Abstract
Despite ample sunshine, 50–90% Indian children have Vitamin D deficiency (VDD). This enigma of widespread VDD needs exploration especially among under-fives as physiological variations in Vitamin D Binding Protein (VDBP) levels could be potential confounders in the interpretation of total 25-hydroxyvitamin D [25(OH)D]. However, there is scarce information about relevance of VDBP levels in under-five age group. We therefore, explored association of VDBP levels among 1–5 year old children with VDD. Serum levels of 25(OH)D, VDBP, calcium, parathyroid hormone (PTH) and alkaline phosphatase were estimated in 210 apparently healthy children in the age group of 1–5 years. VDD was defined as serum 25(OH)D levels < 20 ng/ml as per the IOM classification. VDBP levels were classified as low if levels were < 168 μg/ml as per the kit. The prevalence of VDD was 79.5% (n = 167) and VDBP levels were low in 48.6% (n = 102) of children. 25(OH)D levels correlated positively with VDBP (r = 0.298, p = 0.0001). A significant number of children (52.7%) with VDD had low VDBP (p = 0.015). and despite adequate sun exposure, 43% of children showed VDD and 56.6% had low VDPB levels. The low VDBP levels largely explain low 25OHD levels without necessarily implying VDD. It may add a new dimension for better understanding of widespread VDD among under-five children. It thus, points towards the need for redefining cut offs and complete evaluation of vitamin D status among under-fives including VDBP.
Keywords: Vitamin D, Vitamin D binding protein, Vitamin D deficiency, Under-five children
Introduction
Despite ample sunshine, 50–90% Indian children have Vitamin D deficiency (VDD) [1, 2]. Nearly 90% of Vitamin D requirement is met through adequate exposure of the skin to sunlight by the action of ultraviolet B radiations (between 10 a.m. to 3 p.m.) and 10% is said to meet through diet [3]. Various studies have discussed different aspects of VDD among children. Currently, the plasma concentration of total 25-hydroxyvitamin D [25(OH)D] is considered as an indicator of vitamin D status. The majority (84–90%) of circulating 25(OH)D and 1,25(OH)2D is tightly bound to Vitamin D binding protein (VDBP), 10–15% is to albumin and less than 1% of circulating 25(OH)D exists in an unbound form [4].
VDBP is a polymorphic single chain serum glycoprotein that is primarily produced in the liver. It is encoded by Gc located on chromosome 4q11–q13 and is primarily responsible for preventing Vitamin D from biodegradation, limiting its access to target tissues, and reabsorbing Vitamin D in the kidneys [5]. Internalization of DBP-bound 25(OH)D by the megalin/cubulin endocytic pathway in the proximal tubule epithelium allows hydroxylation of 25(OH)D by 1- hydroxylase to produce 1,25(OH)2D, the active metabolite of vitamin D for systemic/endocrine functions [6]. It is thought that other tissues (non-renal) which do not express megalin depend largely on the diffusion of free 25OHD through the plasma membrane for subsequent conversion to 1,25(OH)2D for autocrine and paracrine effects.
Although, VDBP is relatively stable in healthy population, different physiologic and pathologic conditions such genetic factors, certain drugs (tenofovir, aspirin), smoking, hormonal factors, obesity and insulin resistance, end stage liver disease and nephrotic syndrome can affect VDBP levels [7, 8]. Therefore, the diseases or conditions that affect the synthesis of VDBP or albumin may have a significant impact on the amount of circulating total Vitamin D and may affect the interpretation of 25(OH)D levels [9, 10].
Physiological variations in VDBP have been reported most commonly in children. Low VDBP levels are reported in pediatric critical illness with a strong relationship with GC haplotype [11]. Apart from this, low VDBP levels are also observed in toddlers with a genetic variance of GC T436K SNP affecting circulating levels of the VDBP [12].
However, the biological relevance of the VDBP-bound vitamin D metabolites versus the VDBP-unbound or “free fraction” of vitamin D has not yet been established [6]. Determinants of circulatory levels of Vitamin D metabolites have not been evaluated largely among children. Factors like D binding protein to which majority of 25(OH)D is bound may have some role in Vitamin D deficiency in children. Also, currently used vitamin D assays do not distinguish between the three forms of vitamin D; DBP-bound vitamin D, albumin-bound vitamin D and free, the biologically active vitamin D.
