Abstract
Purpose
The aim of this article was to systematically review the available literature on patient specific total temporomandibular joint total joint replacement (PS-TMJR) implants for their biomaterial, designs, fabrication techniques and their outcomes.
Methods
A literature review was conducted using PubMed, and science direct databases using the key words three-dimensional printing, 3D printing, CAD CAM, computer aided designing, computer aided manufacturing, additive technology, custom made implants, patient specific implants in combination with Temporomandibular joint, TMJ surgery.
Results
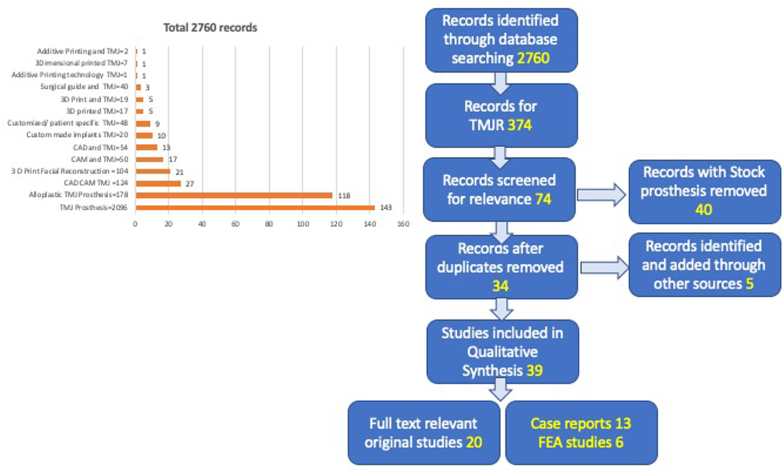
The search revealed 2760 articles, of which 374 were in English and discussed TMJ reconstruction. Further filtering shortlisted 74 articles that discussed PS-TMJR. Duplicates were removed and additional added from article references. 39 articles describing biomaterial, designing and fabrication of PS-TMJR implants and their outcomes were selected for analysis.
Conclusions
Although PS-TMJR implants allow a better anatomical fit, improved fixation, and safeguard various structures such as the inferior alveolar nerve, they vary in designs, material and fabrication techniques. However, PS-TMJR printed with SLM and EBM technologies have yet to be compared with the conventional ones in terms of mechanical strength, and clinical outcome. With emerging bioprinting technologies, even newer biomaterials should be considered for 3D printing of PS-TMJR devices designed to achieve harmony in function between the joint device, bone and masticatory muscles.
Keywords: TMJ, Customised TMJ, PSI TMJ
1. Introduction
The Temporomandibular joint (TMJ) is involved in essential functions like mastication, speech, airway support and deglutition, and is under constant cyclical loading and unloading.1 It also acts as a secondary mandibular growth center in children. It is important that a total TMJ replacement (TMJR) should restore its anatomical form and function, and correct aesthetic discrepancies like posterior vertical height shortening to improve facial aesthetics when concomitant orthognathic procedures are performed.
An ideal TMJR implant prosthesis should include properties for long-term biocompatibility, tribocorrosion, resistance to fatigue from masticatory forces; exhibit minimal joint surface wear and debris, not result in hypersensitivity, and minimise the risk of infection.2,3 Alloplastic stock TMJ implants are commercially available, but in a limited range of sizes, and often not fit the jaw sizes, specially in the asian population or in cases of altered anatomy. Also, normal translational movement and protrusion may not be reproduced even after alloplastic reconstruction, due to the detachment of the lateral pterygoid muscle, especially in bilateral TMJR patients.4 If it is to be placed in a growing child, there is an additional challenge to keep pace with growth. However, literature supports use of TMJR in skeletally immature patients too.5, 6, 7, 8
Use of patient specific (PS) TMJR implant overcomes the limitations with the stock standard size ranges, and has dramatically increased in the last decade as computer assisted design (CAD), and computer assisted manufacture (CAM) software allow to produce complex geometries to match the precise anatomical bony contours for an individual patient.9 Computer assisted manufacturing includes additive manufacturing (AM) and subtractive processes (computer numerical control, CNC milling). While CNC milling remains the stand fabrication procedure, AM is increasingly being used for enabling an extremely high degree of design freedom, production of small detailed structures and incorporation of lattice/honeycomb structures to encourage bone in-growth or tailor mechanical properties.10
The aim of this study was to review the existing literature on PS total TMJR implants for their design considerations, fabrication techniques and outcomes to inform the research necessary to optimize design and process efficiency to meet patient expectations.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to perform the review. Search was performed on PubMed and Science direct to collect articles published for the biomaterial, designs and fabrication techniques of patient specific total temporomandibular joint replacement and their outcomes.
The search terms used were 3D printing, three-dimensional printing, additive printing, additive printing technology, custom made implants, patient specific implants, computer assisted printing, CAD, CAM, computer aided design, computer aided manufacturing, customised implants in combination with temporomandibular joint, TMJ replacement, and TMJ total joint replacement.
Inclusion criteria: Full text papers, case reports, finite element analysis and observational studies (prospective and retrospective) after 1995 till Sep 2020 related to TMJ reconstruction with alloplastic implants in peer reviewed journals. Limits were defined on language and reports published in English language only were considered. The manuscript titles and abstracts were independently screened by two reviewers and irrelevant or duplicate titles were removed. Exclusion criteria were study reviews, in-vitro studies or those involving bioprinting and bioengineering. Included articles underwent a full-text review.
3. Results
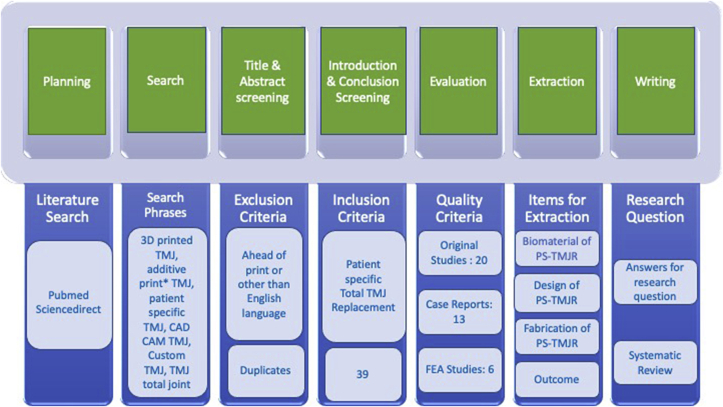
The search revealed 2760 articles. A total of 374 articles were published in, or translated to English language and discussed TMJR implants. Among these 74 were found to be potentially relevant and discussed PS-TMJR implants, of which 38 articles were duplicate and hence removed. An additional 4 articles were extracted by reviewing sources of most relevant articles. A total of 39 articles were selected for full text analysis describing biomaterial, design and fabrication of PS-TMJR implants and their outcomes (Fig. 1, Fig. 2). There were no randomized controlled trials.
Fig. 1.
Research methodology for the literature review which consists of seven steps. Within each of these steps, the results of the process are displayed. The numbers indicate how many documents were retrieved.
Fig. 2.
Distribution of search Records.
20 relevant original studies, 13 case reports and 6 FEA studies reporting new designs were assessed and tabulated in this systematic review, so as not to miss out on the prosthetic designs used so far.
