Abstract
Studies have demonstrated that alfuzosin not only improves lower urinary tract symptoms (LUTS) but also appears to preserve ejaculatory dysfunction (EjD). The objective of this study was to evaluate the impact of alfuzosin on ejaculatory function using the ‘Male Sexual Health Questionnaire (MSHQ)-EjD Short Form’ – a validated, abridged-version of the 25-item MSHQ specifically assessing EjD. A systematic search of MEDLINE, PubMed, Scopus, Embase, and grey literature was performed in January 2017 to identify relevant cohort studies. Search terms were ‘alfuzosin’, ‘benign prostatic hyperplasia’, ‘ejaculatory dysfunction’ and their synonyms without exclusions. Six cohort studies conducted between 2008 to 2015 were selected for analysis. Three of these were conducted in Korea, one in Thailand, one in China, and one in Tunisia. Overall, 1,371 patients were enrolled in these studies with a median age of 62.3 years. All studies quantified patient LUTS and ejaculatory function using the International Prostate Symptom Score (IPSS) and MSHQ-EjD Short Form, respectively. IPSS had a median decrease of 6.6 while MSHQ-EjD had a median increase of 1.9. This review highlights the very real association between sexual function and LUTS. This systematic review confirms that alfuzosin may improve ejaculatory function in addition to LUTS and should be considered in men who are sexually active or who already complain of deteriorating ejaculation.
Keywords: Alfuzosin; Benign prostatic hyperplasia; Ejaculation; Lower urinary tract symptoms; Sexual dysfunction, Physiological
INTRODUCTION
Benign prostatic hyperplasia (BPH) is one of the most common causes of lower urinary tract symptoms (LUTS) with an incidence greater than 80% in men older than age 80 years affected [1]. From longitudinal population-based studies, we know that BPH is an age-related, progressive disease with moderate-severe LUTS noted in 26% of men aged 40 to 49 years and 46% aged 70 to 79 years [2]. While complications, such as acute urinary retention, are relatively low at 2% to 7% with 6% to 10% of men requiring surgery for BPH [2,3,4]. Despite these low rates, BPH-related LUTS do significantly impact a man's quality of life and has led to the ubiquitous use of α1-blocker pharmacotherapy to ameliorate symptoms and reduce the risk of disease progression. While effectively improving LUTS, these benefits often come at a cost of other adverse effects, including sexual dysfunction. Sexual dysfunction is a complex phenomenon characterised not only by erectile dysfunction (ED) but also ejaculatory dysfunction (EjD) and other orgasmic disorders [5,6,7]. The adverse event of EjD is a well recognised amongst α1-blockers. Surveys have shown that up to 70% of sexually active men said they would discontinue BPH therapy that would negatively impact their sex-life [8]. Furthermore, men with LUTS may already complain of significant pre-morbid sexual dysfunction. A large multinational study highlights that EjD was very prominent in men with LUTS in all geographical regions (including Europe, Asia, Latin America, and Russia) with 77.9% complaining of decreased force of ejaculation, 74.4% decreased amount of semen and half of these men considering EjD a significant problem [9]. In one trial, the incidence of EjD in BPH patients treated with Silodosin was up to 22.3% [10]. Another study observing the effect of 0.8 mg tamsulosin revealed that up to 90% of men had reduce ejaculate volume and over one third reported anejaculation [11]. While patients may not be forthcoming about their ejaculations, urologists should carefully consider sexual effects and appropriately counsel patients before initiating therapy.
Interestingly, it appears that not all α1-blockers were made equal. While the mechanism is unclear, alfuzosin's α1-uroselectivity seems to produce less EjD compared to its counterparts that also effect vascular smooth-muscle tone [12]. While the drug shows promise for sexually active men with BPH, the vast majority of available literature lacks a standardised score for quantification of EjD. Most studies simply report rates of ‘abnormal’ ejaculation or utilise the Danish Prostatic Symptom Score (DAN-PSS-1), which is relatively limited in its evaluation of ejaculatory function. Unlike reviews of ED effects of α1-blockers that use the standardised International Index of Erectile Function (IIEF), no such review had been performed on EjD due to lack of standardised quantification.
