Abstract
The elderly population is rapidly increasing; hence, the disability due to age-related diseases has become an important socioeconomic burden. Amongst age-related diseases cardiovascular ones (CVD) have a huge impact on morbidity and mortality and are associated with metabolic syndrome (MetS). Several studies investigated the role of hypovitaminosis D in the pathogenesis of MetS and of CVD, this review unravels the relationship between aging/senescence, vitamin D, gender, and pathogenesis of MetS.
Keywords: Aging, Gender identity, Metabolic syndrome, Vitamin D
INTRODUCTION
The mean age of the population is constantly increasing, in 2050 1 person in 3 will be over 65 and 1 person in 10 will be over 80. Despite the increase in life expectancy, there is no corresponding increase in healthy life expectancy; in 2015, it has been shown that, despite a life expectancy at the age of 65 of 21.2 years for women and 17.9 years for men, only 9.4 years will be healthy years. The discrepancy between increasing life expectancy and life in good health will be one of the major challenge for the health authorities in the near future. Non-communicable chronic diseases (NCDs) are widely diffused, increases with age and have a huge impact on morbidity and mortality, amongst the risk factors for the development of NCDs as cardiovascular diseases (CVD) and type 2 diabetes mellitus the metabolic syndrome (MetS) and vitamin D deficiency may be linked.
Geriatricians and gerontologists differentiate between the terms “aging” and “senescence” that are frequently used as synonyms by the laypersons. Here I'll refer to the term “aging” as to the physiological changes that occur in the organisms at all levels as age increases, without making reference to death and pathological decline. Otherwise, I'll use the term “senescence” to indicate pathological processes associated with aging and ending with organ failure and death [1].
This review focus on the relationship between aging/senescence, vitamin D deficiency, gender, and pathogenesis of MetS.
METABOLIC SYNDROME
The term MetS refers to a cluster of risk factors for CVD and type 2 diabetes mellitus, it has been defined with different criteria, see [2] for a complete review. The International Diabetes Federation, the American Heart Association, and the National Heart, Lung, and Blood Institute defined MetS as the presence of at least three of the following criteria: central obesity, dyslipidemia, impaired glucose metabolism, elevated blood pressure, and low levels of high-density lipoprotein cholesterol [3].
Although there are no global data on MetS prevalence, it has certainly increased over the past several decades worldwide, and it has been estimated that, since MetS is about three times more common than diabetes, the global prevalence should involve over a billion subjects in the world [2]; these impressive numbers allow to define MetS as a “global epidemic” [2]. The prevalence of MetS is generally higher in men than in women [2,4] except in the Middle Eastern countries, were the prevalence is higher in women [5] and, in all the countries, it increases with age.
The prevalence of different characteristics belonging to MetS as overweight, obesity, hypertension, hyperlipidemia, and diabetes increases with age, however the mechanisms linking aging to MetS are fare to be completely elucidated.
The observation of high inter-individual variabilities in metabolic dysregulation in older adults suggest that biological age, rather than chronological age, may be implicated in the pathogenesis of MetS. Studies on different mechanisms of aging suggest an association between high metabolic risk profiles and accelerated senescence.
As it is known, several markers have been proposed in order to measure biological rather than chronological aging [6], unless we are far from having specific and reliable biomarkers of biological aging, several molecules and pathways have been described as possible biomarkers of senescence. Amongst these different biomarkers some have been related to metabolic deregulation and associated to high metabolic risk profiles as telomeres length [7,8], DNA epigenetic modifications [9], and mitochondrial dysfunction [10].
A relationship between aging/senescence, MetS, and vitamin D deficiency may be found in the mechanisms linking these three domains.
SENESCENCE AND METABOLIC SYNDROME
Telomeres protect the chromosomes' integrity and their length becomes shorter with aging due to a decrease in telomerase activity. Cellular replication become impossible under a certain measure of telomere length, this phenomenon is named Hayflick limit and defines cellular senescence and, consequently, biological rather than chronological age. Hence telomere shortening, in particular in leukocytes, has been considered as a marker of cellular senescence and has been associated to several aging-related diseases [11] and with increased metabolic risk and MetS [7,12,13].
Besides telomeres length, epigenetics DNA modification as methylation have been regarded as markers of cellular senescence, aging, and aging-related diseases. The presence of multiple epigenetic changes and in particular of multiple DNA methylation sites on a set of CpG dinucleotides has been defined as “DNA methylation clock” that is an accurate predictor of age, nevertheless different “epigenetic clock” showed different association with senescence and frailty [14].
Mitochondrial dysfunction and oxidative stress have been studied as biomarkers of biological aging and senescence. Aging is associated with a decreased mitochondrial function, number and replication and with an increased oxidative stress, when this phenomenon reached a not yet defined threshold, aging becomes senescence and a progressive decline in different organs begins [15].