Hence, the enigma of widespread VDD needs exploration especially among under-fives as physiological variations in VDBP levels could be potential confounders in the interpretation of plasma total 25-hydroxyvitamin D. However, currently there is scarce information about relevance of VDBP levels in under-five age group.
In the present study, therefore, we explored Vitamin D binding protein levels among 1–5 year old children and its association with Vitamin D deficiency.
Materials and Methods
Subjects
Sample size: Considering an anticipated prevalence of 75% as per our previous findings [13], at 5% level of significance and 5% precision the required sample size was 282. The study protocol was approved by the Institutional Ethics Committee for Clinical Studies. A list of children in the age group of 1–5 years has been obtained from the anganwadi of urban slum, Mumbai. The social staff visited the household for screening of eligible children and requested parents to visit the health facility in the community with their children to participate in the study. Total 282 apparently healthy children in the age group of 1–5 years were included and those with chronic illness, nutritional deficiencies, skeletal diseases, receiving vitamin D supplementation in the past 6 months were excluded. Informed written consent was obtained from the parents/guardian. Information on socio demographic profile, physical activity profile (sun exposure, time spent outdoor, dietary profile (24-h recall of their food intake) was collected using structured questionnaire. Their weight and height was measured in light clothes. Direct sunlight exposure was assessed by documenting average duration of exposure between 10 a.m. and 3 p.m. and percentage of the body surface area exposed daily using the standard method as described in textbook of Bailey and Love’s short practice of surgery [14].
Laboratory Measurements
About 4 ml of venous blood was drawn in the fasting state from the enrolled children in plain vacutainers. Sera were separated by centrifugation of blood samples at 2200 rpm at 4 °C and stored at − 80 °C till assayed. Serum calcium, phosphorus, alkaline phosphatase and albumin were measured by a NABL accredited commercial laboratory using automated blood analyzer (Transia, ERBA, Model Chem-5, PlusV2, India). Commercially available ELISA based diagnostics kits were used for measurement total 25-Dihydroxy Vitamin D (IDS, USA), Vitamin D Binding Protein (R & D System, USA), and intact Parathyroid Hormone (Ray Biotech, USA PTH). The inter-assay and intra assay coefficient of variation (CV) of these parameters were well within accepted limits (intra-assay precision: CV 7.5–8%, inter assay precision: CV 9.5–10%).
For all biochemical parameters, the reference ranges provided by the manufacturers [alkaline phosphatase: 145–320 U/l, calcium: 8.8–10.8 mg/dl, phosphorus: 4.0–7.0 mg/dl, Intact PTH: 14–72 pg/ml] were taken into consideration. VDBP levels were classified as normal if they were between 168 and 367 μg/ml and were classified as low DBP levels if levels were < 168 μg/ml as per the kit.
VDD was defined as serum total 25(OH)D levels < 20 ng/ml as per the IOM(Institute of Medicine) classification [15]. All children were further classified into the following 4 groups as per 25(OH)D levels: Group I: ≥ 20 ng/ml, Group II: 15–20 ng/ml, Group III: 10–15 ng/ml and Group IV: < 10 ng/ml.
Statistical Analysis
Descriptive analysis of variables was presented in terms of percentage or mean (± standard deviation). Chi square test was applied to see the association between explanatory (age, sex sun exposure) and outcome variables (VDD and VDBP). Logistic regression analysis was done to find out the factors associated with VDD. The Pearsons’s correlation was applied to see the relationship between biochemical parameters. The differences in mean level of Vitamin D binding protein among groups (based on 25(OH)D) was estimated using ANOVA. p < 0.05 was considered as significant. Statistical analysis was carried out using IBM SPSS statistical software (version 19, IBM Bangalore).
Results
Out of 282 children, complete information was available for 210 children since 72 parents of children refused blood collection. Hence results have been interpreted for 210 children.
Background Characteristics
All the children included in the study had uneventful birth history, normal development and had completed universal immunization as per age. Their mean age was 36.49 ± 12.68 months. Of these nearly 61% (n = 128) children [boys: 54% (n = 70); girls: 46% (n = 58)] were either going to school or anganwadis (play groups). Mean BMI of the children was 14.6 (± 1.8) kg/m2.
