Abstract
Conventional semen analysis (SA) is an essential component of the male infertility workup, but requires laboratories to rigorously train and monitor technicians as well as regularly perform quality assurance assessments. Without such measures there is room for error and, consequently, unreliable results. Furthermore, clinicians often rely heavily on SA results when making diagnostic and treatment decisions, however conventional SA is only a surrogate marker of male fecundity and does not guarantee fertility. Considering these challenges, the last several decades have seen the development of many advances in SA methodology, including tests for sperm DNA fragmentation, acrosome reaction, and capacitation. While these new diagnostic tests have improved the scope of information available to clinicians, they are expensive, time-consuming, and require specialized training. The latest advance in laboratory diagnostics is the measurement of seminal oxidation-reduction potential (ORP). The measurement of ORP in an easy, reproducible manner using a new tool called the Male Infertility Oxidative Stress System (MiOXSYS) has demonstrated ORP's potential as a feasible adjunct test to conventional SA. Additionally, the measurement of ORP by this device has been shown to be predictive of both poor semen quality and male infertility. Assessing ORP is a novel approach to both validating manual SA results and identifying patients who may benefit from treatment of male oxidative stress infertility.
Keywords: Infertility, Male; MiOXSYS; Oxidation-reduction potential; Semen analysis; Semen analysis subjectivity
INTRODUCTION
Although significant advances have been made in diagnostic laboratory techniques, the cornerstone for the evaluation of male infertility remains manual semen analysis (SA), which is still considered an essential diagnostic tool in the initial male infertility workup despite its intrinsic subjectivity [1]. In an effort to standardize manual SA results and limit inaccuracy, the World Health Organization (WHO) has published a series of laboratory manuals that have since become the gold standard for SA performance and interpretation [2,3]. Nevertheless, errors persist, potentially leading to misdiagnosis and consequently over- or under-treatment, especially since SA plays an important role in guiding management and treatment decisions having to do not only with male factor infertility [4], but also with other potentially serious male health conditions.
There are numerous ways in which systematic and human errors can influence SA results (Fig. 1), including lack of adherence to standardized protocols when measuring individual semen parameters (count, motility, viability, etc.) [5]. Following best practices does not entirely eliminate variability that may result from technician subjectivity and human error. In addition to being vulnerable to variations due to laboratory methods, subjectivity, and human error, semen parameter measurements are associated with biological and lifestyle factors that may vary within an individual over time [5,6], making a single SA an unreliable indicator of underlying pathology.
Fig. 1. Four ways in which error can influence the result gathered during semen analysis.

Clinically, poor semen quality may be an indicator of a variety of medical problems, including varicocele, hormone imbalances, infection, genetic alterations, and testicular cancer. Physicians are responsible for diagnosing and treating these and other mediators of male infertility [7,8,9,10]. From empiric medical therapy to the use of assisted reproductive technology (ART), treatment for male factor infertility and its antecedents may involve pharmaceutical and/or surgical intervention with potentially harmful side effects and significant financial and emotional investment [8,11], underscoring the importance of reliable SA results. At present, clinicians performing infertility workups must consider the intrinsic uncertainties associated with conventional SA that may compromise their ability to make an accurate diagnosis.
Although several technological advances have been made to conventional SA techniques, such as computer-assisted sperm analysis (CASA), these novel developments have significant limitations, including highly variable results, the necessity of frequent recalibration, and costly investment in equipment and training with only marginal improvements in accuracy over manual SA [12,13,14,15]. In addition to these technological advances, sophisticated sperm function tests have been conceived that evaluate parameters, such as sperm DNA fragmentation (SDF), capacitation, acrosome reaction, and the presence of reactive oxygen species (ROS) [16,17]. Although these tests have increased the amount of data by which physicians can make clinical decisions, problems remain. For example, sperm function tests such as SDF are time consuming and time sensitive, and require large financial investments due to their reliance on the use of sperm chromatin structure assays and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) [16,17,18]. Many common ROS measurement techniques only capture the ROS concentration at a single point in time and do not provide a comprehensive evaluation of the relation between oxidant and antioxidant levels, therefore failing to provide a complete picture of the true oxidative stress (OS) environment [16,19]. To manage patients with infertility related to elevated OS, it is important to identify a test that includes all constituents of OS and measures the real-time redox status in a given semen sample.
Due to the aforementioned problems associated with manual SA and the inability of current technology to efficiently mitigate these issues, it is necessary to identify a suitable adjunct test that can indicate when results of a single manual SA may not accurately reflect a patient's underlying reproductive health. We propose the measurement of oxidation-reduction potential (ORP) as a way to validate the results of manual SA. This confirmatory test reliably predicts poor semen quality, is reproducible, easy to use, is cost effective, and can significantly improve the diagnosis and treatment of male infertility.
PROBLEMS OF BASING CLINICAL DECISIONS ON MANUAL SEMEN ANALYSIS ALONE
As part of the first set of diagnostic tools employed to investigate cases of male infertility, manual SA provides clinicians with a snapshot of the overall reproductive health of the patient in the preceding 72 days of spermatogenesis and subsequent epididymal maturation [20]. However, the following section will present how and why the results of manual SA may be inaccurate, incorrectly classified, and subject to human/laboratory error, and what the costs and consequences of these uncertainties may be [7].
1. Errors and subjectivity associated with manual semen analysis
When evaluating each step in the process of performing manual SA (Fig. 2), it becomes clear how human error can influence the results [5]. For example, specimen collection is vulnerable to problems such as incomplete semen collection, short or long abstinence intervals, or delayed delivery to the lab, all of which may affect results. Although the standard protocol prior to SA is to abstain from ejaculation for 2 to 7 days [3], studies have shown that, due to the natural variation in semen parameters within in an individual over time, obtaining a second ejaculate for SA provides physicians the ability to more accurately diagnose patients' fertility status [21,22]. Similarly, the evaluation of sperm concentration, motility, and morphology are vulnerable to human error, especially if technicians are inadequately trained or lack experience, which can lead to observer subjectivity. For example, properly analyzing sperm motility is a consistent challenge in andrology laboratories due to the natural human inclination to fix one's gaze on a moving object. When quickly scanning a single field, as required to gain accurate motility results, this human instinct can interfere with the ability to efficiently count each motile spermatozoa and promptly move to the next one [5]. To improve the technique used by technicians and ultimately improve the accuracy of motility results, it has been suggested that andrology laboratories adopt external quality control monitors to oversee the evaluation process [1].