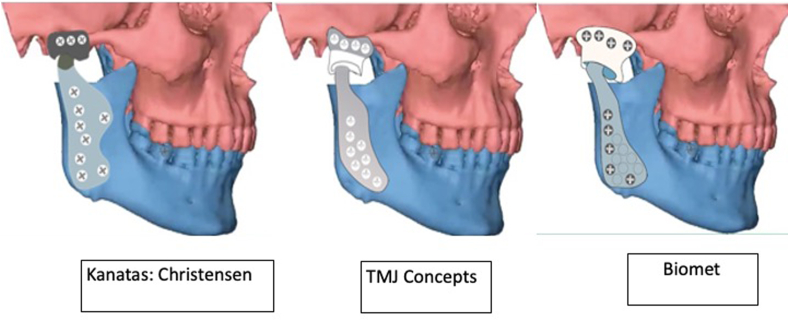
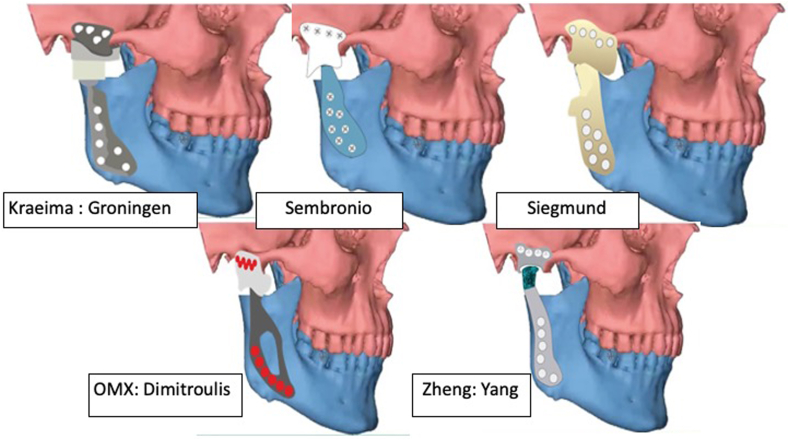
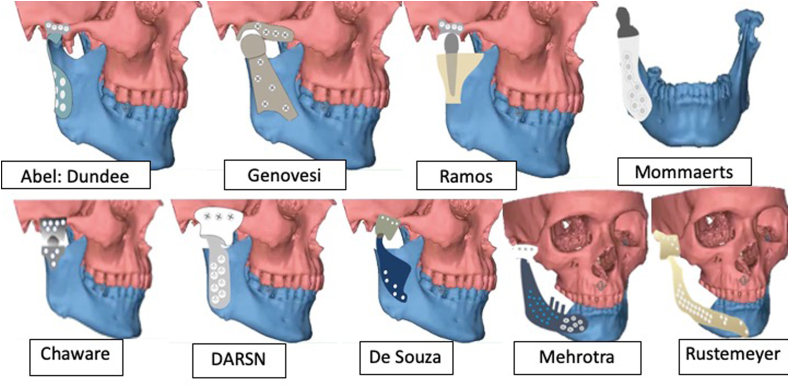
Table 1 describes the material properties of biomaterial used for various PS-TMJR implants. Table 2 describes the biomaterial and design modifications of PS-TMJR devices. The original studies with use of PS-TMJR devices are summarized in Table 3, case reports in Table 4 and FEA studies in Table 5 and describe the outcomes. Fig. 3, Fig. 4, Fig. 5 represent various PS-TMJR implant designs used.
Table 1.
Material properties used in the finite element model reported in studies.
| Young’s modulus (GPa) | Poisson rate | yield strength (ss)/MPa | tensile Strength (sb)/MPa | Elongation | Hardness GPa | |
|---|---|---|---|---|---|---|
| Disc | 0.044 | 0.35 | ||||
| Cancellous bone14,15 | 15.37 ± 2.03 | 0.30 | 7.2 | 7.5 | 0.46 ± 0.08 | |
| Cortical bone2 | 14.5 ± 3.19 | 0.30 | 135 | 150 | 0.43 ± 0.13 | |
| CoCrMo alloy11 | 235–247 | 0.29 | 760–839 | 1290–1420 | 25–29 | 363–402 |
| Titanium alloy2 | 110–119 | 0.33 | 786–910 | 862–1200 | 10–14 | 337–357 |
| UHMWPE2 | 0.8 | 0.4 | 19 | 27 | ||
| PEEK12,13 | 4 | 89.6–380 | 90–100 | 30–150 | 85–109 | |
| CFR PEEK13 | 18 | 120 | ||||
| Zirconia14 | 210 |
Table 2.
Biomaterial & Design modifications of PS-TMJR.
| S No |
Device |
Biomaterial |
Design Modification |
Fabrication technique |
|||
|---|---|---|---|---|---|---|---|
| Glenoid Fossa (GF) | Condyle (C) | Ramus (R) | Fixation Screws(S) | ||||
| 1 | Christensen,15 USA | CoCrMo | CoCrMo | CoCrMo | CoCr | ||
| 2 | Haq,16 UK | UHMWPE | CoCrMo | CoCrMo Medial Ti sprayed | 7 screw holes ramus,5 GF | ||
| 3 | TMJ Concepts,17, 18, 19, 20, 21, 22, 23, 24 USA | UHMWPE, Ti mesh backing | CoCrMo | Ti alloy | Ti alloy | ||
| 4 | Biomet,16,25, 26, 27, 28, 29, 30, 31 USA | UHMWPE | CoCr alloy | CoCr, medial Ti spray | Ti alloy | ||
| 5 | Aftan,32 Iraq | Zr oxide | |||||
| 6 | Butow,33 Africa | nitride coated Ti | CP Ti | Nitride coated Ti | Ti | ||
| 7 | Groningen,11 Netherlands | Ti, UHMWPE | C translation plate zirconia | Ti alloy | Ti alloy | Condylar sphere Translation plate |
CNC GF, DMLS Ti |
| 8 | Abel,34 Dundee | 2 mm | Condylar support prosthesis | FEA Model | |||
| 9 | OMX,35 Melbourne | UHMWPE | Ti alloy | Ti alloy | Ti alloy | Anterior extended GF, flat C, pear-shaped window R for masseter muscle | CNC for GF DMLS Ti |
| 10 | Sembronio,36 Italian | UHMWPE | Ti alloy | Ti alloy | Ti alloy | Posterior lip avoids posterior dislocation C | |
| 11 | Mommaerts,37 Belgian | UHMWPE contained vit E (a-tocopherol) | C coated with carbon | Ti gr 23 alumina sand blast, oxalic acid etch, plasma spray | Ti alloy | Groove C neck for lateral pterygoid, C head coated with carbon | CNC for GF 3D-printed Ti |
| 12 | Siegmund,38 German | UHMWPE | Ti alloy | Ti alloy | Ti alloy | Posterior clip engaged to R | Biomet |
| 13 | Kozakiewicz,39 Polish | UHMWPE | Ti alloy | Ti alloy | Ti alloy | CNC GF DMLS Ti |
|
| 14 | Yang,1 Chinese | UHMWPE, CP Ti mesh | Co–Cr–Mo | Ti alloy | Ti alloy | C cylindrical, hollow, machine taper fits on R | CNC GF,C EBM for R |
| 15 | Chaware40, Indian | ||||||
| 16 | Deshmukh,41 Indian | ||||||
| 17 | Bhargav,42,43 Indian | UHMWPE | Ti alloy | Ti alloy (grade 5) | Ti alloy | 8 counter sinked holes in tripod fashion | |
| 18 | Mehrotra,44 Indian | UHMWPE | Ti alloy | Ti alloy | Ti alloy | SLM | |
| 19 | Sader,45 Germany | new coating | |||||
| 20 | Rustemeyer,46 Germany | UHMWPE | Ti coated C | Extension | Biomet | ||
| 21 | De Souza,47 Brazil | UHMWPE | Promm: C CoCrMo alloy | Pure Ti | pure Ti | CNC | |
| 22 | Olivera,48 Brazil | UHMWPE | CoCrMo alloy | Ti alloy | Ti alloy | Spherical head | |
| 23 | Genovesi,49 Brazil | PEEK LT1 | PEEK LT1 | PEEK LT1 20%Ba | PEEK | ||
Table 3.