Inflammation links aging, MetS and cellular senescence; inflammation increases with aging due to a progressive deregulation of immune function and to the accumulation of senescent cells, these phenomena bring to a chronic, low-grade inflammatory status that progressively contribute to senescence [16]. Immune cells in the inflammatory infiltrates influences metabolism and vice versa, inflammation is influenced by metabolic rate and nutrients availability. As regards MetS, obesity have been associated to a chronic increase in inflammatory status, this further contribute to the deregulation of metabolism [17]. In the same way the telomeres length and the epigenetic clocks are influenced by inflammation, increased oxidative stress [18] and by lifestyle and environmental factors [19,20,21].
The above mentioned biomarkers of cellular senescence and biological aging have been associated to increased metabolic risk profiles and different characteristics of MetS [7,9,12,22,23]. Interestingly a recent experimental study on mice model suggest that amelioration of the mitochondria metabolic profile reduces certain characteristics of MetS as obesity and type 2 diabetes [24].
The complex and bi-directional relationships between the different factors promoting cellular senescence and metabolic dysfunction complicates the understanding of the role of each factor in the development of MetS in aging persons.
Moreover, environmental factors as air pollution [19,20,21], physical activity [25,26], and diet [27,28] greatly influences senescence biomarkers and MetS development.
Several evidences suggested the hypothesis that vitamin D may act as a modulator of different aspects of cellular senescence and metabolic deregulation.
VITAMIN D AND AGING
Vitamin D may be considered a nutrient as it is introduced trough diet, but also as a hormone as it can be synthetized by the skin and, after two hydroxylation in the kidney and in the liver acquires the ability to regulate calcium and phosphate metabolism. About 20% of vitamin D comes from dietary intake, whereas 80% is synthetized by the skin from its precursors 7-dihydrocholesterol thanks to the action of UVB. Despite the ability of the body to actively synthetize vitamin D, hypovitaminosis D is largely prevalent amongst general population and this prevalence increases according with age.
The prevalence of hypovitaminosis depends on the cut-offs used in order to define it; different scientific societies and different countries suggested different threshold for hypovitaminosis D. The majority of the studies agree in defining desirable levels higher than 30 ng/mL or 75 nM/L of blood 25(OH) vitamin D (25(OH)D), under this level the risk of bone metabolism alteration, falls, and myopathy increases, see [29] for a complete review.
The prevalence of hypovitaminosis D augments with aging as elderly subjects are at higher risk for several reasons, as reduced sunlight exposure, reduced intake of foods rich in vitamin D as dairy products due to lactase deficiency, reduction of skin synthesis and reduced gut absorption [30].
AGING, SENESCENCE AND HYPOVITAMINOSIS D: WHAT ARE THE RELATIONSHIPS?
Hypovitaminosis D has been associated with increased mortality for different causes [31], hence it has been suggested that lower levels of 25(OH)D may be regarded as a marker of aging, however the relationship between vitamin D status and other markers of aging are far from being elucidated.
As regards telomeres length the results of different studies are controversial as some papers suggested that 25(OH)D levels are not correlated and do not influence telomeres length [32,33,34,35], whereas others suggest a positive correlation [14,35,36,37,38,39] showing that higher levels of 25(OH)D are associated with longer telomeres and, hence, with lower biological age. Furthermore it has been suggested that the effect of vitamin D on telomeres length may be genetically determined [40] and may start in early life [41] depending also on the maternal vitamin D status.
Association between 25(OH)D and epigenetic modification has been explored with controversial results, a recent cross-sectional study on a large cohort suggest that both epigenetic clock and telomeres length are associated with vitamin D status, however the authors did not observed any clinical correlation with frailty [14]. On the other hand, a large cohort study suggested that vitamin D is a markers of aging and specifically of senescence per se, regardless any correlation with epigenetic clock [32]. A small intervention trial on obese Africans Americans with vitamin D insufficiency shows different effects of vitamin D supplementation on epigenetic clock; the authors observed a slowdown of aging measured by the Horvath, but not by the Hannum epigenetic clock [42]. Controversial results may be due to several techniques used to measure different “clocks” analyzed in various studies.
Besides these effects, vitamin D has some antioxidant effects [43], whereas hypovitaminosis D is associated with a pro-oxidative state due to the decrease in intracellular glutathione [44].