Fig. 2. Ways ‘uncertainty of measurement’ can influence semen analysis.
Inaccuracies may also result from the equipment used to conduct manual SA. For example, the often-used shallow Makler counting chamber allows for only a limited number of spermatozoa (commonly n=10) to enter the microscope viewing field and thus is more prone to errors associated with small sample sizes [7]. In contrast, the deeper haemocytometer counting chamber allows up to 400 spermatozoa to enter the field and provides a more accurate result [7]. Even when quality is monitored and equipment is optimized, poor SA technique and the inherent subjectivity of the tests often remain and may lead to variable results even when a single experienced technician evaluates different aliquots of semen samples from the same patient [6,7].
SA results guide clinical decisions, with semen parameter values often determining treatment trajectories that have very different physical, emotional, and financial costs. For example, the standard of care (SOC) derived cost disparities between procedures such intrauterine insemination (IUI, cost range: $1,275–$3,825) and in vitro fertilization with intracytoplasmic sperm injection (IVF/ICSI, cost range: $8,825–$26,476) are significant [23]. The SOC assumes three cycles of both IUI and IVF/ICSI [23]. The decision about which technique to use is often based on total motile sperm count (TMSC) [24,25,26]. A study of postoperative varicocelectomy patients found that those with TMSC ≥10×106 sperm/mL in their postoperative ejaculate were more likely to achieve pregnancy via IUI than those with TMSC <10×106 sperm/mL, who were more likely to require sperm extraction surgery and IVF/ICSI [8,9]. If the SA inaccurately yields a TMSC of 9×106 sperm/mL vs. 11×106 sperm/mL, the clinician may be inclined to recommend the more costly and invasive procedure.
2. Lack of standardized protocols, training, and quality assurance assessments in andrology laboratories
An increased probability of error often results from lack of adherence to a standardized protocol for the various duties carried out in the andrology laboratory [1,27,28]. A 15-year study of 151 andrology laboratories in Belgium that underwent repeated external quality assessments and controls (EQA/C) revealed many areas for concern, the most notable being limited funding for laboratory training, highly variable frequency of SA performance per month, and completion of proper training courses by only 40% of laboratory staff at the beginning of the study. Only 16.5% of the andrology laboratories evaluated had technicians who were trained in and performed manual SA exclusively [1]. With experience being crucial to carrying out accurate SA, this study makes apparent why manual SA results are often unreliable, giving rise to significant interand intra-laboratory variation [29]. Strictly adhering to an EQA/C regimen, requiring compliance with protocols described in the most recent WHO manual, and consistently training and testing laboratory personnel can alleviate the problem of unacceptably high coefficients of variation (CV) [1,29,30,31]. For example, at the beginning of this 15-year period, the median CV for sperm count across all labs, irrespective of method, was 19.2%; upon implementation of improved techniques and technologies, such as the Neubauer counting chamber, the CV declined to 14.4% [1]. While this represents an improvement of more than 18%, the remaining 14% CV underscores the difficulties that persist, even in the face of increased QC measures.
3. Controversy regarding changing World Health Organization guidelines
Even if manual SA is conducted by trained personnel according to standardized protocols, uncertainty may arise in the interpretation of the results, as the WHO threshold values for abnormal semen quality have shifted over the years, sometimes without adequate explanation [3,28,32,33,34,35,36]. From 1980 to 2010, five editions of the WHO manual for SA have been published, and each time at least some of the reference values have changed (Table 1). The 5th edition of the WHO manual has received extensive criticism for establishing new criteria for assessing sperm morphology and progressive motility, as well as lower reference values based on data obtained only from fertile men, with the reference values being the lowest 5th percentile of the distribution for each semen parameter. Lowering the reference values based on such data potentially increases the number of false-positives and false-negatives for infertility diagnoses [37]. Changing the recommended SA protocol requires andrology laboratories to retrain their staff in the newest methods.
Table 1. Shifting of WHO threshold values for semen parameters per edition.
| Semen parameter | WHO 1980 | WHO 1987 | WHO 1992 | WHO 1999 | WHO 2010 |
|---|---|---|---|---|---|
| Volume (mL) | ND | ≥2 | ≥2 | ≥2 | 1.5 |
| Sperm count (106/mL) | 20–200 | ≥20 | ≥20 | ≥20 | 15 |
| Total sperm count (106) | ND | ≥40 | ≥40 | ≥40 | 39 |
| Total motility (% motility) | ≥60 | ≥50 | ≥50 | ≥50 | 40 |
| Progressive motility (%) | ≥2 | ≥25 | ≥25 (grade a) | ≥25% (grade a) | 32 (grade a+b) |
| Vitality (% alive) | ND | ≥50 | ≥75 | ≥75 | 58 |
| Morphology (% normal forms) | 80.5 | ≥50 | ≥30 | 14 | 4 |
WHO: World Health Organization, ND: not defined.
Adapted from the article of Esteves et al (Urology 2012;79:16-22) [28] with original copyright holder's permission.
The 5th edition simplifies the motility grading system by reducing the number of categories of sperm velocity from four to three [5], eliminating the need to distinguish between fast and slow moving spermatozoa, a discrimination that has demonstrated clinical utility [3,5,38]. To investigate and address the controversial changes made in the 5th edition of the WHO manual, the European Society for Human Reproduction and Embryology (ESHRE) formed a subcommittee, the ESHRE Special Interest Group for Andrology (SIGA) [3,38]. Among the eight areas the SIGA identified as requiring further explanation by the WHO were the decisions to eliminate the distinction between slow and rapidly-progressive spermatozoa and to only report the percent total progressive motility.