Original studies of various PS-TMJR devices and their outcomes.
| SN | Author, year | Number of patients (Joint) | Females | System | FU | Outcome |
|---|---|---|---|---|---|---|
| 1 | Wolford, 199417 | 56 (100) | Techmed | 30 mon | 58% good outcome, 26% fair, 16% poor. 5 R prostheses removed, 30% re-surgery | |
| 2 | Mercuri, 199518 | 215 (363), 296 B/l | Techmed | 48 mon | Decreased pain, increased function, diet, mandibular vertical range of motion. | |
| 3 | Butow, 200133 | (27) | 6 yr | |||
| 4 | Kanatas, 201215 | 31 (44); 13 B/l | 22 | Christensen | 1 yr | Improvement in pain, MO |
| 5 | Briceno, 201320 | 27 (41), 14 B/L | 25 | TMJC | 6 mon | Improved MO, pain relief, satisfaction with surgery and diet consistency. |
| 6 | Haq, 201416 | (5) | 3 | Biomet | 6 mon | MO increased, minimal surgical morbidity, fewer operations, hospital stays |
| 7 | Aagaard, 201425 | 64 (81), 17 B/L | (74) | Biomet | 14 yrs | Adverse events low (7 of 81 alloplastic joints); 2 revision. Almost normal range MO, significant reduction in pain. |
| 8 | Wolford, 201524 | 56 (99) | TMJ Concepts | 21 yrs | 48 patients improved QOL, 6 same, 2 worse. Increased previous surgeries, lower improvement in pain and MO. | |
| 9 | Perez, 201626 | 57 (7 P S/75) 18 B/l) | 38 | Biomet | Compared stock and PS, no statistically significant differences for pain and MO improvement | |
| 10 | Gerbino, 201727 | 38 (13 P S/55) | 29 | Biomet | 45 yrs | Complications: bleeding, malocclusion, postop infection, heterotopic bone formation, contralateral TMJ overload. QoL, MO improved. stock and custom consistent results |
| 11 | Kozakiewic, 201739 | 11 (11) | 4 | 6 mon | Rough surface with DMLS, finishing required. Both had similar clinical outcomes. DMLS more vulnerable to fracture. | |
| 12 | Dimitroulis, 201835 | 38 (50), 12 B/l | 31 | OMX | 2 yrs | 10 (20%) PS, rest patient matched. 74.4% reduction in joint pain, improvements (p < 0.05) in MO (30.8%), diet (77.1%), function (59.2%). |
| 13 | Genovesi, 201849 | 4 (7), 3 B/l | 10 yr | Function close to physiological movements, adequate MO, lateral and protrusive movements preserved. | ||
| 14 | Mehrotra, 201944 | 7 (7) | Improved esthetics, function, QoL, symmetry of face, occlusion, MO, closing, lateral movements with no Jaw deviation during movements. | |||
| 15 | Siegmund, 201938 | 28 (stock, PS) | Biomet | 6 mon | MO, pain, diet improved. 66% stock and 100% PS-TMJ-TJR patients improved well-being. Stock and CAD/CAM comparable result. | |
| 16 | Sembronio, 201936 | 10 (11), 1B/l | No statistically significant deviation between preoperative virtual surgical planning and postoperative results | |||
| 17 | Zheng, 20191 | 12 (12) | Yang’s TMJR | 1 yr | Pain, diet, mandibular function, MIO improved. Lateral movement limited to non-operated side, mandible deviated towards operated side on opening. | |
| 18 | Sahdev 201921 | 95 (108) | TMJC | 4.5 yrs | MO improved by a mean of 7.7 ± 10.27 mm, pain decreased by 1.5 ± 3.29 | |
| 19 | Mommaerts 201937 | 5 | Jaw Function, MO, Diet intake, QoL improved | |||
| 20 | Bhargava, 202043 | 20 (8 F) | Jaw Function, MO, Diet intake, QoL improved |
TMJR: Temporomandibular joint reconstruction, U/l: Unilateral, B/l: Bilateral, MIO: Maximal interincisal mouth opening, QoL: Quality of life, FU: Follow up, mon: months, yr: years, TMJC:TMJ Concepts.
Table 4.
Case reports for PS-TMJR.
| S No | Author | TMJR Biomaterial | Age/Sex | TMJ Disorder | Outcome |
|---|---|---|---|---|---|
| 1 | Chaware 200940 | – | 12 yr F | U/l TMJ ankylosis, FU 3yr | Painless jaw movements. MIO 4.0 cm, normal diet, excellent clinical results. |
| 2 | Sader 200 845 | coated | – | – | Stability improved, implanted body could be miniaturized, inserted quasi minimal- invasively without visible scar formation. The head and socket design enabled good physiologic rotation & translation |
| 3 | Pearce 200919 | TMJ Concepts | 59/M, 59/F, 24/M, 40/F, 21/F | TMJ ankylosis | Single stage management of ankylosis |
| 4 | Aftan 201332 | Zirconium oxide ceramic | TMJ ankylosis, congenital dysmorphisim | Improved esthetics, function, MIO | |
| 5 | de Oliveira-Neto 201448 | 38/M | Ankylosing spondylitis | 20 mm at 2 years | |
| 6 | Neelakandan 201442 | Ti alloy | Recurrent TMJ ankylosis | – | |
| 7 | Lindell 201729 | Biomet | 15/F | TMJ Ankylosis, FU 4 yrs |
No pain, excellent jaw function. |
| 8 | de Souza, 201747 | 67 F | osteoma FU: 2 years, | No painful symptoms, mouth opening of 32 mm | |
| 9 | Sarlabous, 201830 | Biomet | Ameloblastoma | Mandibular function preserved. | |
| 10 | Fernández, 201828 | Biomet | 45/M | Fibrous condylar dysplasia | Satisfactory results for aesthetic, functional outcome. |
| 11 | Rustemeyer 201946 | Biomet | 50/F | SAPHO syndrome with Diffuse sclerosing osteomyelitis | outcome satisfactory |
| 12 | Abou-Foul 201931 | Biomet | 56/M | Calcium pyrophosphate Deposition in TMJ | 24 mon FU 40 mm MO |
| 13 | Wang 201923 | TMJ Concepts | 14/F | Bilateral idiopathic condylar resorption | – |
Table 5.
FEA models of PS-TMJR.
| S No | Author | Model | Outcome |
|---|---|---|---|
| 1 | Ramos 201450 | 2 FEA models of TMJR cadaveric mandible; external connection with screws internal intramedullary fix | Strain distributions significantly differ at external surface of mandible in displacements. Intramedullary fixation increased strains in cancellous tissue. Strain distribution influenced by number and distribution of screws. Intramedullary fixation better, as generates less strain |
| 2 | Ramos, Mesnard 201651 | FEA model of a cadaveric condyle innovative intramedullary implant | Increased strains in proximal region of condyle (140%). Maximum strain and stress generated with implant less than 2200με and 75 MPa in posterior region of cortical bone. Shortly after implant placement, stress and strain results within normal limits, similar to intact condyle. |
| 3 | Bekcioglu 201752 | FEA model of TMJR Biomet | Intact model maximum von Mises stress: 252.697 N/mm sq at condyle and 5.418 N/mm sq at disc. At TMJ TJR implant 792.681 N/mm sq and at contralateral joint, 268.908 N/mm sq at condyle and 8.357 N/mm sq at disc. Unilateral TMJR cause increased stress at disc and contralateral condyle. |
| 4 | Abel 201534 | Condylar support prosthesis (CSP) | Much lower stresses as allows physiological load transfer, reduces strain on the bone around the screws. |
| 5 | Kraeima 201811 | 5 human cadavers | Groningen TMJ prosthesis can be placed with great accuracy. |
| 6 | Chen53 | 2 models of mandible, intact one-side, implanted other side | Maximum stress on UHMWPE surface 19.61 MPa, in mandible at condylar neck, 170.01 MPa. Peak von Mises stress on top screw of mandible, 236.08 MPa. For intact model, strain distribution symmetrical, for model with prosthesis, strain consistent, except last 24 mm, strain 41.4–58.3%. |
Fig. 3.