The presence of vitamin D receptor (VDR) in mitochondria from platelets and megakaryocytes and its relation with different diseases have been demonstrated by my lab [45]; however, VDR function within the organelles remains unclear. Studies on the role of vitamin D on mitochondria function are contradictive, the results are particularly different depending on the tissue analyzed, see [46] for a complete review. Interesting studies show that the active form of vitamin D, calcitriol, enhance mitochondrial function in animal, and in vitro models [47,48]. Moreover, the administration of paricalcitol or of calcipotriol, analogous of vitamin D, in animals and in in vitro models have protective effect on mitochondrial function [49,50]. The protective effect have been shown in different cells and organs as kidney, melanocytes, endothelial cells, hepatocytes, astrocytes, and neurons, however there are not human studies confirming these effects.
The antioxidant and anti-inflammatory effects of vitamin D [29] may explain its relation with aging and senescence markers, thus it is not clear which come first, the chicken or the egg?
VITAMIN D DEFICIENCY AND METABOLIC SYNDROME
Vitamin D has been implicated in the regulation of several pathways, besides its well-known role as regulator of the calcium-phosphate metabolism, it has been suggested that it may be implicated in immune system modulation [29], in the regulation of muscle strength and metabolism and in the cognitive decline [51]. Moreover, hypovitaminosis D has been considered risk factors for CVD [52]. Both cross-sectional [53,54,55] and longitudinal studies [56] suggested a role for hypovitaminosis D in predicting the development of cardio metabolic risk factors as MetS and diabetes.
Despite these studies, the causal direct role of vitamin D in the development of MetS and CVD has not been clearly demonstrated in humans, in fact confounding factors as obesity [53] and dietary intake [55] have been evoked to explicate this association. Moreover, recently a Mendelian randomization study performed on a cohort of more than 33,000 subjects does not confirm the association between 25(OH)D levels and CVD [57]. As regards intervention studies, recent clinical trials did not demonstrate any positive effect of vitamin D supplementation on cardiovascular health [58,59,60].
Taking into accounts different features of MetS, low levels of vitamin D have been associated with obesity, impaired glucose metabolism, and elevated blood pressure.
As regards obesity a recent meta-analysis showed that low 25(OH)D levels are associated with increased body mass index in both diabetic and non-diabetic subjects [61], interestingly hypovitaminosis D is associated especially with visceral fat accumulation and android obesity [62,63]. The android obesity has also been defined as “metabolically unhealthy obesity” as respect to “metabolically healthy obesity”. Subjects with unhealthy obesity are at higher risk for CVD and are characterized by higher liver and visceral fat, but lower subcutaneous fat, lower cardiorespiratory capacity, higher insulin sensitivity, and higher grade of inflammation [64].
Hypovitaminosis D have been associated to an impaired glucose metabolism and with the development of diabetes in some cross-sectional and prospective studies [65,66]. A biological role of vitamin D in maintaining pancreatic β-cells function has been postulated and related to its antioxidant and anti-inflammatory effects [67]. The postulated role of vitamin D in the homeostasis of the epigenome may further explain is protective effect on diabetes onset as diabetes-related genes are inactivated by hypermethylation [68].
As regards hypertension a specific association with sunlight exposure and vitamin D status has been suggested [69,70]. The mechanism evoked in order to explain this association is the role of vitamin D in the regulation of endothelial cells. In in vitro and in vivo models Vitamin D exerts protective effects on endothelial cell reducing apoptosis and autophagy, through its antioxidant effect [71,72,73]. Despites these experimental evidences and the association between low level of 25(OH)D and hypertension showed by observational studies, some studies are discordant and obtained opposite results [74,75]. Interventional trials reported controversial results, some studies suggested the efficacy of vitamin D supplementation in reducing blood pressures [76,77], however others did not [78,79,80].
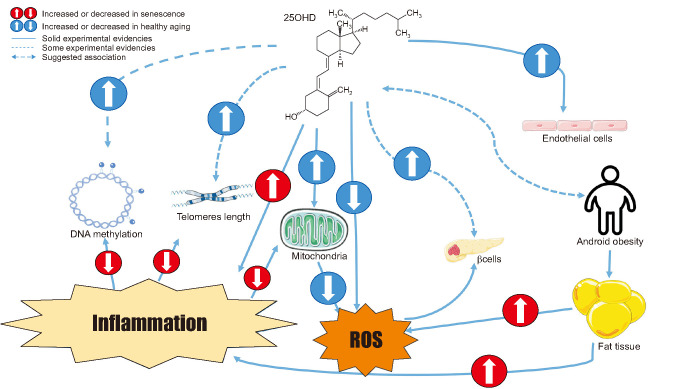
Pathways linking hypovitaminosis D, senescence, and MetS are summarized in Fig. 1.
Fig. 1. The cartoon summarizes the relationship between vitamin D markers of senescence and features of metabolic syndrome. ROS: reactive oxygen species.