The data used to determine the reference levels in the 5th edition came from five studies conducted among 1,953 men in seven countries, only one of which (Australia) was not located in the northern hemisphere [28]. The limited diversity of the populations from which the semen samples were obtained fails to address the known ethnic differences found in semen parameters on a global scale [39,40]. The reference levels may therefore lead to increased misdiagnosis in men from ethnic backgrounds not represented in the WHO sample.
The 5th edition not only makes it difficult to accurately classify patients, especially those from diverse backgrounds, but also reduces the diagnostic abilities of clinicians due to the simplification of motility grading. Clearly, the potential for errors in clinical decision making do not solely arise from the laboratory technician.
4. Variability of semen samples over time
Another factor that limits the accuracy of diagnosis from a single SA is that semen parameters vary over time. In a study involving 20 participants, each provided a semen sample weekly for 10 weeks. The within-subject CV was calculated based upon the fluctuation of each semen parameter within an individual over the course of the study [6]. The CV for sperm concentration was 26.8%, total motility was 18.4%, and morphology was 19.6% (Table 2) [6]. A related study of 5,132 semen samples obtained from 2,566 men who each donated two samples a month apart found that after an initial normozoospermic (as defined by the WHO 5th edition) finding was obtained, 27% of the second samples were pathologic. Additionally, only 51.2% of the initial normozoospermic findings were confirmed by the second SA [3,22]. Physicians evaluating a patient's fertility status or selecting spermatozoa for ART must therefore consider the variability of sperm parameters over time within the same patient [6].
Table 2. Intra-individual sample variation by semen parameter.
| Semen parameter | Mean | Total coefficient of variability within-subject |
|---|---|---|
| Concentration (×106/mL) | 68.1 | 28.1 |
| Total motility (%) | 45.8 | 20.4 |
| Progressive motility (%) | 36.1 | 17.8 |
| Progressive rapid motility (%) | 17.8 | 22.8 |
| Morphology (% normal) | 12.7 | 20.9 |
| Vitality (%) | 62.4 | 12.4 |
Data extracted from the article Alvarez et al (Hum Reprod 2003;18:2082–8) [6].
ADVANCES IN SEMEN ANALYSIS
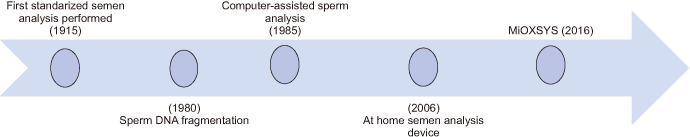
Because of the above-described variability in how semen is collected and analyzed, methods to improve the reliability of results and help avoid inaccuracies in clinical decision making are needed. Over the past several decades, technological advances in SA have been made that have attempted to reduce human subjectivity and increase the range of measures used to assess fertility potential (Fig. 3); however, there is still much progress to be made.
Fig. 3. Timeline of the advances made in analyzing semen samples. MiOXSYS: Male Infertility Oxidative System.
1. Computer-assisted sperm analysis
One such development made more than 40 years ago is the CASA system. CASA is an instrument that converts the motion patterns and morphology of spermatozoa into usable semen parameter data, data that previously could only be gathered by manual SA [41]. At its inception, the CASA device was often referred to as a “Black Box,” as a sample was inserted into the machine and, after a few minutes, a report was spit out. It was difficult to use and early results were unreliable [42]. However, over the past four decades, great strides have been made to increase the computational power, improve the software, and reduce the size of CASA systems [41]. Although much progress has been made, CASA results are still subject to high inter-observer variability due to a lack of frequent and uniform standardization of CASA techniques, CASA's requirement for precise recalibration (suggested at 6-month intervals), and the need to consistently retrain laboratory staff when software updates are implemented. Additionally, variations in technician proficiency in other laboratory techniques (e.g., pipetting, loading the counting chamber) can contribute to variations in CASA results [15,43]. Although SA performed by a technician highly trained in the use of CASA has shown improved precision over manual methods [44], proper operation of this technology requires constant supervision and calibration, which is costly and inefficient [12].
2. At-home semen analysis
Currently there are many at-home SA devices on the market, each with its own unique operational platform designed for the measurement of different semen parameters. The smartphone platform has achieved substantial popularity in recent years. This newly developed technology facilitates the ability for men to carry out simple SA in the comfort of their homes, eliminating any fear or embarrassment associated with providing a sample in an andrology laboratory [45]. The YO Home Sperm Test (Medical Electronics Systems) utilizes the YO device's microscope together with the phone's camera and light source to record a 30-second video of the sperm, which is then analyzed by the built-in software [46]. The YO Home Sperm Test measures a semen sample's motile sperm concentration (MSC), a composite value of the sample's concentration and motility. Like TMSC, MSC provides insight into a patient's fertility outlook by quantifying the concentration of sperm that have sufficient motility to potentially initiate fertilization. When compared with the laboratory-based automated sperm quality analyzer (SQA-Vision) [46], a device that differs from CASA in its use of spectrophotometry and electro-optical signal detection to measure sperm parameters [2], the YO Home Sperm Test's results on both Galaxy and iPhone were highly correlated with the SQA-Vision (Pearson correlation coefficients 0.95 and 0.93, respectively, p<0.0001) [46].
The Trak Male Fertility Testing System is a microfluidic centrifugal-based device that measures sperm concentration and divides the results into three categories: low (≤15×106 sperm/mL), moderate (15–55×106 sperm/mL), and optimal (>55×106 sperm/mL). Using a battery-powered engine to propel spermatozoa through a test cartridge (prop) containing a series of chambers, Trak provides results in approximately 6.5 to 7 minutes [47]. First, a semen sample is ejaculated into the Trak collection cup, which is coated in a liquefaction-inducing enzyme. Next, 0.25 mL of the sample is loaded into the inlet chamber of the test cartridge, which is attached to the engine, and the lid of the device is closed. Once closed, the spin sequence initiates for approximately 6.5 minutes. While spinning, a precise volume of sample is propelled from the inlet chamber (which contains a preloaded density medium), into a metered chamber, through a progressively narrowing collection channel. Lastly, the sample presents as a white column at the end of the cartridge. The length of the white column is proportional to the concentration of the sample and the results can be obtained by visual inspection. However, a significant limitation of Trak is that it can only measure sperm concentration. With only one evaluated parameter, it must be stressed to the user that normal results do not fully rule out infertility [47].