Commercial PS-TMJR systems.
Fig. 4.
Design modifications of PS-TMJR.
Fig. 5.
Some other design modifications of PS-TMJR.
4. Discussion
Patient specific TMJR are indicated for severely degenerated, anatomically distorted joints operated several times or stock implant does not fit, or a simultaneous correction of the mal-aligned jaws is required. They have gained appreciation even in growing children or skeletally immature patients.5,8,54,55 The only disadvantage of patient specific implants is the increased up-front cost. The extra time spent in preoperative planning is, however, potentially compensated by reduction in the duration of surgery and hospital stay.
The fabrication of PS devices through computer aided technologies can be broken down into four steps: medical image acquisition from computer tomography or cone beam computer tomography (CT/CBCT) in digital imaging and communications in Medicine (DICOM) format; processing of the slice images into a 3D reconstruction of the anatomical volume and clean-up of artefact; 3D surgical planning and implants designing; implant production and post processing.1,14,24,34,35,37,45,.
Precise fit of a PS device reduces the chance of micromovement, and the stress generated on the fixation system under loading, and increase the life span of the implant; else these stresses and strains directly or eccentrically vector against the device-to-host-bone interface creating wear.5 Moreover, it minimises the risk of damage to inferior alveolar neurovascular bundle during screw placement, allows maximal screw placement for initial stability by pre-determining the appropriate screw length and preventing use of longer screws, which can irritate the temporalis or medial pterygoid muscles during mandibular function causing pain.
Elledge56 reviewed both stock and custom made globally emerging TMJR devices from 15 countries and found 27 systems. Our review reports only the PS-TMJR devices used till now, which include 8 reports from TMJ Concepts, 9 from Zimmer Biomet, 3 from Brazil, 5 from India, 3 from Germany, 2 from UK, and 1 each from Groningen, Christensen, OMX (Australia), Yang (China), Italy, Poland, Belgium, Africa and Iraq.
4.1. Biomaterial
Employing the most advantageous physical characteristics of biocompatible materials is an essential consideration while designing and manufacturing a PS-TMJR.14 Devices must be designed to deliver good primary stability, reduced micromotions, and osseointegration to withstand the loads acting over full range of function. Good osseointegration depends on the implant’s biocompatibility, surface topography, energy and surface chemistry. In general, material with high wear resistance like Cobalt-chromium-molybdenum (CoCrMo) and titanium alloys with the required strength and fatigue resistance for implant body, should be used for joint surfaces.57
If mechanical properties between the bone and implant material do not match, stress shielding lead s to severe bone density loss; and to reduce this stress-shielding effect, the elastic modulus of the device material should be low.57 Moreover, material with lower stiffness share the load better with the surrounding bone. Either geometry or material should accommodate reduced stiffness in areas prone to stress shielding and subsequent bone resorption.57 Stiffness is also controlled by the geometry of an implant. Making implants hollow or porous, decreases its stiffness. The third property required is osseointegration that can also be encouraged through material and geometries for better bone ingrowth or osseointegration.57,58 Table 1.
The most common alloys used for maxillofacial implant applications are medical grade 23 titanium alloy Ti6Al4VELI alloy (89.0–91.0 wt% Ti, 5.5–6.5 wt% Al, and 3.5–4.5 wt% V, 0.13% (maximum) Oxygen, where ELI stands for Extra Low Interstitial59; and Cobalt-Chrome-Molybdenum (CoCrMo) composed of 58.9–69.5% Co, 27.0–30% Cr, 5.0–7.0% Mo, small amount of other elements (Mn, Si, Ni, Fe, C).60 Mostly, the mandibular component of a PS-TMJR device is machined in titanium alloy with a condylar head of CoCrMo. The inner surface of the ramal component is plasma sprayed with titanium for better bony adherence.1,16,26 Fixation screws require a high strength and fatigue resistance, hence are made in titanium alloy. CoCrMo-on-UHMWPE is preferred over titanium alloy for use as the articular component.
Recent research illustrates the use of polyether-ether-ketone (PEEK), a semi-crystalline, polyaromatic, thermoplastic polymer, for PS devices.48 PEEK was first used for implantation as a spine cage for lumbar fusion, by Brantigan and Steffee in 1989.61 It is radiolucent, hence good for radiographic imaging of implants post-surgery. PEEK is less prone to oxidative degradation, and fewer free radicals are produced during irradiation of PEEK as compared with UHMWPE. PEEK has lower mechanical strengths, which are also influenced by its layer thickness, printing speed, temperature, and heat treatment. Although PEEK is bioinert, compared with titanium, it has limited properties for osseointegration. Hence, surface modification is required to influence cell adhesion, and more binding sites for cell attachment.
Although PEEK reinforced with carbon fibre (CFR) has improved tensile strength and fatigue limit, the bone formation ability and biocompatibility still remains similar to that of PEEK.57,61,62 CFR-PEEK has an elastic modulus close to the human cortical bone (14 GPa) depending upon the amount of reinforced carbon fibres and manufacturing methods. Scientific reports on use of PEEK and CFR PEEK in maxillofacial region as an articulating load bearing surface are limited. However, orthopedic literature shows that the wear resistance of CFR PEEK, is superior to that of UHMWPE when used in hip joint simulations, but has conflicting reports as a bearing surface in the knee joint.62
Use of a thin, hard, wear-resistant protective coat of materials such as Titanium nitride, Titanium carbide, or diamond-like carbon, can significantly improve friction, lubrication and wear properties of Titanium implants.63 Butow changed the titanium coating to titanium nitride.33 Bioinert ceramics like alumina and zirconia have been widely used as articulating surfaces in orthopedic joint replacement systems.31 Aftan used zirconium oxide coating in his TMJR implants.32 The fracture toughness and flexural strength of zirconia are superior to those of alumina, and zirconia displays high resistance to crack propagation and a failure rate of 0.002%. The Groningen device11 had titanium alloy mandibular part with Zirconia condylar sphere and disc made of UHMWPE, over which was the zirconia translation plate and the fossa in titanium. However, on clinical examination, zirconia condylar heads showed accelerated aging and high fracture rates, that led to its withdrawal. Recently, a combination of alumina (70%–95%) and zirconia (30%–5%) known as zirconia-toughened alumina (ZTA) has been used for their enhanced strength and fracture toughness. The addition of chromium and strontium oxides in very low quantities, prevents the loss in hardness of ZTA and crack propagation. Literature reveals lower wear rates for ZTA-on-ZTA and ATZ-on-ATZ compared to Alumina-on-Alumina. However, in vivo studies and clinical data with long-term follow up are currently limited.32
Also, inorganic materials such as hydroxyapatite, if added at the implant surface to mimic natural bone, show better osseointegration. Recently, magnesium phosphate doped with biologically active ions like strontium have shown to significantly enhance bone formation.64,65 It is important to remember that rough surfaces promote cell adhesion or bone in-growth, while smooth surfaces are preferred wherever adhesion of bacteria is to be avoided. So micro-topography can be altered to favour cellular adhesion, macro-roughness (porous coatings) to offer bone ingrowth, mechanical locking and a hydrophilicity to enable cell attachment.63
In this review, we found 6 different types of implant material used for mandibular condylar head, CoCrMo alloy, titanium alloy, carbon coated, commercially pure titanium, PEEK and Zirconia. Mandibular ramus was observed to be fabricated either in titanium alloy, nitride coated titanium, pure titanium, titanium grade 23 alumina sand blasted, oxalic acid etched, plasma sprayed to promote secondary stability via osteointegration,37 CoCrMo alloy or PEEK. The mandibular component was coated with diamond-like carbon to provide an extra area of scaffolding for deep reattachment of the lateral pterygoid muscle; and a groove around the neck to fix the lateral pterygoid muscle.37 Glenoid fossa had UHMWPE with or without titanium mesh, one with reinforced with vitamin E37 as an antioxidant to counter aging, nitride coated titanium, CoCrMo or PEEK; while the fixation screws were mostly titanium alloy.
