Abstract
Allogeneic transplantation remains the most definitive curative option for patients with acute myeloid leukemia (AML). However, given the median age of diagnosis of AML in the late 60s, patients and clinicians have been reluctant to offer transplant to many in the older population. In this age group, AML presents with higher risk molecular and cytogenetic phenotype and patients’ comorbidities, performance status, frailty and life views all impact the decision-making about whether to proceed with transplantation. Recent analyses suggest promising outcomes and thus acknowledgement of chronological age should be tempered with assessments of performance status, frailty, donor availability and careful balancing of a patient’s wishes, life goals and understanding of the risks before restricting access of older patients to the curative potential of allotransplantation.
Keywords: allogeneic transplant, age, frailty, non-relapse mortality
Decision-making about hematopoietic cell transplantation (HCT) for older patients is challenging. It depends on actual or perceived risks, potential benefits, patient choice and available options for any patient in question. Balancing these hazards requires good communication and data sharing to yield proper decision-making that fits patients’ needs and offers realistic expectations of outcome.
The median age at diagnosis of AML is ~68 years and 75% are diagnosed over the age of 55. Life expectancy for patients in the late 60s is longer than one may anticipate. In the United States, published data on life expectancy for males age 70 is ~15 years and even at age 75 is still 11 years (Social Security Tables, 2020). In contrast, life expectancy for patients with AML at age 75 is only 20% at one year and only 4% at three years (1-13).
Risk factors that directly impact the likelihood of survival after HCT include age, but also the patients’ accumulated comorbidities (14). Physical assessments of frailty are often measured by get up and go, 6 meter walk and measures of balance plus the all-encompassing performance score reflecting ability to perform the activities of daily living without assistance (15). In addition, at all ages, the disease phenotype, molecular and cytogenetic characteristics as well as response to initial therapy determine the suitability to consider allogeneic transplantation (16). To decide about transplantation there also needs to be a healthy donor with suitable histocompatibility and availability in the timeframe needed for the patient care. Finally, a transplant center needs to be willing to consider allografting for patients with any and all of these relevant risk considerations (17-23).
Perhaps most importantly, the patient must be able to understand, weigh the options and accept the risks accompanying allotransplantation in comparison to the alternative treatment or supportive care measures proposed. Potential post-HCT morbidity of GVHD, infections and ongoing complications must be understood (1-3).
Older age interacts with many of the factors influencing outcomes of treatment for leukemia or other hematologic malignancies. Older patients often present with a disease phenotype expressing higher genetic and molecular abnormalities. They may, of course, have defined comorbidities, a greater likelihood of physiologic frailty and may be less willing to except high risk treatments. Additionally, older patients are less likely to have healthy siblings suitable to be donors and thus more often need alternative donor HCT including unrelated donors (URD), umbilical cord blood (UCB) donors or more recently haploidentical, partially matched relatives. Alternative donors often need modified GVHD prophylaxis and older patients frequently require reduced intensity conditioning (RIC) to limit the morbidities and toxicity accompanying HCT. Any pre-existing organ dysfunction may limit their tolerance of intensive therapy. Finally, the higher-risk phenotypes occurring in older patients often require urgent transplantation if the patient achieves an initial remission. Reluctance to consider transplantation right at the time of diagnosis often delays initiation of HLA typing and donor identification. This further compromises the urgency of HCT if the donor search is initiated later after diagnosis or only after remission is achieved.
New options of therapy (molecularly targeted treatment, venetoclax plus hypomethylating agents or others) have been recognized as potentially suitable for older patients (24). Yet recently published guidelines from ASH did not discuss transplantation fitness, but only stated that if patients are not allotransplant candidates, after CR they will need post remission therapy (25).
Outcomes of transplant for patients with AML have been reviewed and summarized. As an example, a meta-analysis published in 2016 reported 13 studies with 749 patients with AML undergoing allotransplants over the age of 60 years and described 35 to 40% relapse free and overall survival at three years (12). Nearly all relapses occurred in the first six months following HCT, yet non-relapse mortality continued to rise to nearly 40% by three years post-HCT.