The end goal of these “point-of-care” tests is to identify patients who need to be seen by a specialist for further evaluation, not to replace laboratory-based SA and certainly not to diagnose infertility [13]. At-home SA devices currently only measure concentration and/or motility; they are not able to measure morphology or any of the other basic parameters included in manual SA, such as pH, liquefaction, and vitality. Without having the complete picture provided by SA performed by a trained technician and interpreted by an infertility specialist, a conclusive diagnosis cannot be made.
3. DNA fragmentation, acrosome reaction, and capacitation tests
Advanced SA methods, including SDF, capacitation, and acrosome reaction tests, are often used to supplement manual SA in the evaluation of male infertility. However, the difficulties associated with performing these sperm function tests and the challenges experienced in interpreting their results have prevented their widespread use [48]. Several techniques are available to measure SDF. They include evaluation for strand breaks by probes or dyes, or measuring the susceptibility of DNA to denaturation. In men who are varicocelectomy candidates, couples with recurrent pregnancy loss, or couples with failed IVF, there may be a role for SDF tests. However, the cost of equipment (e.g., flow cytometers for TUNEL assays, terminal deoxynuleotidyl transferase enzyme, fluorophore modified dUTPs, etc.), need for skilled technicians, and lack of a clear-cut clinical reference value have impeded the routine application of SDF assays. The acrosome reaction test is carried out in the laboratory by artificially inducing sperm cells to lose the “cap” portion of the head (acrosome) by exposing the cells to a follicular fluid-like substance (Tyrode's Solution). Subsequently, sperm are evaluated by fluorescence microscopy to determine which ones have or have not successfully shed their cap. A study has shown this test, with a receiver operating characteristic (ROC) curve cutoff of 0.65, to be a valid predictor of IVF fertilization, with 63.2% sensitivity, 80.6% specificity, 80% positive predictive value, and 64.1% negative predictive value [49]. However, the methods required to perform the test are costly and labor intensive [50]. Lastly, the sperm capacitation test evaluates the physiological changes that naturally occur once a sperm cell is inside the female reproductive tract. Capacitation is deduced by measuring the localization trend of the membrane protein ganglioside monosialotetrahexosylganglioside (GM1) in spermatozoa, and the number of successfully capacitated sperm cells are then counted to provide the “Cap-Score” [51]. In a study of 91 men, those whose semen had a Cap-Score >26.7% were 2.78 times as likely to achieve pregnancy over the course of ≤3 IUI cycles than those with a Cap-Score <26.7% [51]. As with the acrosome reaction test, capacitation testing requires extensive training, monitoring, and is not a cost-effective adjunct to manual SA.
4. Oxidative stress tests
OS and its deleterious influence on male fertility have been widely studied [52,53,54]. OS results when an excess of oxidants (i.e., ROS) or a deficiency in antioxidants disrupts the homeostasis of the redox system and pushes it out of balance. When a biological system is in a state of OS, pathological events may occur such as apoptosis, DNA damage, and lipid peroxidation [52].
Currently, several methods exist to measure the concentration of ROS and antioxidants, but they are often costly, time consuming, require extensive training, or necessitate a large sample volume [54]. Chemiluminescence, which measures light emitted by a reaction between chemical reagents and ROS in the sample, is widely used in the evaluation of seminal ROS, but has several limitations. First, chemiluminescence cannot measure ROS in frozen samples [15]. Second, due to the 800 µL sample requirement to run the test, patients with low semen volume cannot be evaluated. Third, chemiluminescence does not account for the potential presence of ROS-producing leukocytes in the sample and therefore is not a reliable measure of ROS generated by sperm. The common laboratory-based Total Antioxidant Capacity (TAC) assay evaluates the total antioxidants available in a semen sample. To measure the antioxidant concentration only in the clear seminal fluid, TAC first requires the removal of all cellular bodies from the sample. Subsequently, an oxidation reaction is artificially induced and the sample's ability to neutralize this reaction by its native antioxidants is measured by rapid colorimetry [16]. A low TAC value (<1,950 µM of TAC buffer standard Trolox equivalent) indicates OS due to a deficiency in the sample's antioxidant concentration and thus the inability to scavenge the excess ROS [16]. Although TAC provides valuable data on the presence of OS in a sample, this assay has its share of limitations. First, TAC only measures the antioxidants in the seminal plasma and not the presence of enzymatic antioxidants or individual antioxidants in the entire ejaculate. Second, the TAC assay kit is not cost effective when compared to other fertility diagnostic tools available. Third, the TAC assay only indirectly measures OS [16]. It is therefore important to identify a test that provides a comprehensive measure of both oxidant and antioxidant activity [54], thereby facilitating our understanding of the real-time redox status in a given semen sample.
A POTENTIAL SOLUTION—MEASURING OXIDATION-REDUCTION POTENTIAL
Measuring ORP, or redox potential, is the most recent advance in identifying a single direct marker of male factor infertility. ORP provides a snapshot of the oxidant-to-antioxidant relationship and yields insight into the redox equilibrium and the state of OS in each sample [55,56,57]. ORP levels in whole blood have been shown to increase due to trauma [58,59], intense exercise [60], and organ dysfunction [61]. In cases of male infertility, ORP levels have also been found to be elevated [55,56,57]. ORP has been utilized in the clinical andrology setting as a reliable marker of OS in human semen samples [62]. Studies are currently underway evaluating the use of seminal ORP as a marker for embryo development and pregnancy outcomes.