The Male Sexual Health Questionnaire Ejaculatory Function Short Form (MSHQ-EjD-SF) is a validated abridged version of the 25-item MSHQ, first introduced in 2007 [13]. It is a four-item questionnaire that assesses EjD, with three questions on function and one question on bother. Compared to the DAN-PSS-1 and International Continence Society sex questionnaire, the MSHQ also evaluates psychometric properties such as force, delay and pleasure of ejaculation addressing the previous limitations of these scores [9,14].
The aim of the present review is to evaluate clinical trials assessing ejaculatory function and alfuzosin treatment using the MSHQ-EjD-SF.
METHODS
We conducted a systematic review of Medline, PubMed, Pre-Medline, Embase, Scopus, and ‘grey literature’ (sourced from scientific meeting abstracts and Google Scholar) to identify original research articles that measured the outcomes of LUTS and EjD using validated questionnaires (International Prostate Symptom Score [IPSS] and MSHQ-EjD-SF) in men with BPH after treatment with alfuzosin. Keywords used included ‘benign prostatic hyperplasia’, ‘lower urinary tract symptoms’, ‘alpha blockers’, ‘alfuzosin’, ‘ejaculatory dysfunction’ and their synonyms (Appendix). The reference lists of identified studies were also manually searched to see if they met the inclusion criteria.
Given that the 25-item MSHQ was only validated for use in 2004 [14], and the abridged MSHQ-EjD-SF in 2007 [13], the present systematic review was limited to studies published between January 2007 and January 2017. There was no restriction on language and non-English publications were translated via Google Translate (Google LLC, Mountain View, CA, USA). Studies were excluded if only qualitative descriptors of EjD were used, if they were non-human studies or if conference abstracts did not have access to full-text. All studies were reviewed independently by two reviewers and any discrepancies resolved by consensus.
The primary outcome measure was change in MSHQ-EjD-SF scores following treatment with alfuzosin in males with BPH. Data extracted from selected studies included study design and sample size, demographic and patient characteristics information, inclusion/ exclusion criteria used, duration of treatment, and the mean change in symptom scores from baseline to after treatment with alfuzosin. These changes were derived from ‘overall’ score, rather than the specific domains of the SHQ-EjD-SF (ability to ejaculate during sexual activity, force of ejaculation, volume of ejaculation, bother). If data required for the review was not published, authors were contacted to clarify these results. Statistical imputation was not used for any missing data.
As a systematic review of the literature, institutional research ethics review approval was not required.
RESULTS
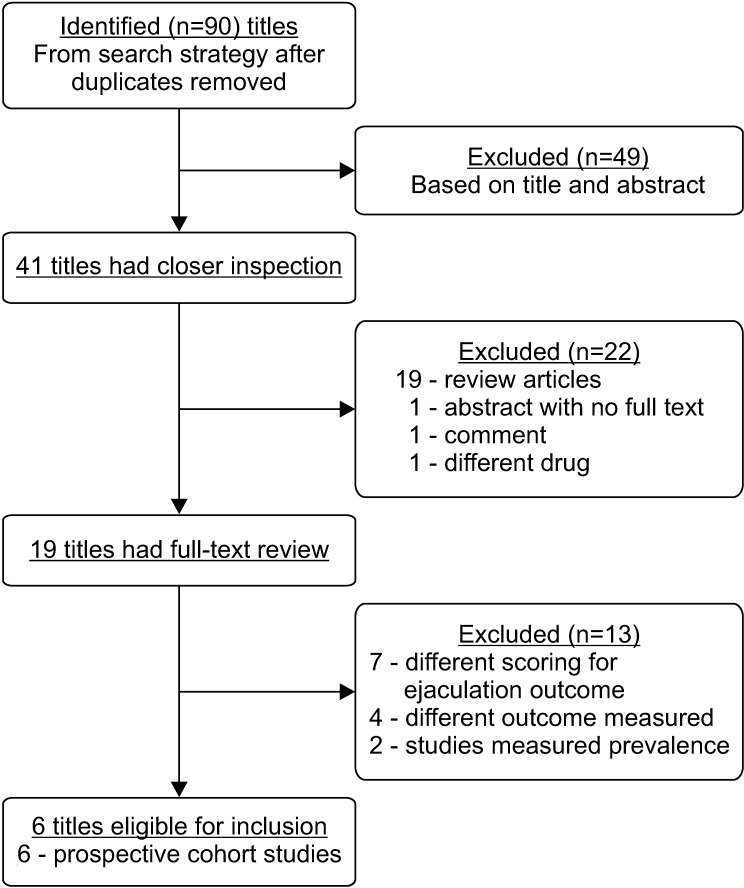
The initial search strategy yielded 90 original articles. After removal of duplicates, 13 studies were included for a full text review. Six of these 13 studies satisfied the final inclusion criteria (Fig. 1) with combined sample size of 1,371 men.
Fig. 1. Search results from systematic search strategy.
1. Study characteristics