A recent unpublished analysis of data from the CIBMTR examining all allotransplants in the United States between 2005 and 2019 (n=80,281 cases) showed ~70% one year survival for patients under 40 with only a modest decrement in one year survival; roughly 4% lower 1 year survival per decade of age for those over 40 (Table 1). In contrast, as previously shown (27) autologous transplantation showed no fall off in one year survival for older patients in a similar analysis of over 130,000 patients.
Table 1.
US Allogeneic Transplants by Donor type: 2005-2019: 1 year Survival by Donor Type
| HLA-identical sibling (N = 27406) |
Haploidentical (N = 7624) |
Unrelated donor (N = 39912) |
Cord blood (N = 5339) |
Total (N = 80281) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall survival at 1 year |
N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) |
| Age 20-30 | 2133 | 77.4 (75.8-78.9)% |
710 | 74.8 (72.1-77.3)% |
3056 | 71.3 (70-72.6)% |
547 | 58.9 (55.8-62)% |
6446 | 72.3 (71.4-73.2)% |
| 31-40 | 2161 | 75.9 (74.4-77.5)% |
562 | 71.6 (68.6-74.6)% |
2910 | 69.9 (68.6-71.3)% |
512 | 59.6 (56.4-62.8)% |
6145 | 71 (70.1-72)% |
| 41-50 | 3833 | 70.5 (69.3-71.7)% |
713 | 69.7 (67-72.4)% |
4223 | 66.6 (65.4-67.7)% |
518 | 54.9 (51.858)% |
9287 | 67.6 (66.8-68.3)% |
| 51-60 | 5796 | 66.7 (65.8-67.7)% |
1136 | 65.4 (63.2-67.5)% |
6506 | 62.7 (61.8-63.6)% |
635 | 52 (49.2-54.7)% |
14073 | 63.9 (63.3-64.5)% |
| 61-70 | 3472 | 63.8 (62.6-65.1)% |
1060 | 59.6 (57.4-61.7)% |
6291 | 60.5 (59.6-61.4)% |
444 | 46.1 (43-49.2)% |
11267 | 60.6 (60-61.3)% |
| >=71 | 226 | 57.6 (52.8-62.3)% |
201 | 57.2 (52.3-62)% |
967 | 59.3 (57-61.6)% |
34 | 38.4 (29.2-48.1)% |
1428 | 57.9 (56.1-59.8)% |
Data from CIBMTR (Center for International Blood and Marrow Transplant Research)
In these allogeneic recipients, outcomes in older patients were similar for those receiving matched sibling, haploidentical or matched URD, though a bit worse for the few cord blood recipients. Only a small decline in one year survival was noted for those over 60 or even over 70 in all donor groups, though the cohorts over age 70 are quite small.
A recent focused analysis restricted to patients over 60 with AML in first complete remission analyzed 1330 patients transplanted from 2007 to 2017. Their outcomes (adjusted for cytogenetics, measurable residual disease prior to HCT, donor type and HCT in more recent years) showed no meaningful differences in either 5-year survival (~40%), incidence of relapse (~35%) or treatment related mortality (~25%) comparing those between age 60 to 64, 65 to 69 or over 70 (26).