The enrolled children were from urban slums with closely packed overcrowded tenements. In 42.4% (n = 89) of their houses, sunrays were reaching only up to verandas between 10 a.m. and 3 p.m. It was observed that 43.8% (n = 92) children used to play outside houses between 10 a.m. and 3 p.m.
Biochemical Parameters
The mean 25(OH) D level of these children was 14.58 ± 7.76 ng/ml whereas that of VDBP was 171.01 ± 56.1 ng/ml. The mean levels of PTH, calcium, phosphorus and alkaline phosphatase were 48.6 ± 63.9 pg/ml, 9.2 ± 0.5 mg/dL, 7.7 ± 1.4 mg/dL and 226.8 ± 78.7 IU/l respectively. The prevalence of VDD amongst children was found to be 79.5% (n = 167; 95% CI 73.4–84.7) and prevalence of low VDBP levels was found to be 48.6% (n = 102; 95% CI: 41.6–55.5%).
The distribution of VDD and low VDBP levels according to age, sex of children and sun exposure has been shown in Table 1. There was no significant difference in VDBP levels between age groups (1–2 years and of 2–5 years) and sex of children. A negative correlation was observed between the BMI and VDBP levels (r = − 0.46, p = 0.05), however, it was not statistically significant. The sun exposure of < 10 min from 10 a.m. to 3 p.m. was significantly associated with VDD, but it had no relation with low VDBP levels.
Table 1.
The distribution of VDD and low VDBP levels according to age, sex of children and sun exposure
| Age group (in months) | Sex | Sun exposure (in mins) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 12–23 (n = 42) | 24–59 (n = 168) | p value | Boys (n = 114) | Girls (n = 96) | p value | < 10 (n = 110) | 10–45 (n = 100) | p value | |
| VDD (%) (< 20 ng/ml) | 78.6 | 81.0 | 0.73 | 77.2 | 84.4 | 0.191 | 86.2 | 73.7 | 0.02 |
| Low VDBP (%) < 168 μg/ml | 50.0 | 48.2 | 0.84 | 47.4 | 50.0 | 0.70 | 48.6 | 49.5 | 0.9 |
The 25(OH)D levels were significantly and positively correlated with VDBP. Though, not significant, PTH and alkaline phosphatase correlated negatively with VDBP and there was no correlation of VDBP with calcium or phosphorus.
Upon further assessing the level of VDBP according to the level of 25(OH)D, it was observed that VDBP levels in Group I [25(OH)D ≥ 20 ng/ml] differ significantly from those of Group III [25(OH)D 10–15 ng/ml] and Group IV[25(OH)D < 10 ng/ml], as depicted in Fig. 1 which shows VDBP levels (mean ± SD) in four groups according to 25(OH)D level.
Fig. 1.
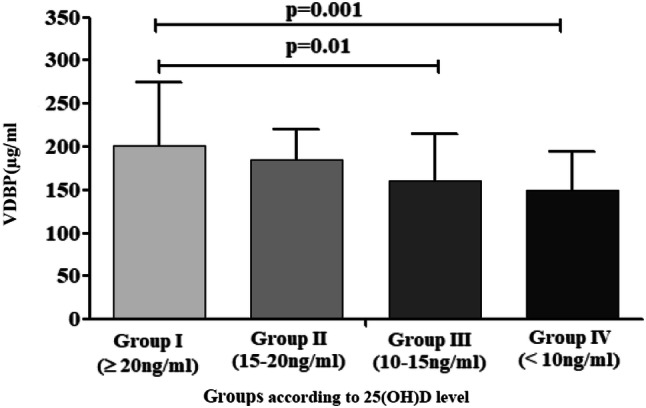
VDBP levels (mean ± SD) in four groups according to 25(OH)D levels
Nearly 50.7% children with VDD had low VDBP levels. Additionally, Group IV [(25(OH)D level < 10 ng/ml], has 60.3% children with low VDBP than their counterparts in Group I (p = 0.015) (Fig. 2).
Fig. 2.