The characteristics and methodology of these cohort studies are summarised in Table 1. All six studies were open labelled, non-comparative prospective cohort studies that solely investigated changes in LUTS and ejaculatory function after treatment with alfuzosin. Ejaculatory function, erectile function and LUTS were measured in these studies using the MSHQ-EjD SF, the IIEF-5 and IPSS, respectively. The treatment protocol was identical across all studies with 10 mg of alfuzosin taken once daily. MSHQ-EjD and IPSS were measured at baseline and after treatment was commenced, however follow-up time varied. All trials were conducted over multiple centres across Asia with the exception of Yoon et al [15]. Individual sample sizes varied with largest trial in Tunisia assessing 730 patients [16], while the others had less than 200 patients each. A number of similarities between these studies regarding patient selection criteria, intervention protocol and outcomes measured was observed. Kim et al [17] had the shortest intervention time, re-assessing men after just 3 months of treatment, while Yoon et al [15] followed their patients up to 2 years after commencement of treatment. In addition to the above six papers, Martín-Morales et al [18] also met inclusion criteria, however published data was incomplete. Reasonable efforts were made to contact the authors unsuccessfully, thereby excluding this study from final selection.
Table 1. Study characteristics.
| Study | Ben Rhouma et al [16] (2015) | Yoon et al [15] (2014) | Hwang et al [22] (2012) | Leungwattanakij et al [20] (2010) | Kim et al [17] (2010) | Chung et al [21] (2009) |
|---|---|---|---|---|---|---|
| Type | Open, non-comparative prospective cohort | Open, non-comparative prospective cohort | Open, non-comparative prospective cohort | Open, non-comparative prospective cohort | Open, non-comparative prospective cohort | Open, non-comparative prospective cohort |
| Size (n) | 730 (234 drop out) | 30 (18 drop out) | 279 (123 drop out) | 99 | 135 (25 drop out) | 148 (25 drop out) |
| Location (no. of center) | Multicenter, Tunisia | Single center, South Korea | Multicenter (9), Taiwan | Multicenter (13), Thailand | Multicenter (9), South Korea | Multicenter (4), South Korea |
| Intake period | June 2009.Jull 2015 | 2010.2012 | September 2006.May 2008 | June 2006.December 2007 | Unclear | June 2006.October 2007 |
| Population studied | Male patients with LUTS suggestive of BPH | Male patients with IPSS>8, prostate volume>20, sexually active | Males >40 with moderate to severe LUTS suggestive of BPH, IPSS>8 | Males >50 with moderate to severe LUTS suggestive of BPH, IPSS>8, sexually active | Males >40 with mild to moderate LUTS with BPH, prostate volume>20 | Males >50 with moderate to severe LUTS suggestive of BPH, IPSS>8, sexually active |
| Intervention group | Alfuzosin 10 mg daily | Alfuzosin 10 mg daily | Alfuzosin 10 mg daily | Alfuzosin 10 mg daily | Alfuzosin 10 mg daily | Alfuzosin 10 mg daily |
| Comparator group | Baseline scores | Baseline scores | Baseline scores | Baseline scores | Baseline scores | Baseline scores |
| Primary outcomes | IPSS and MSHQ-EjD after 6 months | IPSS, IIEF and MSHQ-EjD after 2 years | IPSS, MSHQ-EjD, IIEF after 6 months | IPSS, IIEF, MSHQ-EjD after 6 months | IPSS, MSHQ-EjD after 3 months | IPSS, MSHQ-EjD after 6 months |
LUTS: ower urinary tract symptoms, BPH: benign prostatic hyperplasia, IPSS: International Prostate Symptom Score, MSHQ-EjD: Male Sexual Health Questionnaire ejaculatory dysfunction, IIEF: International Index of Erectile Function.