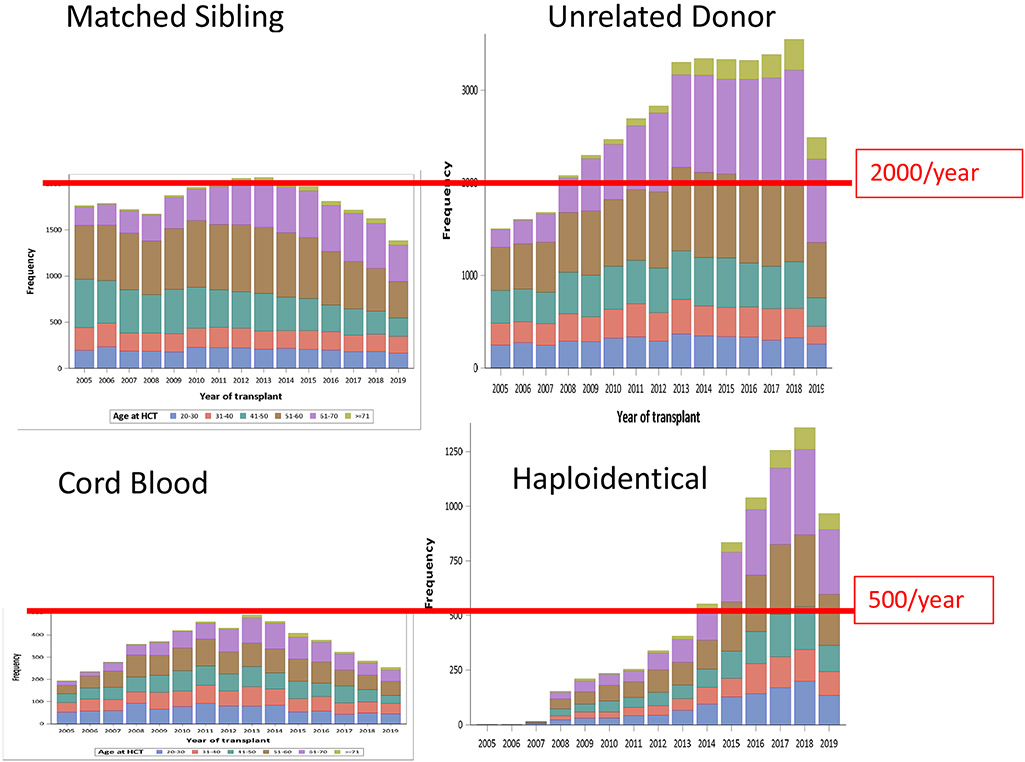
These developing and encouraging data are not fully reflected in national utilization of transplantation for those over age 60 or 70. Data from the CIBMTR examining allografts in the United States between 2005 and 2019 (Figure 1) showed a modest recent fall in transplantation numbers for recipients of sibling grafts age 40 to 60 (from 1000 down to ~700 per year), but a slight recent increase over age 60 (~500 per year since 2013 and >100/year for patients over age 70 groups including those in the 50s 60s and 70s with ~1000/y, 1200/y and 200/y in the 3 age cohorts, respectively. Even in these older age groups, twice as many received URD compared to sibling transplants. The less frequently chosen donor options showed progressively smaller numbers of cord blood grafts (~200/y over age. 50) with a rise in haploidentical HCTs (~750/y over age 50), and ~50/y over age of 70. Though increasing, these numbers are still dwarfed by sibling and particularly URD HCTs done yearly.
Figure 1. Frequency of Allogeneic Transplants 2005-2019 by Donor Type*.
*data from CIBMTR 2005-2019
To conclude, it is necessary to emphasize that age remains important, but it is far from the only barrier to successful HCT for older patients. Comorbidities, performance status and frailty are all measures of recipient fitness for transplantation that should be directly considered in treatment decision-making. In addition, the higher risk molecular and cytogenetic phenotypes occurring in older patients need direct consideration. In general, for older recipients HCT only in early disease status can yield meaningful benefit. HCT during later remission yields more toxicity as well as greater risks of relapse. Patient goals and willingness to except risk and contend with ongoing post-transplant morbidity need clear discussion and assured understanding. This may be, perhaps, even more important for the older recipient population. Therefore, while observational data provides evidence of increasing success for transplantation, even beyond the age of 70, selecting HCT as the best option requires caution, care and collaborative wisdom between recipients and their transplant team.