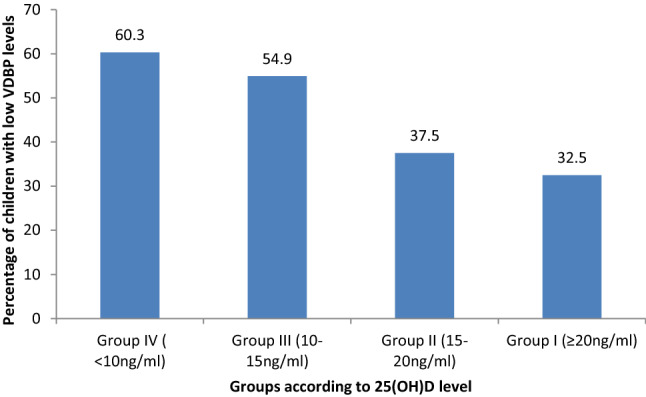
Percentage of children with low VDBP levels according to 25(OH) D Levels
Association VDBP Levels with VDD with Respect to Sun Exposure
Though, VDD was significantly associated with duration of sun exposure, approximately, 73.4% children had VDD despite sun exposure of 10–45 min in a day (Table 1). Hence, the association of low VDBP levels in VDD was assessed after stratification of duration of sun exposure (< 10 min and 10–45 min) during 10 a.m. to 3 p.m.
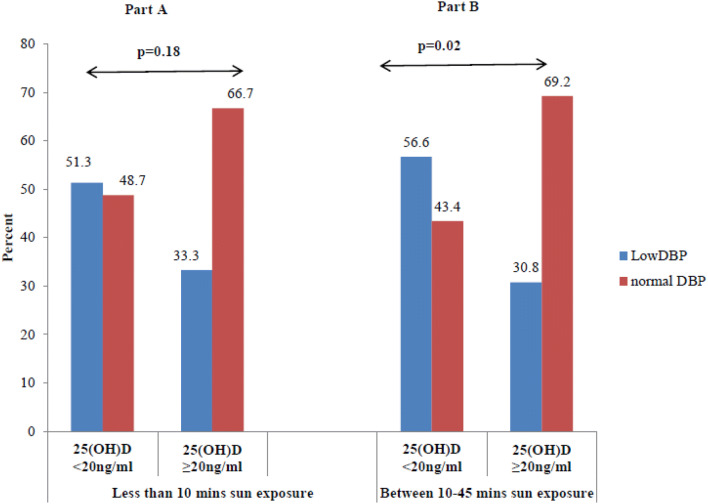
Figure 3 shows percentage distribution of children with low VDBP with respect to 25(OH) D levels according to duration of sun exposure between 10 a.m. and 3 p.m. Part A of Fig. 3 shows Percentage of children with low and normal VDBP with respective to 25(OH) D levels with less than 10 min of sun exposure and Part B of Fig. 3 shows percentage of children with low and normal VDBP with respective to 25(OH) D levels having sun exposure between 10–45 min with a significant difference (p = 0.02) between 25(OH)D,20 ng/ml and 25(OH)D ≥ 20 ng/ml.
Fig. 3.
Percentage distribution of children with low VDBP with respect to 25(OH) D levels according to duration of sun exposure between 10 a.m. and 3 p.m.
It was observed that in more than half (56.6%) of children with VDD [25(OH) D < 20 ng/ml] and sun exposure of 10–45 min in a day had significantly (p = 0.022) low VDBP levels than children with no VDD.
Further applying logistic regression, as shown in Table 2, it was observed that Children who had sun exposure for less than 10 min were 2.26 times significantly more likely to have vitamin D deficiency than the children who had sun exposure more than 10 min. Odd of vitamin D deficiency was 2.12 times more in children having VDBP level low than their counterparts.
Table 2.
Unadjusted and adjusted odds ratio of Vitamin D deficiency with selected background characteristics
| Variables | Unadjusted odds ratio (95% CI) | Adjusted odds ratioa (95% CI) |
|---|---|---|
| Sun exposure | ||
| < 10 min | 2.18 (1.09–4.35) | 2.26 (1.12–4.59) |
| > 10 min | 1.00 | 1.00 |
| DBP level low | ||
| Yes | 2.03 (1.01–4.08) | 2.12 (1.04–4.30) |
| No | 1.00 | 1.00 |
aAdjusted for age and gender. Dependent variable: vitamin D deficiency [25(OH)D < 20ng/ml]
Discussion
The study assessed VDD in children below 5 years and evaluated its association with VDBP levels. The prevalence of VDD (79.5%) amongst children of age group 1–5 years was high along with low VDBP levels (48.6%).