2. Study appraisal
Each cohort study was appraised via the Cochrane Risk of Bias Tool for Non-Randomised Trials (ROBIN-1) (Table 2). This tool stratifies a trial's overall risk of bias using domain-based assessments of different bias types [19]. All the trials except Leungwattanakij et al [20] were deemed to have an overall moderate risk of bias. Leungwattanakij et al [20] did not report the proportion of missing patients, their reasons for drop out and whether they were included in analysis. Ben Rhouma et al [16] did not specify what their patient inclusion criteria was and whether it was developed retrospectively or prospectively. None of the studies identified relevant cofounding factors or explicitly state whether adjustments were made on this basis. Despite this, the studies were consistent in so far as their results were likely effected by similar biases. For example, Kim et al [17], Chung et al [21], and Hwang et al [22] had near identical patient selection criteria, treatment protocol, and treatment length. All studies reported their patient exclusion criteria.
Table 2. Study appraisal using ROBINS-1 tool to determine risk of bias in non-randomised studies.
| Bias due to | Ben Rhouma et al [16] (2015) | Yoon et al [15] (2014) | Hwang et al [22] (2012) | Leungwattanakij et al [20] (2010) | Kim et al [17] (2010) | Chung et al [21] (2009) |
|---|---|---|---|---|---|---|
| Confounding | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk |
| Selection | Moderate risk | Low risk | Low risk | Low risk | Moderate risk | Low risk |
| Classification of interventions | Low risk | Low risk | Low risk | Low risk | Low risk | Low |
| Deviations from intended interventions | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Missing data | Low risk | Moderate risk | Moderate risk | Serious risk | Low risk | Low risk |
| Measurement outcome | Moderate risk | Low risk | Moderate risk | Moderate risk | Moderate risk | Moderate risk |
| Selection of reported result | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Overall | Moderate risk | Moderate risk | Moderate risk | Serious risk | Moderate risk | Moderate risk |
3. Changes in International Prostate Symptom Score after treatment
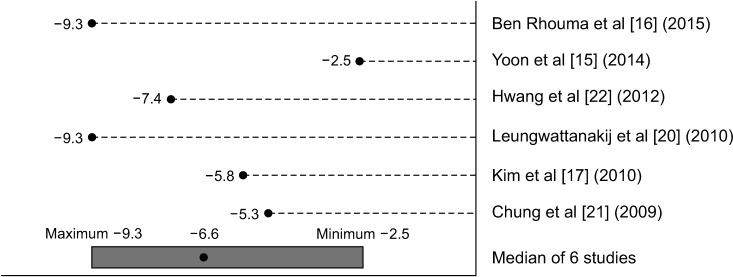
Change in LUTS scores from baseline demonstrated a significant decrease in IPSS score after commencement of alfuzosin treatment, with a median decrease of 6.6 points, range: 2.5 to 9.3 points (Fig. 2). All studies showed that LUTS improved after treatment of 6 months (except Kim et al [17] which demonstrated improvement after 3 months). While the change in score after 6 months was used for Yoon et al's study [15] for uniformity, their results showed a continuous improvement in IPSS score at 2 years of treatment. In this study, the mean IPSS at month of treatment was 20, compared to 11.5 after 2 years of treatment. A visual summary detailing the spread of means was used rather than a meta-analysis given the studies were mostly descriptive ordinal scales and lacked measures of variability. The median of all six studies is less susceptible to outliers due to the small sample size.