1. Introduction to the Male Infertility Oxidative System
Among the laboratory instruments designed to measure OS in a biological sample, one recent advancement is the Male Infertility Oxidative System (MiOXSYS). MiOXSYS is based on a galvanostatic measure of the electron movement and provides information on the complete oxidation-reduction activity within a given sample [55]. MiOXSYS requires only a small amount of semen (30 µL) and yields results in <5 minutes. In order to improve the ease of use, standardized laboratory protocols and consistent device software upgrades have been implemented. MiOXSYS produces results based on the patients' physiological balance of oxidants vs. antioxidants, represented by ORP, which has been shown to be both stable and reproducible [55,56,57]. Due to these qualities, MiOXSYS has the potential to mitigate the problems of unreliability inherent in manual SA as well as the issues of extensive training, cost, and oversight associated with advanced SA tests. Although manual SA is vulnerable to subjectivity and human error, when carried out properly, the multifaceted insights it provides are crucial to patient diagnosis; thus, it is a step too far to eliminate this manual SA altogether. Rather, the measurement of ORP by MiOXSYS could serve as an adjunct test to manual SA that reliably discriminates between the presence and absence of abnormal semen parameters.
2. Validation of oxidation-reduction potential as an indicator of semen quality and fertility potential
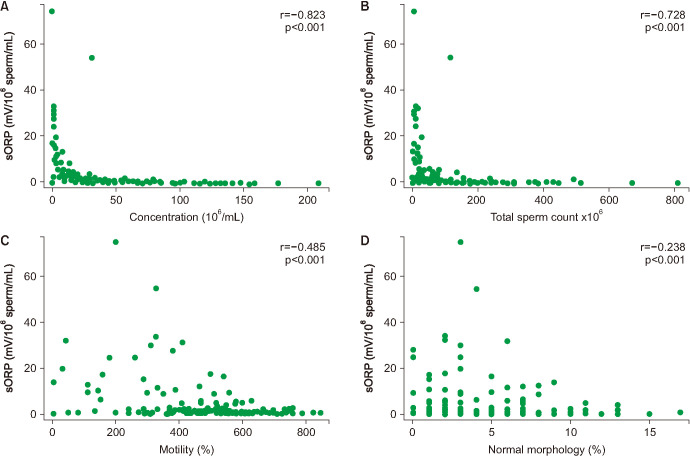
In a study of 106 infertile men and 51 healthy controls (proven and unproven fertility) from our clinic, we recently reported a negative correlation between ORP and semen parameters across all participants (sperm concentration [r=−0.823; p<0.001], total sperm count [r=−0.728; p<0.001], percent total motility [r=−0.485; p<0.001], and percent normal morphology [r=−0.238; p=0.020]) (Fig. 4). Infertile men also had higher ORP levels compared with the control group (6.22±1.10 mV/106 sperm/mL vs. 1.59±0.29 mV/106 sperm/mL (p=0.004) [55]. Of the 157 men, 65 participants had normal semen parameters and 92 presented as abnormal for one or more semen parameters based on WHO 5th edition criteria [3,55]. A ROC curve analysis yielded a cutoff value of 1.36 mV/106 sperm/mL that distinguished between those with normal and any abnormal semen parameters with 69.6% sensitivity, 83.1% specificity, 85.3% positive predictive value, and 65.9% negative predictive value. The median ORP was below this cutoff for infertile participants and above it for healthy controls (Fig. 5). As quality control measures, we measured intra- and inter-observer variability and found that, using this cutoff, there was strong intra-observer reliability (average CV=8.39%) and inter-observer reliability (correlation coefficients >0.97, CV across observers=3.61%) among our three technicians [55]. These findings were corroborated by a study we published the following year in which a static ORP cut-off value of 1.38 mV/106 sperm/mL was determined via ROC curve analysis to differentiate normal from abnormal semen samples within a sample of 365 infertile men (Fig. 6), suggesting that ORP could be a valuable addition to SA as a useful marker of semen quality [56].
Fig. 4. Correlation of oxidation-reduction potential (ORP) with sperm parameters. (A) Sperm Concentration. (B) Sperm count. (C) Motility. (D) Morphology. sORP: static ORP. Adapted from the article of Agarwal et al (Reprod Biomed Online 2017;34:48–57) [55] with original copyright holder's permission.
Fig. 5. Distribution of oxidation-reduction potential (ORP) values in controls and patients with the established cut-off values. Results shown in box-plot showing median and the 25th, 75th percentile. The whiskers represent the 95% confidence intervals. sORP: static ORP. Adapted from the article of Agarwal et al (Reprod Biomed Online 2017;34:48–57) [55] with original copyright holder's permission.

Fig. 6. Higher oxidation-reduction potential (ORP) values were measured in abnormal parameters. Shown: Semen with abnormally low numbers of spermatozoa had higher ORP values, semen with abnormally few motile spermatozoa was also related to higher ORP values and, semen with fewer morphologically normal spermatozoa had higher ORP values. *Significant at p<0.05. Adapted from the article of Arafa et al (Andrologia 2018;50) [56] with original copyright holder's permission.
Finally, we conducted a multi-center study with 2,092 participants from nine institutions located in seven different countries including USA, Qatar, Japan, UK, Turkey, Egypt, and India. In this study we evaluated 1) the variability of all measures according to WHO criteria for the normal and abnormal SA groups and 2) verified the ORP cut-off value to distinguish men with normal semen parameters from those with abnormal semen parameters. Mean semen quality measures differed significantly between those with no abnormal parameters and those with at least one abnormal parameter (concentration, motility, or morphology) according to WHO 5th edition criteria (Table 3). An ORP cutoff value of 1.34 mV/106 sperm/mL was derived to distinguish between the two groups and the median ORP was below this cutoff for those with at least one abnormal semen parameter and above it for those with normal semen parameters (Fig. 7) [63]. The consistency of this cutoff value with those of our prior studies indicates the reliability of ORP as a tool to evaluate patients from diverse ethnic backgrounds, whose standard semen parameter measures may have different distributions [64,65].
