R, Magill A, Miller R. Update on Rapid Diagnostic Testing for Malaria. Clin Microbiol Rev. 2008;21(1):97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong R, Tse H. Lateral flow immunoassay. New York: Springer; 2010. [Google Scholar]
- 8.Posthuma-Trumpie G, Korf J, van Amerongen A. Development of a competitive lateral flow immunoassay for progesterone: influence of coating conjugates and buffer components. Anal Bioanal Chem. 2008;392(6):1215–1223. doi: 10.1007/s00216-008-2362-8. [DOI] [PubMed] [Google Scholar]
- 9.Hristov D, Rodriguez-Quijada C, Gomez-Marquez J, Hamad-Schifferli K. Designing paper-based immunoassays for biomedical applications. Sensors. 2019;19(3):554. doi: 10.3390/s19030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linares E, Kubota L, Michaelis J, Thalhammer S. Enhancement of the detection limit for lateral flow immunoassays: evaluation and comparison of bioconjugates. J Immunol Methods. 2012;375(1–2):264–270. doi: 10.1016/j.jim.2011.11.003. [DOI] [PubMed] [Google Scholar]