ROLE OF LIFESTYLE AND ENVIRONMENT
Lifestyle and environment further complicate the study of the relationship between hypovitaminosis D, aging/senescence, and development of MeTS. As it is known, a healthy diet and a good level of physical activity are associated with higher levels of 25(OH)D [55], with lower incidence of MeTS and CVD [55,81], and with an healthy aging.
Furthermore, biological pathways leading to senescence are influenced by physical activity [25,26,82] and nutritional intake [27,28,51]. Also air pollution has been associated with accelerated senescence, lower 25(OH)D levels, and increased risk of MeTS [19,83].
Recently we demonstrated that mitochondria bioenergetics can be improved by supplementation with essentials aminoacids this improvement leads to reduction of oxidative stress, increased muscle performance, and improvement of cognitive performance [84].
The complex influence of lifestyle and environment in the pathogenesis of MeTS, its relationships with hypovitaminosis D and senescence add complexity in the unravelling of the role of hypovitaminosis D as risk factor for MeTS.
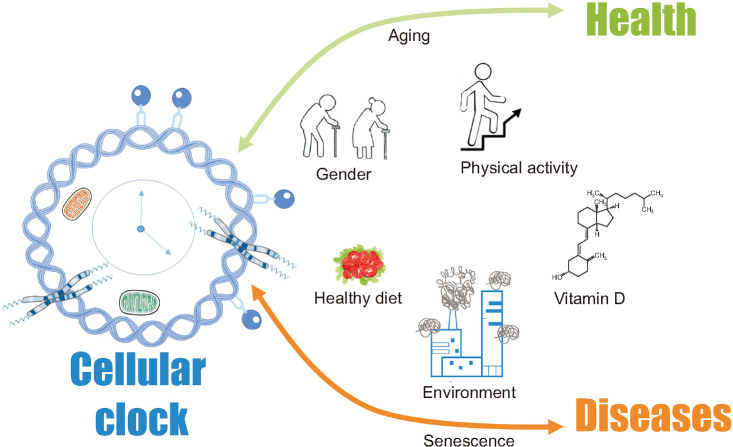
Relationships between aging/senescence and lifestyle/environment are summarized in Fig. 2.
Fig. 2. The cartoon summarizes the relationship between lifestyle, environment, senescence, and aging.
IS THERE A ROLE FOR GENDER?
It is known that MetS has a higher prevalence in men than in women [2,4], however this gender-related difference may vary according with countries; as in the north Africa is has been reported that MetS has a higher prevalence in women [5]. Moreover, MetS is globally increasing regardless to gender [2]. Nevertheless some interesting gender differences have been reported on the effect of MetS on vascular aging, and in particular on arterial stiffness; Kruszyńska et al [85] recently reported an accelerated vascular aging in women affected by MetS as compared to men in the middle-aged population. This difference may be due to the drop of estrogen levels at the onset of menopause, as estrogens modulate arterial stiffness over the lifespan [86]
According with the above-described evidences one may hypothesize that the difference in the prevalence of MetS in men and women may be explained with the different prevalence of hypovitaminosis D or with different biological aging rate according with genders.
The majority of studies dealing with hypovitaminosis D have been performed in postmenopausal women; this is mainly due to the historical role of vitamin D in the control of bone turnover and to the higher incidence of osteoporosis in women. A systematic review on the prevalence of hypovitaminosis D involving more than 168,000 participants does not find any gender related differences in the prevalence of hypovitaminosis D, however, amongst the studies included in the review, only 10 were focused on male and only 3,143 participants were men [87]. Subsequent studies in Chile [88] and in Saudi Arabia [89] showed that 25(OH)D levels were lower in men than in women, even after correction for lifestyle factors. Hence, we have no conclusive data on a possible difference in vitamin D levels across genders and consequently it is not possible to hypothesize a role for hypovitaminosis D in explaining different gender prevalence of MetS.
As regards biological aging and its different markers, a gender difference have been observed as regards telomeres length, oxidative stress [90], and inflammation [91] that are generally lower in women than in men. These differences have been mainly attributed to the action of estrogens that reduce reactive oxygen species and telomerase attrition through multiple mechanisms. Some studies suggested that estrogens are potent antioxidants and simulates antioxidant genes [92], affects DNA repair [93] and stimulate telomerase activity [94]. On the contrary, testosterone has been associated to increased oxidative stress in animal models, this may contribute to telomeres shortening [95].
The mechanisms underlying the difference in telomeres length in men and women are not completely clear. In fact, even after menopause, when the levels of estrogens drop, women had lower telomeres length as compared to age-matched men [96]. Solid data obtained by a meta-analyses on more than 36,000 individuals confirm the association between senescence markers and gender, showing a generally lower biological age in women [97]. In addition, a study on heterozygotes twins confirmed that leucocytes telomeres length is higher in female than in male twin [98].