As TMJR is a biomechanical rather than a biological solution for the diseased joint, attempts are still on for finding a solution with new materials having improved mechanical properties or can be bioprinted with autologous stem cells and support them to facilitate creation of soft tissues.9
4.2. Fabrication process
Although the standard fabrication technique uses CNC milling, recent reports show use of additive technologies for device production66,67 as they may be of greater advantage when used to handle complex cases with less experienced surgeons.67 Metal AM technologies fall under three broad categories: Selective laser melting (SLM), direct metal laser sintering (DMLS) and electron beam melting (EBM). SLM and DMLS fabricate parts in layers from powdered metal alloy using CO2 Laser and require a low melting temperature 200 °C, while EBM fabricates layer by layer either using wire or powder and requires a high melting temperature (680–720 °C). DMLS process allows fabrication of human implants in minimum processing time, directly from computer-aided design models with no requirement for sintering later as the parts are produced with 95% density.68
The major advantage of DMLS is that it produces objects free from the residual stresses and internal defects. SLM is a high-powered laser that fully melts each layer of metal powder rather than just sintering it; and prints dense and strong objects. It is a very high-energy process, as each layer of metal powder must be heated above the melting point of the metal; and can lead to stresses and dislocations inside the final product, which can compromise its physical properties.69 The most accurate and predictable technique has been CNC milling, followed by DMLS, and then metal casting35
However, there are a few biomechanical concerns that have arisen70,71
Porosity: Pores can cause low density, more the pores, lower is the density of part.
Density: When a part undergoes cyclic stresses, its density determines whether or not the part will fail under load. The lower the density of part, the more likely it is to crack under pressure.
Residual stress: Variable thermal changes during printing can lead to residual stress. Residual stress has an unfavourable impact on the integrity of the part, resulting in deformation.
Cracking and Warping: When melted metal cools after printing, causes contraction and curling up of edges and deformation. If stress exceed the part’s strength, leads to cracking. Cracking can also occur if powder is not melted properly.
Post processing surface roughness: Rough surfaces may require machining, grinding, or polishing to achieve better finish, which may again damage the part.
Laser-based additive technologies like SLM and DMLS have inherent problems such as high porosity, residual stress, cracking, warping, and surface roughness that may increase the likelihood of mechanical failure, screws becoming loose and hence require long-term studies.56 Appropriate design, build strategies and post-processing such as vacuum heat treatment are used to overcome these potential limitations.
EBM, a technology originally developed by Arcam AB (Sweden), now part of GE Additive (USA), is one of the earliest adopted AM technologies for implant production.66 EBM overcomes the need for heat treatment by incorporating a combination of vacuum and a high build chamber temperatures during the fabrication process. The trade-off is a higher surface roughness over SLM and DMLS, which requires a higher level of post-processing and makes it unfeasible to use for small features typically required for maxillofacial implants.
In general, all AM-produced implants require post-processing either by CNC or hand polishing to achieve a smooth surface finish and create accurate features such as screw holes and threads.38 The biggest future challenges facing the 3D printing industry include equipment costs, manufacturing resources and costs, limited materials availability, lack of in-house additive manufacturing expertise, longer production times, liability implication and lack of formal standards.63
Selective laser sintering (SLS) has been the most popular technology for fabricating PEEK, while fused deposition modeling (FDM) is one of the fastest growing, cost effective, easier to use with minimal risk of contamination. However, semicrystalline structure and high melting temperature of PEEK makes it difficult to process PEEK objects by FDM printing and the process is liable to cause excessive thermal stress and thermal cracks.62
4.3. Design considerations
The designing of TMJR is an interdisciplinary activity, that requires an understanding of the engineering concepts, implant properties, a detailed knowledge of anatomy, surgical expertise and experience.5,24 TMJR implants should be capable of rotational and translational movements, allowing more than 2000 hinge and sliding movements per day while eating, speaking, swallowing.24 Care should be taken to ensure no rocking movement to prevent failure of the implant later. It has been seen that if the condyle position in the reconstructed joint is displaced inferiorly by 3–4 mm, thereby lowering its point of rotation, the use of a unilateral prosthesis would no longer overload the contralateral healthy joint.16 It is this reason why the thickness of UHMWPE should be at least 3 mm.16,36,63
Sembronio designed a UHMWPE Fossa to completely enclose the condylar head and emphasized a posterior lip to avoid posterior dislocation of the condyle.36 Ackland’s design of the OMX device had the mandibular component following the contour of the ascending ramus with a pear-shaped window in the condylar component to facilitate reattachment of masseter muscle.2,34,35 The articular head of the condylar component was circular and slightly flat to facilitate better translation during mastication. The concavity of the fossa was hemispherical to match the condylar head anatomy and allow translation of the condyle.2 The screw holes were placed close to the posterior and inferior border of the mandible to avoid damaging inferior alveolar nerve.
Siegmund emphasized a posterior clip to hold the posterior border of ramus, Kozakiewicz39 design of mandibular component wrapped the entire mandibular ramal stump posteriorly. Abel designed a condylar support prosthesis in the Dundee device34 and Ramos introduced a press-fit stem into the condyle. Zheng designed a patient specific TMJR implant considering the dimension and slope of articular surface of the fossa, a cylindrical hollow condylar head, a machine taper connection on the top of the mandibular handle, and sandblasted the inner surface of the mandible.1
In India, Chaware and Deshmukh designed in stainless steel with a spherical condyle, Bhargav designed DARSN device very similar to Biomet, while in Brazil de Souza designed Promm prosthesis, Olivera48 had spherical condyle though both had UHMWPE fossa CoCrMo condylar head and mandibular component in titanium. Genovesi49 designed the entire device in zirconia. Rustemeyer46 designed extended devices, Mehrotra44 introduced dental implant abutment studs for dental rehabilitation.
Mommaerts37 created a lattice structure of 500 mm diamond units in the condylar neck of a titanium, to reattach lateral pterygoid to resume normal protrusion and lateral excursions and AM fabricated mandibular component to house bone graft and growth factors to promote bone formation.
Finite element analysis of the temporomandibular joint and the custom TMJ devices showed that the strain distribution can be influenced by number and distribution of screws.49 Using a larger number of screws decreases stresses at each individual screw. However, an intramedullary fixation may be better, as it generates less strain.49 Concentration of maximum stress during function is at the most superior screw hole in the ramus.44,72 According to Hsu,73 3 staggered screws can provide optimal implant stability and stress strain distributions in a TMJ condylar prosthesis. However, TMJ Concepts recommends use of 100% screw holes for screw fixation, although use of fewer screw holes does not predispose it to hardware loss. Others recommend use of more than 50% screw holes or a minimum of 5 screws.64,74 If a device does not use the full length of the vertical mandibular ramus for screw fixation, it may have problems with stability leading to micromotion, osteolysis, or failure.66 Appropriate screw length can be predetermined through the CT data, to avoid damage to the neurovascular bundle. At least five screw holes are recommended in the PS-TMJR to fit the patient’s zygomatic arch, and at least seven screw holes in the ramal component.16 Each screw hole should be positioned away from vital structures and into the best possible bone for the fixation.16 Depth measurements for each screw hole should be provided to confidently place bicortical screws in the respective holes without damaging the adjacent nerves or vessels.