Nearly 50.7% children with VDD had low VDBP levels. Among different categories of VDD (< 10, 10–15, 15–20 and ≥ 20), children with 25(OH)D < 10 ng/ml had significant percentage of low VDBP levels. The study did not find gender based differences in VDBP status. There was no significant difference in VDBP status between younger children (12–23 months) and comparatively older children (24–60 months).
There was no significant association of VDBP levels with Calcium, phosphorus, alkaline phosphatase and PTH. In contrary to previous studies which have reported its association with PTH levels, our study did not observe any association with PTH levels probably because of physiological variation in PTH response among children. No association of VDBP with BMI was observed. Earlier studies have had conflicting results on the relationship between BMI and VDBP. Some studies have shown no association between VDBP and BMI [16–18] whereas some have reported a positive correlation between VDBP concentrations and BMI [19, 20].
Significant prevalence of low VDBP levels among under-five children highlights the need of re-defining Vitamin D deficiency among under-five children. Physiological variations in VDBP levels and low levels among toddlers have been reported in some studies [7, 12]. VDBP status among young Indian males has also been explored in past [21]. However, this is the first study to our knowledge among under-five children in India which has explored VDBP levels.
The FAO/WHO Expert Consultation has stated that in most locations in the world around the equator (between latitudes 42° N and 42° S) the most physiologically relevant and efficient way of acquiring vitamin D is by 10–45 min of skin exposure (without sunscreen) of the arms and face to sun (Ultraviolet spectrum of wavelength 290–310 nm) [3]. Upon assessing Vitamin D status among children who had 10–45 min of sun exposure between 10 and 3 p.m., it was observed that VDD was unexplained among 34% of children despite adequate sun-exposure. However, amongst them, 56.6% had low DBP levels. This significant prevalence (p = 0.012) of low VDBP levels among children having VDD despite adequate sun exposure 10–45 min points towards the need of exploring bioavailable Vitamin D levels among under-five age group.
VDBP levels may be low among under-five children due to physiological variation. Low VDBP levels among under-five children can certainly affect 25(OH) D levels. Alterations in VDBP levels may be potential confounders in the interpretation of total 25(OH) D concentrations among children and therefore estimation of VDBP holds important. Children with low VDBP may not show any change in total 25(OH) D levels after treatment. The decline in PTH which generally occurs in response to treatment after correction of VDD may not be observed in such cases as the free or unbound form will remain unaltered. Hence this may further also have treatment implications.
According to the free hormone hypothesis, the unbound form of 25(OH)D would correlate better with the biological actions of vitamin D than the bound form [22, 23]. Bioavailable 25(OH)D (the free plus albumin bound portions) has been proposed as a better indicator of vitamin D activity in some of the studies [3, 23, 24], possibly explaining discrepancies between findings of vitamin D effect on health outcomes in studies relying on total 25(OH)D. Several studies have identified a strong relationship between bioavailable vitamin D and indicators of vitamin D status such as bone mineral density and parathyroid hormone [24–26]. The difference in PTH response in children on basis of elevation in PTH to low 25(OH)D levels has been reported in some studies in past [13, 27]. Therefore, A “functional health-based reference value” which physiologically defines hypovitaminosis D as the concentration of 25(OH)D at which PTH begins to increase may be important among children in view of its physiological variations [1, 13].
Proposed Conceptual Framework for Understanding VDD Among Under-Five Children
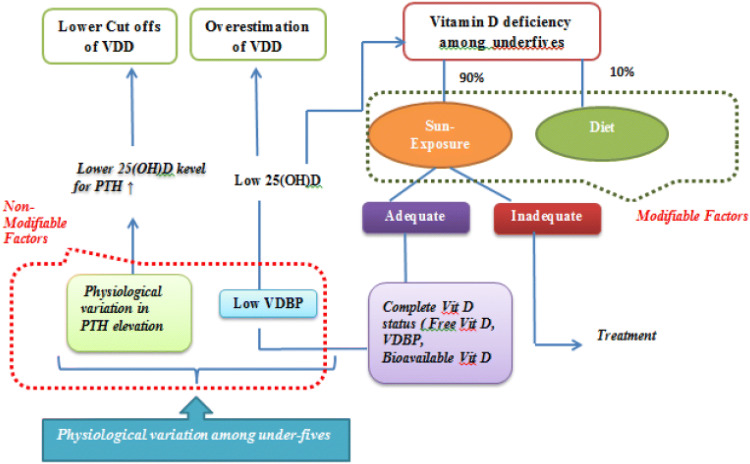
In view of physiological variation in VDBP levels and the difference in PTH response in children the cut offs for paediatric population especially under-fives may differ. We propose a conceptual framework for understanding VDD among under-five children as shown in (Fig. 4).