As regards the role of chronic low-grade inflammation, the different fat distribution in men and women may play an important role, as it is known that men have a predominant “unhealthy obesity” with increased visceral fat as respect to women, this is associated with increased inflammation [64]. Obese men exposed to a high-fat meal produced elevated levels of inflammatory cytokines [99], moreover experimental mice models showed that an high-fat diet induced more inflammation in males than in females, this phenomenon is only partially reduced by ovariectomy, showing that estrogens explains only partially this difference [100]. Furthermore, a sex-difference in immune response showing a higher propensity of immune cells from male to produce inflammatory cytokines has been described, interestingly this different answer to immune stimuli is not totally explained by sex hormones, see for a complete review [91].
According to this data is possible to hypothesize a role for senescence rate in explaining different gender prevalence of MetS, however, there are no direct evidences of an association between senescence and gender in MetS.
CONCLUSION
Vitamin D deficiency is highly prevalent, particularly amongst older person and hypovitaminosis D may accelerate senescence. Despite some evidences linking hypovitaminosis D and MetS, observational studies cannot prove causality and there are not convincing data from intervention studies showing than the administration of vitamin D in different forms is effective in reducing MetS and CVD.
Aging and, in particular, senescence is associated with an increased risk of MetS.
Gender differences in the biological mechanisms leading to senescence have been described and these differences may influence different prevalence of MetS according to gender.
Multiple and bi-directional relationships between hypovitaminosis D, aging/senescence, MetS, lifestyle, and environment complicate the study of this interesting topic, greatly increasing the risk of biases, further intervention studies taking into account these multiple confounding factors are needed in order to clarify this topic.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. 2018;320:1319–1320. doi: 10.1001/jama.2018.12440. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia X, Chen W, McDermott J, Han JJ. Molecular and phenotypic biomarkers of aging. F1000Res. 2017;6:860. doi: 10.12688/f1000research.10692.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Révész D, Milaneschi Y, Verhoeven JE, Penninx BW. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J Clin Endocrinol Metab. 2014;99:4607–4615. doi: 10.1210/jc.2014-1851. [DOI] [PubMed] [Google Scholar]
- 8.Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, et al. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity (Silver Spring) 2015;23:2165–2174. doi: 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- 9.Nannini DR, Joyce BT, Zheng Y, Gao T, Liu L, Yoon G, et al. Epigenetic age acceleration and metabolic syndrome in the coronary artery risk development in young adults study. Clin Epigenetics. 2019;11:160. doi: 10.1186/s13148-019-0767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-García FJ, Monistrol-Mula A, Cardellach F, Garrabou G. Nutrition, bioenergetics, and metabolic syndrome. Nutrients. 2020;12:2785. doi: 10.3390/nu12092785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee S. Telomeres in health and disease. J Oral Maxillofac Pathol. 2017;21:87–91. doi: 10.4103/jomfp.JOMFP_39_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M, Martin H, Firpo MA, Demerath EW. Inverse association between adiposity and telomere length: the Fels longitudinal study. Am J Hum Biol. 2011;23:100–106. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonfigli AR, Spazzafumo L, Prattichizzo F, Bonafè M, Mensà E, Micolucci L, et al. Leukocyte telomere length and mortality risk in patients with type 2 diabetes. Oncotarget. 2016;7:50835–50844. doi: 10.18632/oncotarget.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetter VM, Spira D, Banszerus VL, Demuth I. Epigenetic clock and leukocyte telomere length are associated with vitamin D status but not with functional assessments and frailty in the Berlin aging study II. J Gerontol A Biol Sci Med Sci. 2020;75:2056–2063. doi: 10.1093/gerona/glaa101. [DOI] [PubMed] [Google Scholar]
- 15.Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Zhao J, Meng H, Zhang X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front Immunol. 2019;10:1173. doi: 10.3389/fimmu.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 19.Niehoff NM, Gammon MD, Keil AP, Nichols HB, Engel LS, Taylor JA, et al. Hazardous air pollutants and telomere length in the Sister study. Environ Epidemiol. 2019;3:e053. doi: 10.1097/EE9.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med. 2013;70:743–749. doi: 10.1136/oemed-2012-101350. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CA, Tan Q, Munoz E, Jylhävä J, Hjelmborg J, Christiansen L, et al. A decade of epigenetic change in aging twins: genetic and environmental contributions to longitudinal DNA methylation. Aging Cell. 2020;19:e13197. doi: 10.1111/acel.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panov AV, Dikalov SI. Cardiolipin, perhydroxyl radicals, and lipid peroxidation in mitochondrial dysfunctions and aging. Oxid Med Cell Longev. 2020;2020:1323028. doi: 10.1155/2020/1323028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves-Figueiredo H, Silva-Platas C, Lozano O, Vázquez-Garza E, Guerrero-Beltrán CE, Zarain-Herzberg A, et al. A systematic review of post-translational modifications in the mitochondrial permeability transition pore complex associated with cardiac diseases. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165992. doi: 10.1016/j.bbadis.2020.165992. [DOI] [PubMed] [Google Scholar]