Footnotes
The author receives research support from Incyte and from FATE Therapeutics, both unrelated to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152(5):524–542. [DOI] [PubMed] [Google Scholar]
- 2.Deeg HJ, Steuten LM. Therapy for hematologic cancers in older patients, quality of life, and health economics: difficult decisions. JAMA Oncol. 2015;1:571. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun C, Le-Rademacher J, Wang H-L, Othus M, Sun Z, Major B, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60–75 years in first complete remission (CR1): an Alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33:2599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juliusson G, Hagberg O, Lazarevic VL, Ölander E, Antunovic P, Cammenga J, et al. Improved survival of men 50 to 75 years old with acute myeloid leukemia over a 20-year period. Blood. 2019; 134:1558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. JCO. 2013;31:2662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutra Del Galy A, Marouf A, Raffoux E, Robin M, Michonneau D, Sebert M, et al. Allogeneic hematopoietic stem cell transplantation in elderly patients with acute myeloid leukemia or myelodysplastic syndromes: myth and reality. Leukemia 10.1038/s41375-020-1004-9 [DOI] [PubMed] [Google Scholar]
- 10.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010: 28:1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcomes of older patients with Acute Myeloid Leukemia. Cancer 2013: 119: 2720–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016;22(4):651–657. doi: 10.1016/j.bbmt.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClune BL, Ahn KW, Wang HL, Antin JH, Artz AS, Cahn JY, et al. Allotransplantation for patients age ≥40 years with non-Hodgkin lymphoma: encouraging progression-free survival. Biol Blood Marrow Transplant. 2014: 960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, et al. Development and Validation of a Novel Acute Myeloid Leukemia–Composite Model to Estimate Risks of Mortality. JAMA Oncol. 2017;3(12):1675–1682. doi: 10.1001/jamaoncol.2017.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polverelli N, Tura P, Battipaglia G, Malagola M, Bernardi S, Gandolfi L, et al. Multidimensional geriatric assessment for elderly hematological patients (≥60 years) submitted to allogeneic stem cell transplantation. A French–Italian 10-year experience on 228 patients. Bone Marrow Transplant. Published online May 12, 2020:1–10. doi: 10.1038/s41409-020-0934-1 [DOI] [PubMed] [Google Scholar]
- 16.He F, Cao Q, Lazaryan A, Brunstein C, Holtan S, Warlick E, et al. Allogeneic Hematopoietic Cell Transplantation for Older Patients: Prognosis Determined by Disease Risk Index. Biol Blood Marrow Transplant. 2017: 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ditschkowski M, Elmaagacli AH, Trenschel R, Steckel NK, Koldehoff M, Beelen DW. Myeloablative allogeneic hematopoietic stem cell transplantation in elderly patients. Clin Transplant. 2006;20(1):127–131. [DOI] [PubMed] [Google Scholar]
- 18.Finke J, Nagler A. Viewpoint: What is the role of allogeneic haematopoietic cell transplantation in the era of reduced-intensity conditioning – is there still an upper age limit? A focus on myeloid neoplasia. Leukemia. 2007;21(7):1357–1362. [DOI] [PubMed] [Google Scholar]
- 19.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. 2007;109(4):1395–1400. [DOI] [PubMed] [Google Scholar]
- 20.Flannelly C, Tan BE-X, Tan JL, McHugh CM, Sanapala C, Lagu T, et al. Barriers to Hematopoietic Cell Transplantation for Adults in the United States: A Systematic Review with a Focus on Age. Biol Blood Marrow Transplant. Biol Blood Marrow Transplant. 2020. September 20;S1083-8791(20)30585–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt VR, Chen B, Lee SJ. Use of hematopoietic cell transplantation in younger patients with acute myeloid leukemia: A National Cancer Database Study. Bone Marrow Transplant. 2018;53:873–879. [DOI] [PubMed] [Google Scholar]
- 22.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative Allogeneic Hematopoietic Cell Transplantation in Adults 60 Years of Age and Older. J Clin Oncol. 2005;23(15):3439–3446. [DOI] [PubMed] [Google Scholar]
- 23.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. 2007;109:1395–400. [DOI] [PubMed] [Google Scholar]
- 24.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020. 4:3528–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maakaron JE, Chen K, Zhang Mei-Jie, Hourigan CS, Litzow M, Saber W, et al. Impact of Age on the Outcomes of HCT for AML in CR1: Promising Therapy for Older Adults, Blood ASH, 2020. [Google Scholar]
- 27.Munshi PN, Vesole D, Jurczyszyn A, Zaucha JM, St. Martin A, Davila O, et al. Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma. Cancer. 2020. PMID 32965680 [DOI] [PMC free article] [PubMed] [Google Scholar]