Table 3. Background information on the study population with a comparison of semen parameters between the normal and abnormal groups.
| Semen parameter | Normal group (n=199) | Abnormal group (n=1893) | p-value |
|---|---|---|---|
| ORP (mV/106 sperm/mL) | 0.88±1.64 | 5.08±14.24 | 0.001 |
| Sperm total (×106) | 231.01±171.96 | 108.30±147.38 | 0.001 |
| Progressive motility (%) | 48.08±10.87 | 15.81±15.29 | 0.001 |
| Total motility (%) | 57.19±10.44 | 41.26±17.16 | 0.001 |
| Normal morphology (%) | 6.76±3.49 | 4.87±7.46 | 0.001 |
| Sperm concentration (×106/mL) | 70.53±50.82 | 34.72±31.16 | 0.001 |
| Volume (mL) | 3.47±1.41 | 3.18±2.29 | 0.001 |
Values are presented as mean±standard deviation or range. Sperm parameters and ORP values in patients with at least one abnormal semen parameter versus normal semen parameters.
ORP: oxidation-reduction potential.
Data extracted from the article Agarwal et al (Asian J Androl 2019;21:565–9) [63].
Fig. 7. Distribution of oxidation-reduction potential (ORP) in patients with at least one abnormal sperm parameter versus patients with normal sperm parameters, showing the established cut-off value of 1.34 mV/106 sperm/mL. Adapted from the article of Agarwal et al (Asian J Androl 2019 [epub ahead of print]) [63].

These studies represent just a few of the many works either already published or currently underway that demonstrate the capability of ORP measurement to discern between normal and abnormal SA and aid in the diagnosis of male infertility. As an adjunct test, the quick, reliable, non-observer-dependent ORP results produced by MiOXSYS could serve to confirm the validity of manual SA results or shed light on the possibility of subjectivity and human error. Normalized ORP results are influenced by the sperm concentration in a given semen sample. Therefore, ORP testing is not suitable in cases of severe oligozoospermia (≤1×106 sperm/mL) and azoospermia. The conventional SA is recommended in these cases.
3. Clinical utility of measuring oxidation-reduction potential with Male Infertility Oxidative System
In the male infertility workup, elevated ORP can act as a confirmatory test for manual SA, with strong inter- and intra-observer reliability. In cases when manual SA results yield parameters within the normal range but MiOXSYS analysis shows elevated ORP, the high positive predictive value of ORP suggests that clinicians should be wary of the accuracy of the SA results [55,56]. In this situation, manual SA should be repeated and the patient should be assessed for known triggers of OS. Finally, due to its ease of use, ability to analyze fresh or frozen samples, cost effectiveness, and ability to quickly provide insight into a male patient's fertility potential, MiOXSYS analysis alone may be used as a screening tool in areas of the world in which access to a fertility specialist is limited. In general, the actual cost for a routine SA using WHO 2010 criteria may vary anywhere from $140.00 to $300.00. Also, the cost for additional sperm function test to measure the sperm DNA damage can be $175.00 to $400.00 per test. Whereas, the cost to measure ORP is approximately $50.00 which is just one fourth the cost of a sperm DNA test. In the absence of a high-level, quality-controlled infertility lab with technicians trained to do manual SA, ORP measured by MiOXSYS may play an important role in assessing sperm quality and facilitating clinical decision making.
CONCLUSIONS
Manual SA is the first step in determining a couple's fertility status, and yet its results are vulnerable to error from many sources. The low rate of implementation of standardized WHO-informed protocols and adherence to EQA/C in andrology laboratories, as well as the prevalence of human subjectivity and potential error in SA results, point to the urgent need for an additional method to confirm the results of manual SA. Advanced techniques, such as DNA fragmentation, acrosome reaction, and capacitation tests indicate problems associated with reduced fecundity, but all require significant investments of money, time, and training. ORP measured via MiOXSYS predicts abnormal semen parameters with high sensitivity and specificity, and is quick and simple to perform, cost effective, highly reliable, requires little training, and utilizes a small sample size. Furthermore, we have provided evidence supporting the use of seminal ORP as a surrogate measure of OS and abnormal semen parameters across diverse populations. Although the shortcomings of manual SA have been well documented, it is still an essential test in the workup of male patients presenting with infertility. The authors believe that an additional diagnostic tool such as the MiOXSYS can provide valuable feedback to confirm the results of manual SA or highlight inconsistencies that may warrant repeated SA. In doing so, ORP evaluation will improve andrologists' ability to provide SA results that accurately reflect patients' fertility potential and enable clinicians to optimize their treatment decisions.
ACKNOWLEDGEMENTS
This study was supported by American Center for Reproductive Medicine.
The authors thank Scott Lundy, MD, Chak-Lak Cho, MD, Isaac Glatstein, MD, Manesh Kumar Panner Selvam, PhD, and Rakesh Sharma, PhD for review of our manuscript and offering helpful comments. The authors also thank Mr. Ken Abraham, Center for Medical Art & Photography, Cleveland Clinic, for his assistance in preparing the figures for this study.
Footnotes
Conflict of Interest: LGK was supported by the National Institute of Environmental Health Sciences under Award Number K99ES030403. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Other authors have nothing to disclose.
- Conceptualization: AA.
- Data acquisition: all authors.
- Formal analysis: all the authors.
- Writing—original draft: CD.
- Writing—review & editing: all the authors.
- Approval of the final manuscript: all the authors.