- 24.Tavallaie M, Voshtani R, Deng X, Qiao Y, Jiang F, Collman JP, et al. Moderation of mitochondrial respiration mitigates metabolic syndrome of aging. Proc Natl Acad Sci U S A. 2020;117:9840–9850. doi: 10.1073/pnas.1917948117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, et al. Lifelong voluntary aerobic exercise prevents age- and Western diet-induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol. 2020 doi: 10.1113/JP280607. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shadyab AH, LaMonte MJ, Kooperberg C, Reiner AP, Carty CL, Manini TM, et al. Leisure-time physical activity and leukocyte telomere length among older women. Exp Gerontol. 2017;95:141–147. doi: 10.1016/j.exger.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciarretta S, Forte M, Castoldi F, Frati G, Versaci F, Sadoshima J, et al. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa297. [Epub] [DOI] [PubMed] [Google Scholar]
- 28.Ruocco C, Segala A, Valerio A, Nisoli E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr Opin Clin Nutr Metab Care. 2021;24:88–95. doi: 10.1097/MCO.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 29.Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettger SF, Angersbach B, Klimek CN, Wanderley ALM, Shaibekov A, Sieske L, et al. Prevalence and predictors of vitamin D-deficiency in frail older hospitalized patients. BMC Geriatr. 2018;18:219. doi: 10.1186/s12877-018-0919-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot Ld, et al. Consortium on Health and Ageing: Network of Cohorts in Europe and the United States. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schöttker B, Hagen L, Zhang Y, Gào X, Holleczek B, Gao X, et al. Serum 25-hydroxyvitamin D levels as an aging marker: strong associations with age and all-cause mortality independent from telomere length, epigenetic age acceleration, and 8-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2019;74:121–128. doi: 10.1093/gerona/gly253. [DOI] [PubMed] [Google Scholar]
- 33.Mazidi M, Mikhailidis DP, Banach M, Dehghan A. Impact of serum 25-hydroxyvitamin D 25(OH) on telomere attrition: a Mendelian randomization study. Clin Nutr. 2020;39:2730–2733. doi: 10.1016/j.clnu.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Williams DM, Palaniswamy S, Sebert S, Buxton JL, Blakemore AI, Hyppönen E, et al. 25-Hydroxyvitamin D concentration and leukocyte telomere length in young adults: findings from the Northern Finland birth cohort 1966. Am J Epidemiol. 2016;183:191–198. doi: 10.1093/aje/kwv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julin B, Shui IM, Prescott J, Giovannucci EL, De Vivo I. Plasma vitamin D biomarkers and leukocyte telomere length in men. Eur J Nutr. 2017;56:501–508. doi: 10.1007/s00394-015-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazidi M, Michos ED, Banach M. The association of telomere length and serum 25-hydroxyvitamin D levels in US adults: the National Health and Nutrition Examination Survey. Arch Med Sci. 2017;13:61–65. doi: 10.5114/aoms.2017.64714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beilfuss J, Camargo CA, Jr, Kamycheva E. Serum 25-hydroxyvitamin D has a modest positive association with leukocyte telomere length in middle-aged US adults. J Nutr. 2017;147:514–520. doi: 10.3945/jn.116.244137. [DOI] [PubMed] [Google Scholar]
- 38.Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JJ, Cahoon EK, Linet MS, Little MP, Dagnall CL, Higson H, et al. Relationship between plasma 25-hydroxyvitamin D and leucocyte telomere length by sex and race in a US study. Br J Nutr. 2016;116:953–960. doi: 10.1017/S0007114516002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Normando P, Santos-Rebouças C, Leung C, Epel E, da Fonseca AC, Zembrzuski V, et al. Variants in gene encoding for vitamin D binding protein were associated with leukocyte telomere length: the Pró-Saúde study. Nutrition. 2020;71:110618. doi: 10.1016/j.nut.2019.110618. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Kim GJ, Lee D, Ko JH, Lim I, Bang H, et al. Higher maternal vitamin D concentrations are associated with longer leukocyte telomeres in newborns. Matern Child Nutr. 2018;14:e12475. doi: 10.1111/mcn.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2019;74:91–98. doi: 10.1093/gerona/gly223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin AM, Chen KB, Chao PL. Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Ann N Y Acad Sci. 2005;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- 44.Krone B, Grange JM. Paradigms in multiple sclerosis: time for a change, time for a unifying concept. Inflammopharmacology. 2011;19:187–195. doi: 10.1007/s10787-011-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Amelio P, Cristofaro MA, De Vivo E, Ravazzoli M, Grosso E, Di Bella S, et al. Platelet vitamin D receptor is reduced in osteoporotic patients. Panminerva Med. 2012;54:225–231. [PubMed] [Google Scholar]