Fig. 4.
Conceptual framework for understanding VDD among under-five children
As seen in Fig. 4, Sun exposure and diet are primary determinants of vitamin D status. Preventive measures can be taken to ensure adequate vitamin D status. However, certain underlying factors like low VDBP and physiological variation in PTH elevation, though independent of each other, are important determinants of vitamin D status among under-fives. These should be taken into consideration while defining and managing VDD in them.
Currently there are no defined cut offs pertaining to 25(OH)D levels in Paediatric age group in India to classify VDD. The findings of the study throw light on unexplored facet of VDD among under-five children. In view of variations in DBP levels among children, there is an urgent need to review the recommended cut offs pertaining to Paediatric population especially under-fives [28, 29].
There were some limitations of the study such as the seasonal variations were not accounted for and free 25(OH) D by direct method was not measured which would have helped to compare the calculated and direct levels. Being a cross sectional study, it was also not possible to study parameters as response to treatment.
To the best of our knowledge, we believe that, despite these limitations, this is the first study to report that VDBP levels influence Vitamin D status in a relatively large cohort of children and to attempt to address the issue of 25(OH)D bioavailability in context of VDD.
Conclusion
Conclusively physiological variations in PTH and VDBP levels in under-five age group may affect Vitamin D status and needs to be carefully looked into before deciding treatment strategies.
However, it definitely opens a window of research towards complete evaluation of vitamin D status among under-five children and to relook at existing deficiency status. Being an exploratory study, the findings necessitate the need of further research to estimate complete 25(OH)D status including free 25(OH)Dindex, VDBP to understand whether the free 25(OH)D index as compared to total 25(OH)D levels is a better marker of 25(OH)D tissue bioavailability.
Acknowledgements
The authors acknowledge the encouragement and guidance received from Dr. Smita Mahale, Director, ICMR-NIRRH. We thank her for reviewing the article and valuable inputs. The authors would also thank Dr. Yeshwant Amdekar, Former Medical Director Bai Jerbai Wadia Hospital for Children, Mumbai for his critical review. The authors would also like to acknowledge staff (Mrs. Varsha Tryambake, Mrs. Bhagyashree Kanje, Ms. Sharmila Kamat, Mr. Iranna Mashal, Mrs. Shobha Vange, Mrs. Rachana Dalvi, Mrs. Vaishali Chalke) for data collection and data entry. We would also like to thank the parents and children who participated in the study.
Funding
This study was funded by Indian Council of Medical Research (ICMR)-National Institute for Research in Reproductive Health (NIRRH), Mumbai.
Compliance with Ethical Standards
Conflict of interest
Suchitra Surve, Shahina Begum, Beena Joshi, M Ikram Khatkhatay, Seema Kadam and Sanjay Chauhan declare that they have no conflict of interest.
Research Involving Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee, NIRRH ethics committee for clinical studies (275/2015) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from parents of all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suchitra Surve, Email: dr.suchi172@gmail.com, Email: surves@nirrh.res.in.
Shahina Begum, Email: begums@nirrh.res.in.
Beena Joshi, Email: bjoshithane@gmail.com.
M. Ikram Khatkhatay, Email: ikramkhat@gmail.com.
Seema Kadam, Email: seemakadam1209@gmail.com.
Sanjay Chauhan, Email: slchauhan@hotmail.com.
References
- 1.Harinarayan CV, Joshi SR. Vitamin D status in India—its implications and remedial measures. J Assoc Phys. 2009;57:40–48. [PubMed] [Google Scholar]
- 2.Rathi N, Rathi A. Vitamin D and child health in the 21st century. Indian Pediatr. 2011;48:619–625. doi: 10.1007/s13312-011-0107-9. [DOI] [PubMed] [Google Scholar]
- 3.Report of the Joint FAO/WHO Expert Consultation on Vitamin and mineral requirement in human nutrition: Bangkok 1998. 2nd ed. Rome: FAO; 2004.