References
- 1.Punjabi U, Wyns C, Mahmoud A, Vernelen K, China B, Verheyen G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology. 2016;4:1084–1093. doi: 10.1111/andr.12230. [DOI] [PubMed] [Google Scholar]
- 2.Lammers J, Splingart C, Barrière P, Jean M, Fréour T. Double-blind prospective study comparing two automated sperm analyzers versus manual semen assessment. J Assist Reprod Genet. 2014;31:35–43. doi: 10.1007/s10815-013-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. pp. xiv–271. [Google Scholar]
- 4.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson MJ. Uncertainty of measurement and clinical value of semen analysis: has standardisation through professional guidelines helped or hindered progress? Andrology. 2016;4:763–770. doi: 10.1111/andr.12209. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez C, Castilla JA, Martínez L, Ramírez JP, Vergara F, Gaforio JJ. Biological variation of seminal parameters in healthy subjects. Hum Reprod. 2003;18:2082–2088. doi: 10.1093/humrep/deg430. [DOI] [PubMed] [Google Scholar]
- 7.Björndahl L, Barratt CL, Mortimer D, Jouannet P. ‘How to count sperm properly’: checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–232. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
- 8.Meng MV, Greene KL, Turek PJ. Surgery or assisted reproduction? a decision analysis of treatment costs in male infertility. J Urol. 2005;174:1926–1931. doi: 10.1097/01.ju.0000176736.74328.1a. discussion 1931. [DOI] [PubMed] [Google Scholar]
- 9.Pathak P, Chandrashekar A, Hakky TS, Pastuszak AW. Varicocele management in the era of in vitro fertilization/intracytoplasmic sperm injection. Asian J Androl. 2016;18:343–348. doi: 10.4103/1008-682X.178482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasani N, Mohseni Meybodi A, Rafaee A, Sadighi Gilani MA, Mohammadzadeh R, Sabbaghian M. Spermatogenesis disorder is associated with mutations in the ligand-binding domain of an androgen receptor. Andrologia. 2019;51:e13376. doi: 10.1111/and.13376. [DOI] [PubMed] [Google Scholar]
- 11.Patel AS, Leong JY, Ramasamy R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: a systematic review. Arab J Urol. 2017;16:96–102. doi: 10.1016/j.aju.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikka SC, Hellstrom WJ. Current updates on laboratory techniques for the diagnosis of male reproductive failure. Asian J Androl. 2016;18:392–401. doi: 10.4103/1008-682X.179161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vij SC, Agarwal A. Editorial on “An automated smartphonebased diagnostic assay for point-of-care semen analysis”. Ann Transl Med. 2017;5:507. doi: 10.21037/atm.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori Y, Pfanner P, Prins GS, Niederberger C. Novel device for male infertility screening with single-ball lens microscope and smartphone. Fertil Steril. 2016;106:574–578. doi: 10.1016/j.fertnstert.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Talarczyk-Desole J, Berger A, Taszarek-Hauke G, Hauke J, Pawelczyk L, Jedrzejczak P. Manual vs. computer-assisted sperm analysis: can CASA replace manual assessment of human semen in clinical practice? Ginekol Pol. 2017;88:56–60. doi: 10.5603/GP.a2017.0012. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Qiu E, Sharma R. Laboratory assessment of oxidative stress in semen. Arab J Urol. 2017;16:77–86. doi: 10.1016/j.aju.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–1052. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Cho CL, Esteves SC, Majzoub A. Current limitation and future perspective of sperm DNA fragmentation tests. Transl Androl Urol. 2017;6(Suppl 4):S549–S552. doi: 10.21037/tau.2017.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa D, Asaduzzaman M, Young F. Real time monitoring and quantification of reactive oxygen species in breast cancer cell line MCF-7 by 2′,7′-dichlorofluorescin diacetate (DCFDA) assay. J Pharmacol Toxicol Methods. 2018;94(Pt 1):26–33. doi: 10.1016/j.vascn.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Koch A, Horst G. Moving semen analysis into the future. Lancet Laboratories Newsletter. 2017;1:1–2. [Google Scholar]
- 21.Alipour H, Van Der Horst G, Christiansen OB, Dardmeh F, Jørgensen N, Nielsen HI, et al. Improved sperm kinematics in semen samples collected after 2 h versus 4-7 days of ejaculation abstinence. Hum Reprod. 2017;32:1364–1372. doi: 10.1093/humrep/dex101. [DOI] [PubMed] [Google Scholar]
- 22.Blickenstorfer K, Voelkle M, Xie M, Fröhlich A, Imthurn B, Leeners B. Are WHO recommendations to perform 2 consecutive semen analyses for reliable diagnosis of male infertility still valid. J Urol. 2019;201:783–791. doi: 10.1016/j.juro.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Babigumira JB, Sharara FI, Garrison LP., Jr Projecting the potential impact of the Cap-Score™ on clinical pregnancy, live births, and medical costs in couples with unexplained infertility. J Assist Reprod Genet. 2018;35:99–106. doi: 10.1007/s10815-017-1021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges E, Jr, Setti AS, Braga DP, Figueira RC, Iaconelli A., Jr Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016;4:880–886. doi: 10.1111/andr.12199. [DOI] [PubMed] [Google Scholar]
- 25.Van Voorhis BJ, Barnett M, Sparks AE, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001;75:661–668. doi: 10.1016/s0015-0282(00)01783-0. [DOI] [PubMed] [Google Scholar]
- 26.Majzoub A, Arafa M, El Ansari W, Mahdi M, Agarwal A, Al-Said S, et al. Correlation of oxidation reduction potential and total motile sperm count: its utility in the evaluation of male fertility potential. Asian J Androl. 2019 doi: 10.4103/aja.aja_75_19. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatimel N, Mansoux L, Moreau J, Parinaud J, Léandri RD. Continued existence of significant disparities in the technical practices of sperm morphology assessment and the clinical implications: results of a French questionnaire. Fertil Steril. 2017;107:365–372.e3. doi: 10.1016/j.fertnstert.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, Agarwal A. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Keel BA, Stembridge TW, Pineda G, Serafy NT., Sr Lack of standardization in performance of the semen analysis among laboratories in the United States. Fertil Steril. 2002;78:603–608. doi: 10.1016/s0015-0282(02)03296-x. [DOI] [PubMed] [Google Scholar]
- 30.Keel BA, Quinn P, Schmidt CF, Jr, Serafy NT, Jr, Serafy NT, Sr, Schalue TK. Results of the American Association of Bioanalysts national proficiency testing programme in andrology. Hum Reprod. 2000;15:680–686. doi: 10.1093/humrep/15.3.680. [DOI] [PubMed] [Google Scholar]