- 46.Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020;199:105595. doi: 10.1016/j.jsbmb.2020.105595. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Zhu Y, Wang X, Yang Y, Cheng S. Cardioprotective effect of calcitriol on myocardial injury induced by isoproterenol in rats. J Cardiovasc Pharmacol Ther. 2013;18:386–391. doi: 10.1177/1074248413482754. [DOI] [PubMed] [Google Scholar]
- 48.Longoni A, Kolling J, dos Santos TM, dos Santos JP, da Silva JS, Pettenuzzo L, et al. 1,25-Dihydroxyvitamin D3 exerts neuroprotective effects in an ex vivo model of mild hyperhomocysteinemia. Int J Dev Neurosci. 2016;48:71–79. doi: 10.1016/j.ijdevneu.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 49.García IM, Altamirano L, Mazzei L, Fornés M, Cuello-Carrión FD, Ferder L, et al. Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones. 2014;19:479–491. doi: 10.1007/s12192-013-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Q, Li X, Sun J, Ding G, Zhou M, Zhao W, et al. The effects of calcipotriol on the dendritic morphology of human melanocytes under oxidative stress and a possible mechanism: is it a mitochondrial protector? J Dermatol Sci. 2015;77:117–124. doi: 10.1016/j.jdermsci.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 51.D'Amelio P, Quacquarelli L. Hypovitaminosis D and aging: is there a role in muscle and brain health? Nutrients. 2020;12:628. doi: 10.3390/nu12030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latic N, Erben RG. Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int J Mol Sci. 2020;21:6483. doi: 10.3390/ijms21186483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mousa A, Naderpoor N, de Courten MPJ, Scragg R, de Courten B. 25-hydroxyvitamin D is associated with adiposity and cardiometabolic risk factors in a predominantly vitamin D-deficient and overweight/obese but otherwise healthy cohort. J Steroid Biochem Mol Biol. 2017;173:258–264. doi: 10.1016/j.jsbmb.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Srimani S, Saha I, Chaudhuri D. Prevalence and association of metabolic syndrome and vitamin D deficiency among postmenopausal women in a rural block of West Bengal, India. PLoS One. 2017;12:e0188331. doi: 10.1371/journal.pone.0188331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chun H, Kim GD, Doo M. Differences in the association among the vitamin D concentration, dietary macronutrient consumption, and metabolic syndrome depending on pre- and postmenopausal status in Korean women: a cross-sectional study. Diabetes Metab Syndr Obes. 2020;13:3601–3609. doi: 10.2147/DMSO.S275847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pham TM, Ekwaru JP, Setayeshgar S, Veugelers PJ. The effect of changing serum 25-hydroxyvitamin D concentrations on metabolic syndrome: a longitudinal analysis of participants of a preventive health program. Nutrients. 2015;7:7271–7284. doi: 10.3390/nu7095338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet. 2016;9:349–356. doi: 10.1161/CIRCGENETICS.116.001396. [DOI] [PubMed] [Google Scholar]
- 58.Djoussé L, Cook NR, Kim E, Bodar V, Walter J, Bubes V, et al. VITAL Research Group. Supplementation with vitamin D and omega-3 fatty acids and incidence of heart failure hospitalization: VITAL-heart failure. Circulation. 2020;141:784–786. doi: 10.1161/CIRCULATIONAHA.119.044645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rafiq S, Jeppesen PB. Body mass index, vitamin D, and type 2 diabetes: a systematic review and meta-analysis. Nutrients. 2018;10:1182. doi: 10.3390/nu10091182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Fan D, Yin F. The relationship between vitamin D status and visceral fat accumulation in males with type 2 diabetes. J Nutr Sci Vitaminol (Tokyo) 2020;66:396–401. doi: 10.3177/jnsv.66.396. [DOI] [PubMed] [Google Scholar]
- 63.Lampignano L, Zupo R, Donghia R, Guerra V, Castellana F, Murro I, et al. Cross-sectional relationship among different anthropometric parameters and cardio-metabolic risk factors in a cohort of patients with overweight or obesity. PLoS One. 2020;15:e0241841. doi: 10.1371/journal.pone.0241841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41:405–420. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kositsawat J, Freeman VL, Gerber BS, Geraci S. Association of A1C levels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care. 2010;33:1236–1238. doi: 10.2337/dc09-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Berridge MJ. Vitamin D deficiency and diabetes. Biochem J. 2017;474:1321–1332. doi: 10.1042/BCJ20170042. [DOI] [PubMed] [Google Scholar]