4.4. Cost
Multiple factors influence the lifetime cost of treatment. This includes costs associated with the implant cost (including design and fabrication, which is typically far higher with custom implants than stock alternatives), the cost of the procedure itself and costs related to post-surgery outcomes and quality of life. The major advantages of alloplastic over autogenous TMJ reconstruction is that a second surgical site with its associated morbidity is avoided, expensive operative time is reduced and postoperative hospital stay decreased.63 Therefore, although these devices appear expensive, the overall cost is either equal or less than that of autogenous reconstruction.63
A single stage bilateral PS-TMJR implants costs from £15 000 to £20 000 in the UK, (20 000 USD per joint)1,19 while a stock prostheses costs almost half.17 However, if the costs are holistically assessed, PS-TMJR implants have the potential to be less expensive due to reductions in surgical and postoperative hospital stay time, smaller bone resections, less blood loss and lower blood transfusion rates, leading to an early discharge straight to home rather than to a rehabilitation or post-acute care facility, improved outcomes, better quality of life, and substantial cost-savings.74
A recent report by Yang undertaken in 16 patients requiring mandibular reconstruction, showed that the time spent by a surgeon on virtual surgery and plate design was 18.83 ± 13.19 h, and the time taken for 3D printing, post-processing, and product delivery was 162.9 ± 55.15 h.75 Ballard reported a mean 62 min of time saved ($3720/case saved) when 3D printed anatomic models were used in surgical care in 7 patients, and a mean 23 min time saved ($1488/case saved) when 3D printed surgical guides were used in 25 studies, if per minute OT time saves $62 (range 22–133$ saved/minute).76
4.5. Outcomes and complications
Patients with loss of a large portion of the mandibular ramus are usually not candidates for reconstruction with stock devices, and a patient specific implant should be considered. PS-TMJR implants overcome the limitations of stock devices and provide absolute fit and accuracy, leading to better and long-term clinical outcomes16,57
Outcomes studies with TMJ Concepts at twenty years have shown them as safe and predictable devices although they may have longevity issues due to material wear or failure, material hypersensitivity, and cost.24,63 The most common reported complications include infection (1–3%), periprosthetic heterotopic ossification (2%), persistent pain (1%), and material sensitivity (0.5%).68 Other complications (5%) include facial nerve damage, alveolar nerve injuries, neuropathic pain, loss of sensation in the skin, hearing problems, and infection.15 Complications with stock as well as PS-TMJR include hardware failure, infection, and heterotopic bone formation.77, 78, 79, 80 No evidence has been found that compares infection rates associated with patient specific and stock.63 Also PS-TMJR printed with SLM and EBM are yet to be compared with the conventional milled ones in terms of mechanical strength, stress and strain as well as their clinical outcome.
Evaluation of the Groningen TMJ prosthesis at 8-year follow-up showed 87.5% success, with high patient satisfaction.32 Most studies have reported acceptable outcomes with PS-TMJR22,35,37 even in the long term. The precise design and fit makes it less likely to exhibit micromovement under loading, gives it longevity and reduces the rate of failure.14
5. Conclusions
Although PS-TMJR implants allow a better anatomical fit, improved fixation, and safeguard various structures such as the inferior alveolar nerve, they vary in designs, material and fabrication techniques. However, PS-TMJR printed with SLM and EBM technologies have yet to be compared with the conventional ones in terms of mechanical strength, and clinical outcome. With emerging bioprinting technologies, even newer biomaterials should be considered for 3D printing of PS-TMJR devices designed to achieve harmony in function between the joint device, bone and masticatory muscles.
Declaration of competing interest
None.
Acknowledgement
Collaborative Medical Device Design Initiative: Thematic partnership, 184–1/2018(IC) funded by UK India Educational Research Initiative, University grants Commission India).
References
- 1.Zheng J., Chen X., Jiang W., Zhang S., Chen M., Yang C. An innovative total temporomandibular joint prosthesis with customized design and 3D printing additive fabrication: a prospective clinical study. J Transl Med. 2019;17(1):4. doi: 10.1186/s12967-018-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew M.T., Kerwell S., Lundberg H.J., Sukotjo C., Mercuri L.G. Tribocorrosion and oral and maxillofacial surgical devices. Br J Oral Maxillofac Surg. 2014;52:396–400. doi: 10.1016/j.bjoms.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri L.G. Alloplastic TMJ replacement. Rationale for custom devices. Int J Oral Maxillofac Surg. 2012;41:1033–1040. doi: 10.1016/j.ijom.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Ackland D.C., Robinson D., Redhead M., Lee P.V.S., Moskaljuk A., Dimitroulis G. A personalized 3D-printed prosthetic joint replacement for the human temporomandibular joint: from implant design to implantation. J Mech Beh Biomed Mat. 2017;69:404–411. doi: 10.1016/j.jmbbm.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Mercuri L.G., Swift J.Q. Considerations for the use of alloplastic temporomandibular joint replacement in the growing patient. J Oral Maxillofac Surg. 2009;67:1979–1990. doi: 10.1016/j.joms.2009.05.430. [DOI] [PubMed] [Google Scholar]
- 6.Resnick C.M. Temporomandibular joint reconstruction in the growing child. Oral Maxillofac Surg Clin. 2018;30:109–121. doi: 10.1016/j.coms.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Cascone P., Basile E., Angeletti D., Vellone V., Ramieri V., Pecram Study Group TMJ replacement utilizing patient fitted TMJ TJR devices in a re-ankylosis child. J Cranio-Maxillo-Fac Surg. 2016;44:493–499. doi: 10.1016/j.jcms.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Sidebottom A.J. Alloplastic or autogenous reconstruction of the TMJ. J Oral Biol Craniofac Res. 2013;3:135–139. doi: 10.1016/j.jobcr.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamidi S.K., Klutcharch K., Rao S., Souza J.C.M., Mercuri L.G., Mathew M.T. Advancements in temporomandibular joint total joint replacements. Biomedical Engineering Letters. 2019;9:169–179. doi: 10.1007/s13534-019-00105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan X.P., Tan Y.J., Chow C.S.L., Tor S.B., Yeong W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: a state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater Sci Eng C. 2017;76:1328–1343. doi: 10.1016/j.msec.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 11.Kraeima J., Merema B.J., Witjes M.J.H., Spijkervet F.K.L. Development of a patient-specific TMJ prosthesis according to the Groningen principle through a cadaver test series. J Cranio-Maxillo-Fac Surg. 2018;46(5):779–784. doi: 10.1016/j.jcms.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 12.http://www.matweb.com/search/datasheet_print.aspx?matguid=2164cacabcde4391a596640d553b2ebe.