- 31.Filimberti E, Degl’Innocenti S, Borsotti M, Quercioli M, Piomboni P, Natali I, et al. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): the experience of an external quality control (EQC) programme. Andrology. 2013;1:401–407. doi: 10.1111/j.2047-2927.2012.00042.x. [DOI] [PubMed] [Google Scholar]
- 32.Alshahrani S, Aldossari K, Al-Zahrani J, Gabr AH, Henkel R, Ahmad G. Interpretation of semen analysis using WHO 1999 and WHO 2010 reference values: abnormal becoming normal. Andrologia. 2018;50:e12838. doi: 10.1111/and.12838. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Singapore: Press Concern; 1980. [Google Scholar]
- 34.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 2nd ed. Cambridge: Cambridge University Press; 1987. p. 67. [Google Scholar]
- 35.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999. p. 128. [Google Scholar]
- 36.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3rd ed. Cambridge: Cambridge University Press; 1992. p. 107. [Google Scholar]
- 37.Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98:1428–1431. doi: 10.1016/j.fertnstert.2012.07.1130. [DOI] [PubMed] [Google Scholar]
- 38.Barratt CL, Björndahl L, Menkveld R, Mortimer D. ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance. Hum Reprod. 2011;26:3207–3212. doi: 10.1093/humrep/der312. [DOI] [PubMed] [Google Scholar]
- 39.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443–453. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 40.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 41.Amann RP, Waberski D. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology. 2014;81:5–17.e1-3. doi: 10.1016/j.theriogenology.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Mortimer ST, van der Horst G, Mortimer D. The future of computer-aided sperm analysis. Asian J Androl. 2015;17:545–553. doi: 10.4103/1008-682X.154312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehlers J, Behr M, Bollwein H, Beyerbach M, Waberski D. Standardization of computer-assisted semen analysis using an e-learning application. Theriogenology. 2011;76:448–454. doi: 10.1016/j.theriogenology.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Dearing CG, Kilburn S, Lindsay KS. Validation of the sperm class analyser CASA system for sperm counting in a busy diagnostic semen analysis laboratory. Hum Fertil (Camb) 2014;17:37–44. doi: 10.3109/14647273.2013.865843. [DOI] [PubMed] [Google Scholar]
- 45.Sigman M. Cell phone microscope for semen analysis. Fertil Steril. 2016;106:549. doi: 10.1016/j.fertnstert.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal A, Panner Selvam MK, Sharma R, Master K, Sharma A, Gupta S, et al. Home sperm testing device versus laboratory sperm quality analyzer: comparison of motile sperm concentration. Fertil Steril. 2018;110:1277–1284. doi: 10.1016/j.fertnstert.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 47.Schaff UY, Fredriksen LL, Epperson JG, Quebral TR, Naab S, Sarno MJ, et al. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2017;107:358–364.e4. doi: 10.1016/j.fertnstert.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Majzoub A, Esteves SC, Gosálvez J, Agarwal A. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl. 2016;18:205–212. doi: 10.4103/1008-682X.172642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tello-Mora P, Hernández-Cadena L, Pedraza J, López-Bayghen E, Quintanilla-Vega B. Acrosome reaction and chromatin integrity as additional parameters of semen analysis to predict fertilization and blastocyst rates. Reprod Biol Endocrinol. 2018;16:102. doi: 10.1186/s12958-018-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeginiadou T, Papadimas J, Mantalenakis S. Acrosome reaction: methods for detection and clinical significance. Andrologia. 2000;32:335–343. doi: 10.1046/j.1439-0272.2000.00359.x. [DOI] [PubMed] [Google Scholar]
- 51.Schinfeld J, Sharara F, Morris R, Palermo GD, Rosenwaks Z, Seaman E, et al. Cap-Score™ prospectively predicts probability of pregnancy. Mol Reprod Dev. 2018;85:654–664. doi: 10.1002/mrd.23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal A, Patnaik P, Shaw D, Rathee V, Khan SW, Jain M, et al. Influence of demographic and clinical factors on surgical outcomes of the transobturator tape procedure in patients with Stress Urinary Incontinence. Curr Urol. 2015;8:126–132. doi: 10.1159/000365703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014;60:206–216. doi: 10.3109/19396368.2014.918675. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12:33. doi: 10.1186/1477-7827-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Arafa M, Agarwal A, Al Said S, Majzoub A, Sharma R, Bjugstad KB, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2018:50. doi: 10.1111/and.12881. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal A, Chandrakumar R, Arafa MM, Elbardisi H, Okada H, Suzuki K, et al. Multi-center evaluation of oxidation reduction potential assay in the infertile male. Fertil Steril. 2017;108(Suppl 3):e317 [Google Scholar]
- 58.Rael LT, Bar-Or R, Mains CW, Slone DS, Levy AS, Bar-Or D. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma. 2009;26:1203–1211. doi: 10.1089/neu.2008.0816. [DOI] [PubMed] [Google Scholar]
- 59.Rael LT, Bar-Or R, Salottolo K, Mains CW, Slone DS, Offner PJ, et al. Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: a retrospective analysis. Scand J Trauma Resusc Emerg Med. 2009;17:57. doi: 10.1186/1757-7241-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stagos D, Goutzourelas N, Bar-Or D, Ntontou AM, Bella E, Becker AT, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015;20:154–162. doi: 10.1179/1351000214Y.0000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar-Or R, Rael LT, Curtis CG, Mains CW, Slone DS, Bar-Or D. Raman spectral signatures of human liver perfusates correlate with oxidation reduction potential. Mol Med Rep. 2009;2:175–180. doi: 10.3892/mmr_00000080. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–318. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal A, Panner Selvam MK, Arafa M, Okada H, Homa S, Killeen A, et al. Multi-center evaluation of oxidationreduction potential by the MiOXSYS in males with abnormal semen. Asian J Androl. 2019;21:565–569. doi: 10.4103/aja.aja_5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glazer CH, Li S, Zhang CA, Giwercman A, Bonde JP, Eisenberg ML. Racial and sociodemographic differences of semen parameters among US men undergoing a semen analysis. Urology. 2019;123:126–132. doi: 10.1016/j.urology.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Henkel R, Elbardisi H, Agarwal A, Majzoub A, Mostafa Arafa M. Ethnic differences in male fertility parameters in 3,915 men examined for infertility in a single center introduction. Paper presented at: The American Society of Andrology 44th Annual Conference; 2019 Apr 6-9; Chicago, IL, USA. Andrology. 2019;7(Suppl 1):91. [Google Scholar]