- 4.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP. The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144:132–137. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 6.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 7.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014 doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jassil NK, Sharma A, Bikle DD, Wang X. Vitamin D binding protein and 25-hydroxyvitamin D levels: emerging clinical applications. Endoc Pract. 2017;23:605–613. doi: 10.4158/EP161604.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanton D, Han Z, Bierschenk L, Linga-Reddy MV, Wang H, Clare-Salzler M, et al. Reduced serum vitamin D-binding protein levels are associated with type 1 diabetes. Diabetes. 2011;60:2566–2570. doi: 10.2337/db11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denburg MR, Kalkwarf HJ, de Boer IH, Hewison M, Shults J, Babette SZ, et al. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28(9):1843–1853. doi: 10.1007/s00467-013-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden K, Feldman HA, Chun RF, Smith EM, Sullivan RM, Agan AA, et al. Critically ill children have low vitamin D-binding protein, influencing bioavailability of vitamin D. Ann Am Thorac Soc. 2015;12:1654–1661. doi: 10.1513/AnnalsATS.201503-160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter TO, Zhang JH, Parra E, Ellis BK, Simpson C, Lee WM, et al. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. J Bone Miner Res. 2013;28:213–221. doi: 10.1002/jbmr.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surve S, Begum S, Chauhan S, Khatkhatay I, Joshi B. Discrepancy between the recommended and functional cut offs of Vitamin D among under-five children: experiences from a pilot study. Indian J Endocr Metab. 2018;22:473–478. doi: 10.4103/ijem.IJEM_574_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey H, Love RJMN, Russell RCG, Williams NS, Bulstrode CJK. Bailey and Love’s short practice of surgery. London: Arnold; 2000. [Google Scholar]
- 15.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. Washington: National Academies Press; 2011. [PubMed] [Google Scholar]
- 16.Walsh JS, Evans AL, Bowles S, Naylor KE, Jones KS, Schoenmakers I, et al. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016;103:1465–1471. doi: 10.3945/ajcn.115.120139. [DOI] [PubMed] [Google Scholar]
- 17.Holmlund-Suila E, Pekkinen M, Ivaska KK, Andersson S, Mäkitie O, Viljakainen H. Obese young adults exhibit lower total and lower free serum 25-hydroxycholecalciferol in a randomized vitamin D intervention. Clin Endocrinol (Oxf) 2016;85(3):378–385. doi: 10.1111/cen.13093. [DOI] [PubMed] [Google Scholar]
- 18.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D—binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism. 2009;58:438–442. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Saarnio E, Pekkinen M, Itkonen ST, Kemi V, Karp H, Ivaska KK, et al. Low free 25-hydroxyvitamin D and high vitamin D binding protein and parathyroid hormone in obese Caucasians. A complex association with bone? PLoS ONE. 2018;13(2):e0192596. doi: 10.1371/journal.pone.0192596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taes YE, Goemaere S, Huang G, Van Pottelbergh I, De Bacquer D, Verhasselt B, et al. Vitamin D binding protein, bone status and body composition in community-dwelling elderly men. Bone. 2006;38(5):701–707. doi: 10.1016/j.bone.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Goswami R, Saha S, Sreenivas V, Singh N, Lakshmy R. Vitamin D-binding protein, vitamin D status and serum bioavailable 25(OH)D of young Asian Indian males working in outdoor and indoor environments. J Bone Miner Metab. 2017;35:177–184. doi: 10.1007/s00774-016-0739-x. [DOI] [PubMed] [Google Scholar]
- 22.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 23.Tsuprykov O, Chen X, Hocher CF, Skoblo R, Yin L, Hocher B. Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol. 2018;180:87–104. doi: 10.1016/j.jsbmb.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naderpoor N, Shorakae S, Abell SK, Mousa A, Joham AE, Moran LJ, et al. Bioavailable and free 25-hydroxyvitamin D and vitamin D binding protein in polycystic ovary syndrome: relationships with obesity and insulin resistance. J Steroid Biochem Mol Biol. 2018;177:209–215. doi: 10.1016/j.jsbmb.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Marwaha RK, Sripathy G. Vitamin D & bone mineral density of healthy school children in Northern India. Indian J Med Res. 2008;127:239–244. [PubMed] [Google Scholar]
- 28.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol. 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surve S, Chauhan S, Amdekar Y, Joshi B. Vitamin D deficiency in children: an update on its prevalence, therapeutics and knowledge gaps. Indian J Nutr. 2017;4(3):167. [Google Scholar]