- 69.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 70.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 71.Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99:1367–1374. doi: 10.1210/jc.2013-2103. [DOI] [PubMed] [Google Scholar]
- 72.Sturza A, Văduva A, Uțu D, Rațiu C, Pop N, Duicu O, et al. Vitamin D improves vascular function and decreases monoamine oxidase A expression in experimental diabetes. Mol Cell Biochem. 2019;453:33–40. doi: 10.1007/s11010-018-3429-2. [DOI] [PubMed] [Google Scholar]
- 73.Hussien NI, El-Wakeel HS, Souror SM, Ahmed IA. Alleviation of cardiac mitochondrial dysfunction and oxidative stress underlies the protective effect of vitamin D in chronic stress-induced cardiac dysfunction in rats. Gen Physiol Biophys. 2019;38:51–61. doi: 10.4149/gpb_2018036. [DOI] [PubMed] [Google Scholar]
- 74.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261:558–565. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 75.Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 76.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 77.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 78.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 79.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014;29:1–14. doi: 10.1007/s10654-013-9874-z. [DOI] [PubMed] [Google Scholar]
- 81.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 83.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring) 2014;22:1580–1589. doi: 10.1002/oby.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buondonno I, Sassi F, Carignano G, Dutto F, Ferreri C, Pili FG, et al. From mitochondria to healthy aging: the role of branched-chain amino acids treatment: MATeR a randomized study. Clin Nutr. 2020;39:2080–2091. doi: 10.1016/j.clnu.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Kruszyńska E, Łoboz-Rudnicka M, Palombo C, Vriz O, Kozakova M, Ołpińska B, et al. Carotid artery stiffness in metabolic syndrome: sex differences. Diabetes Metab Syndr Obes. 2020;13:3359–3369. doi: 10.2147/DMSO.S262192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oneglia A, Nelson MD, Merz CNB. Sex differences in cardiovascular aging and heart failure. Curr Heart Fail Rep. 2020;17:409–423. doi: 10.1007/s11897-020-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl D, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 88.Vallejo MS, Blümel JE, Arteaga E, Aedo S, Tapia V, Araos A, et al. Gender differences in the prevalence of vitamin D deficiency in a southern Latin American country: a pilot study. Climacteric. 2020;23:410–416. doi: 10.1080/13697137.2020.1752171. [DOI] [PubMed] [Google Scholar]
- 89.AlQuaiz AM, Kazi A, Fouda M, Alyousefi N. Age and gender differences in the prevalence and correlates of vitamin D deficiency. Arch Osteoporos. 2018;13:49. doi: 10.1007/s11657-018-0461-5. [DOI] [PubMed] [Google Scholar]
- 90.Lulkiewicz M, Bajsert J, Kopczynski P, Barczak W, Rubis B. Telomere length: how the length makes a difference. Mol Biol Rep. 2020;47:7181–7188. doi: 10.1007/s11033-020-05551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bachmann MC, Bellalta S, Basoalto R, Gómez-Valenzuela F, Jalil Y, Lépez M, et al. The challenge by multiple environmental and biological factors induce inflammation in aging: their role in the promotion of chronic disease. Front Immunol. 2020;11:570083. doi: 10.3389/fimmu.2020.570083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viña J, Borrás C, Gambini J, Sastre J, Pallardó FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005;579:2541–2545. doi: 10.1016/j.febslet.2005.03.090. [DOI] [PubMed] [Google Scholar]
- 93.Sengupta S, Wasylyk B. Physiological and pathological consequences of the interactions of the p53 tumor suppressor with the glucocorticoid, androgen, and estrogen receptors. Ann N Y Acad Sci. 2004;1024:54–71. doi: 10.1196/annals.1321.005. [DOI] [PubMed] [Google Scholar]
- 94.Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, et al. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res. 2008;103:34–42. doi: 10.1161/CIRCRESAHA.107.169037. [DOI] [PubMed] [Google Scholar]
- 95.Alonso-alvarez C, Bertrand S, Faivre B, Sorci G. Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct Ecol. 2007;21:873–879. [Google Scholar]
- 96.Mayer S, Brüderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, et al. Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res. 2006;112:194–201. doi: 10.1159/000089870. [DOI] [PubMed] [Google Scholar]
- 97.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Halcyon Study Team. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brüderlein S, Müller K, Melzner J, Högel J, Wiegand P, Möller P. Different rates of telomere attrition in peripheral lymphocytes in a pair of dizygotic twins with hematopoietic chimerism. Aging Cell. 2008;7:663–666. doi: 10.1111/j.1474-9726.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 99.Blackburn P, Després JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006;14:1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 100.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]