- 13.Najeeb S., Zafar M.S., Sultan Z.K., Siddiqui F. Applications of PEEK in oral implantology and prosthodontics. Prosthodont Res. 2016;60(1):12–19. doi: 10.1016/j.jpor.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Han X., Yang D., Yang C. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J Clin Med. 2019;8(2):240. doi: 10.3390/jcm8020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanatas A.N., Needs C., Smith A.B., Moran A., Jenkins G., Worrall S.F. Short-term outcomes using the Christensen patient-specific TMJ implant system: a prospective study. Br J Oral Maxillofac Surg. 2012;50:149–153. doi: 10.1016/j.bjoms.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Haq J., Patel N., Weimer K., Matthews N.S. Single stage treatment of ankylosis of TMJ using patient-specific total joint replacement and virtual surgical planning. Br J Oral Maxillofac Surg. 2014;52(4):350–355. doi: 10.1016/j.bjoms.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Wolford L.M., Cottrell D.A., Henry C.H. Temporomandibular joint reconstruction of the complex patient with the Techmedica custom-made total joint prosthesis. J Oral Maxillofac Surg. 1994;52:2–10. doi: 10.1016/0278-2391(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 18.Mercuri L.G., Wolford L.M., Sanders B., White R.D., Hurder A., Henderson W. Custom CAD/CAM total temporomandibular joint reconstruction system: preliminary multicenter report. J Oral Maxillofac Surg. 1995;53(2):6. doi: 10.1016/0278-2391(95)90381-x. [DOI] [PubMed] [Google Scholar]
- 19.Pearce C.S., Cooper C., Speculand B. One stage management of ankylosis of the temporomandibular joint with a custom-made total joint replacement system. Br J Oral Maxillofac Surg. 2009;47(7):530–534. doi: 10.1016/j.bjoms.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Briceno F., Ayala R., Delgado K., Pinango S. Evaluation of temporomandibular joint total replacement with alloplastic prosthesis: observational study of 27 patients. Craniomaxillofacial Trauma Reconstr. 2013;6(3):171–178. doi: 10.1055/s-0033-1343779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahdev R., Wu B.W., Anderson N., Khawaja S.N., Kim S., Keith D.A. A retrospective study of patient outcomes after temporomandibular joint replacement with alloplastic total joint prosthesis at Massachusetts general hospital. J Oral Maxillofac Surg. 2019;77(2):280–288. doi: 10.1016/j.joms.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Gruber E.A., McCullough J., Sidebottom A.J. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg. 2015;53:412–415. doi: 10.1016/j.bjoms.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Veiszenbacher E., Waite P.D. Kau CH Comprehensive treatment approach for bilateral idiopathic condylar resorption and anterior open bite with customized lingual braces and total joint prostheses. Am J Orthod Dentofacial Orthop. 2019;156(1):125–136. doi: 10.1016/j.ajodo.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Wolford L.M., Mercuri L.G., Schneiderman E.D., Movahed R., Allen W. Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ Concepts device. J Oral Maxillofac Surg. 2015;73(5):952–960. doi: 10.1016/j.joms.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Aagaard E., Thygesen T. A prospective, single-centre study on patient outcomes following temporomandibular joint replacement using a custom-made biomet TMJ prosthesis. Int J Oral Maxillofac Surg. 2014;43:1229–1235. doi: 10.1016/j.ijom.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Perez L.M.G., Somarriba B.G.P., Centeno G., Vallellano C., Carmona J.F.M. Evaluation of total alloplastic temporo-mandibular joint replacement with two different types of prostheses: a three-year prospective study. Med Oral Patol Oral Cir Bucal. 2016;21(6):e766–e775. doi: 10.4317/medoral.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbino G., Zavattero E., Bosco G., Berrone S., Ramieri G. Temporomandibular joint reconstruction with stock and custom-made devices: indications and results of a 14-year experience. J Cranio-Maxillo-Fac Surg. 2017;45:1710–1715. doi: 10.1016/j.jcms.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Fernández F.M., Fernández S.J., Costas L.A., López B.A. Fibrous condylar dysplasia: resection and reconstruction with a custom-made TMJ prosthesis using virtual surgical planning. J Stomatol Oral Maxillofac Surg. 2018;119(2):135–139. doi: 10.1016/j.jormas.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Lindell B., Thor A. A Case of glenoid fossa fracture, progressive ankylosis, total joint reconstruction with alloplastic prosthesis to normalized function including evaluation with F18-PET/CT–a four year follow-up. Craniomaxillofacial Trauma Reconstr. 2017;10(1):60–65. doi: 10.1055/s-0036-1572493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarlabous M., Psutka D.J. Treatment of mandibular ameloblastoma involving the mandibular condyle. J Craniofac Surg. 2018;29(3):e307–e314. doi: 10.1097/SCS.0000000000004362. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Abou-Foul A.K., Saeed N.R. Treatment of calcium pyrophosphate deposition in the temporomandibular joint with resection and simultaneous reconstruction using a custom joint prosthesis. J Oral Maxillofac Surg. 2020;24(2):235–238. doi: 10.1007/s10006-019-00825-7. [DOI] [PubMed] [Google Scholar]
- 32.Aftan K. Total TMJ replacement with zirconium oxide ceramic prosthesis. Int J Oral Maxillofac Surg. 2013;42(10):1356. [Google Scholar]
- 33.Bütow K.W., Blackbeard G.A., van der Merwe A.E. Titanium/titanium nitride temporo mandibular joint prosthesis: historical background and a six-year clinical review. SADJ. 2001;56(8):370–376. [PubMed] [Google Scholar]
- 34.Abel E.W., Hilgers A., McLoughlin P.M. Finite element analysis of a condylar support prosthesis to replace the TMJ. Br J Oral Maxillofac Surg. 2015;53(4):352–357. doi: 10.1016/j.bjoms.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Dimitroulis G., Austin S., Sin Lee P.V., Ackland D. A new three-dimensional, print-on-demand temporomandibular prosthetic total joint replacement system: preliminary outcomes. J Cranio-Maxillo-Fac Surg. 2018;46(8):1192–1198. doi: 10.1016/j.jcms.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Sembronio S., Tel A., Costa F., Isola M., Robiony M. Accuracy of custom-fitted temporomandibular joint alloplastic reconstruction and virtual surgical planning. Int J Oral Maxillofac Surg. 2019;48(8):1077–1083. doi: 10.1016/j.ijom.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Mommaerts M.Y. On the reinsertion of the lateral pterygoid tendon in total temporomandibular joint replacement surgery. J Cranio-Maxillo-Fac Surg. 2019;47:1913–1917. doi: 10.1016/j.jcms.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Siegmund B.J., Winter K., Meyer-Marcotty P., Rustemeyer J. Reconstruction of the temporomandibular joint: a comparison between prefabricated and customized alloplastic prosthetic total joint systems. Int J Oral Maxillofac Surg. 2019;48:1066–1071. doi: 10.1016/j.ijom.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Kozakiewicz M., Wach T., Szymor P., Zieliński R. Two different techniques of manufacturing TMJ replacements - a technical report. J Cranio-Maxillo-Fac Surg. 2017;45(9):1432–1437. doi: 10.1016/j.jcms.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Chaware S.M., Bagaria V., Kuthe A. Application of the rapid prototyping technique to design a customized TMJ to treat temporomandibular ankylosis. Indian J Plast Surg. 2009;42(1):85–93. doi: 10.4103/0970-0358.53031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshmukh T.R., Kuthe A.M., Chaware S.M., Bagaria V., Ingole D.S. A novel rapid prototyping and finite element method-based development of the patient-specific temporomandibular joint implant. Comput Methods Biomech Biomed Eng. 2012;15(4):363–370. doi: 10.1080/10255842.2010.538385. [DOI] [PubMed] [Google Scholar]
- 42.Neelakandan R.S., Raja A.V.D.K., Krishnan A.M. Total alloplastic TMJ reconstruction for management of TMJ ankylosis. J Maxillofac Oral Surg. 2014;13(4):575–582. doi: 10.1007/s12663-013-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhargava D., Neelakandan R.S., Sharma Y., Dalsingh V., Beena S., Gurjar P. Predictability and feasibility of total alloplastic temporomandibular joint reconstruction using DARSN TM joint prosthesis for patients in Indian subcontinent-A prospective clinical study. J Stomatol Oral Maxillofac Surg. 2020;121(1):2–8. doi: 10.1016/j.jormas.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Vignesh U., Mehrotra D., Howlader D., Singh P.K., Gupta S. Patient specific three-dimensional implant for reconstruction of complex mandibular defect. J Craniofac Surg. 2019;30(4):e308–311. doi: 10.1097/SCS.0000000000005228. [DOI] [PubMed] [Google Scholar]
- 45.Sader R., Leiggener C.S., Zeilhofer H.F. A new innovative solution for total TMJ replacement. J Cranio-Maxillo-Fac Surg. 2008;36(1) · S 159. [Google Scholar]
- 46.Rustemeyer J., Siegmund B.J., Okcu Y., Busch A. Total mandibular reconstruction following diffuse sclerosing osteomyelitis. Oral Maxillofac Surg. 2019;23(1):95–99. doi: 10.1007/s10006-018-0731-9. [DOI] [PubMed] [Google Scholar]
- 47.de Souza N.T., Cavalcante R.C.L., Cavalcante M.A.A. An unusual osteoma in the mandibular condyle and the successful replacement of the temporomandibular joint with a custom-made prosthesis. BMC Res Notes. 2017;10:727. doi: 10.1186/s13104-017-3060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira-Neto P.J., Marchiori E.C., de Almeida Lopes M.C., Moreira R.W. Bilateral alloplastic prostheses for temporomandibular joint reconstruction in patient with ankylosing spondylitis. Craniomaxillofacial Trauma Reconstr. 2014;7(2):149–153. doi: 10.1055/s-0034-1371546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genovesi W. A new concept and design for an alloplastic total TMJ prosthesis using PEEK LT1 20% BA. J Oral Maxillofac Surg. 2018;76(10):e75–e76. [Google Scholar]
- 50.Ramos A., Mesnard M. Theoretical assessment of an intramedullary condylar component versus screw fixation for the condylar component of a hemi arthroplasty alloplastic TMJ replacement system. J Cranio-Maxillo-Fac Surg. 2014;42:169–174. doi: 10.1016/j.jcms.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Ramos A., Mesnard M. A new condyle implant design concept for an alloplastic TMJ in bone resorption cases. J Cranio-Maxillo-Fac Surg. 2016;44(10):1670–1677. doi: 10.1016/j.jcms.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Bekcioglu B., Bulut E., Bas B. The effects of unilateral alloplastic TMJ replacement on the opposite-side natural joint: a finite-element analysis. J Oral Maxillofac Surg. 2017;75:2316–2322. doi: 10.1016/j.joms.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Wang Y., Mao Y. Biomechanical evaluation of Chinese customized three-dimensionally printed total TMJ prostheses: a finite element analysis. J Cranio-Maxillo-Fac Surg. 2018;46(9):1561–1568. doi: 10.1016/j.jcms.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Keyser B.R., Banda A.K., Mercuri L.G., Warburton G., Sullivan S.M. Alloplastic total temporomandibular joint replacement in skeletally immature patients: a pilot survey. Int J Oral Maxillofac Surg. 2020;49(9):1202–1209. doi: 10.1016/j.ijom.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Humphries L.S., Shakir A., Figueroa A.A. Custom alloplastic temporomandibular joint reconstruction: expanding reconstructive horizons. J Craniofac Surg. 2020;31(6):1651–1658. doi: 10.1097/SCS.0000000000006595. [DOI] [PubMed] [Google Scholar]
- 56.Elledge R., Mercuri L.G., Attard A., Green J., Speculand B. Review of emerging temporomandibular joint total joint replacement systems. Br J Oral Maxillofac Surg. 2019;57:722–728. doi: 10.1016/j.bjoms.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Meurechy N De, Braem A., Mommaerts M.Y. Biomaterials in temporomandibular joint replacement: current status and future perspectives—a narrative review. Int J Oral Maxillofac Surg. 2018;47(4):518–533. doi: 10.1016/j.ijom.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Brizuela A., Herrero-Climent M., Rios-Carrasco E. Influence of the elastic modulus on the osseointegration of dental implants. Materials. 2019;12(6):980. doi: 10.3390/ma12060980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.http://asm.matweb.com/search/SpecificMaterial.asp?bassnum=MTP641.
- 60.http://www.supraalloys.com/titanium-grades.php.
- 61.Han X., Yang D., Yang C. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J Clin Med. 2019;8:240–257. doi: 10.3390/jcm8020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brockett C.L., Carbone S., Fisher J., Jennings L.M. PEEK and CFR-PEEK as alternative bearing materials to UHMWPE in a fixed bearing total knee replacement: an experimental wear study. Wear. 2017;374–375:86–91. doi: 10.1016/j.wear.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercuri L.G. Total joint reconstruction- autologous or alloplastic. Oral Maxillofac Surg Clin. 2006;18:399–410. doi: 10.1016/j.coms.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Kim J.W., Yang B.E., Hong S.J. Bone regeneration capability of 3D printed ceramic scaffolds. Int J Mol Sci. 2020;21(14):4837. doi: 10.3390/ijms21144837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golafshan N., Vorndran E., Zaharievski S. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials. 2020;261:120302. doi: 10.1016/j.biomaterials.2020.120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martelli N., Serrano C., van den Brink H. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery. 2016;159(6):1485–1500. doi: 10.1016/j.surg.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 67.Tack P., Victor J., Gemmel P., Annemans L. 3D printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15(1):115. doi: 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.https://www.sciencedirect.com/topics/materials-science/direct-metal-laser-sintering.
- 69.https://www.element.com/nucleus/2016/06/29/dmls-vs-slm-3d-printing-for-metal-manufacturing.
- 70.https://www.autodesk.com/redshift/5-problems-with-3d-printing-and-how-to-fix-them/.
- 71.https://amfg.ai/2019/10/08/10-of-the-biggest-challenges-in-scaling-additive-manufacturing-for-production-expert-roundup/.
- 72.Abramowicz S., Barbick M., Rose S.P., Dolwick M.F. Adaptability of stock TMJ prosthesis to joints that were previously treated with custom joint prosthesis. Int J Oral Maxillofac Surg. 2012;41:518–520. doi: 10.1016/j.ijom.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Hsu J.T., Huang H.L., Tsai M.T., Fuh L.J., Tu M.G. Effect of screw fixation on temporomandibular joint condylar prosthesis. J Oral Maxillofac Surg. 2011;69:1320–1328. doi: 10.1016/j.joms.2010.05.074. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor M.I., Blau B.E. The economic value of customized versus off-the-shelf knee implants in medicare fee-for-service beneficiaries. Am Health Drug Benefits. 2019;12(2):66–73. [PMC free article] [PubMed] [Google Scholar]
- 75.Yang W.F., Zhang C.Y., Choi W.S. A novel ‘surgeon-dominated’ approach to the design of 3D-printed patient-specific surgical plates in mandibular reconstruction: a proof-of- concept study. Oral Maxillofac Surg. 2020;49(1):13–21. doi: 10.1016/j.ijom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Ballard D.H., Mills P., Duszak R., Jr., Weisman J.A., Rybicki F.J., Woodard P.K. Medical 3D printing cost-savings in orthopedic and maxillofacial surgery: cost analysis of operating room time saved with 3D printed anatomic models and surgical guides. Acad Radiol. 2020;27:1103–1113. doi: 10.1016/j.acra.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanatsios S., Breik O., Dimitroulis G. Biomet stock temporomandibular joint prosthesis: long-term outcomes of the use of titanium condyles secured with four or five condylar fixation screws. J Cranio-Maxillo-Fac Surg. 2018;46(10):1697–1702. doi: 10.1016/j.jcms.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Wolford L.M., Dingwerth D.J., Talwar RM, Pitta MC. Comparison of 2 temporomandibular joint total joint prosthesis systems. J Oral Maxillofac Surg. 2003;61:685–690. J Oral Maxillofac Surg. 2003;61:685–690. doi: 10.1053/joms.2003.50112. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez-Perez L.M., Gonzalez-Perez-Somarriba B., Centeno G., Vallellano C., Montes-Carmona J.F. Evaluation of total alloplastic temporomandibularjoint replacement with two different types of prostheses: a three-year prospective study. Med Oral Patol Oral Cir Bucal. 2016;21:e766–775. doi: 10.4317/medoral.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson N.R., Roberts M.J., Doi S.A., Batstone M.D. Total temporomandibular joint replacement prostheses: a systematic review and bias-adjusted meta-analysis. Int J Oral Maxillofac Surg. 2017;46:86–92. doi: 10.1016/j.ijom.2016.08.022. [DOI] [PubMed] [Google Scholar]