Fig. 2. Graphical summary of mean change from baseline in International Prostate Symptom Score with alfuzosin 10 mg daily treatment.
4. Changes in Male Sexual Health Questionnaire ejaculatory dysfunction after treatment
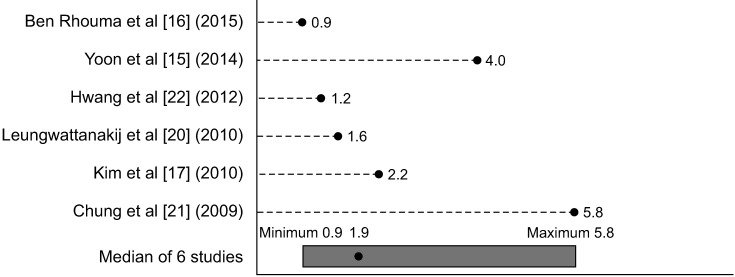
MSHQ-EjD scores also increased after alfuzosin treatment, correlating to an improvement in ejaculatory function. Median increase of MSHQ-EjD SF score was 1.9, range: 0.9 to 5.8 (Fig. 3). As above, Yoon et al [15] also demonstrated a continued improvement in MSHQ-EjD-SF scores from 6 months to 2 years with a mean increase of 5.
Fig. 3. Graphical summary of mean change from baseline in functional Male Sexual Health Questionnaire ejaculatory dysfunction score with alfuzosin 10 mg daily treatment.
DISCUSSION
The present study is the first review that links alfuzosin and improved ejaculatory function using the validated MSHQ-EjD questionnaire. As well as providing a more ejaculate-friendly option to the medical management of BPH, the review further consolidates the growing body of evidence that BPH and sexual dysfunction are inherently linked. In order to better appreciate the reason why these symptoms coexist, it is imperative to expel the idea that EjD is merely an effect of ‘retrograde’ ejaculation. While 0.8 mg of tamsulosin resulted in anejaculation in one third of patients and reduced ejaculatory volume in 90%, there was no significantly difference in post-ejaculation urinary sperm concentrations when compared to placebo or patients prescribed alfuzosin [11]. Another study performed by 17 Korean urologists also demonstrated that daily 0.2 to 0.4 mg tamsulosin significantly decreased ejaculate volume with no sperm found in mid-stream urine samples collected after ejaculation [23]. While some may consider this reduced post-coital ‘clean-up’ an advantage, decreased stimuli in the posterior urethra can significantly dampen the male orgasm and have pervasive effects on relationships and quality of life [24].
While the precise mechanism of alfuzosin and other α1-blockers on EjD is unclear, the effect is likely multifactorial. Alpha1-blocking medications for BPH differentially bind to α1A, α1B, and α1D receptor subtypes thereby producing different side effects. For example, the majority (70%) of the α1-adrenergic receptors in the prostate are of the α1A subtype [25]. Tamsulosin's differential affinity for α1A-adrenoreceptors in the prostate and α1B-receptors of cardiovascular smooth muscle is approximately 15.8:1 [26], thereby improving LUTS without significant hypotensive effects. As such, tamsulosin is thought to reduce pressure generated within the vas deferens and seminal vesicles thereby impairing emission phase of ejaculation [27]. This was demonstrated in animal models, where tamsulosin administration significantly decreased intra-seminal vesicle pressure of rats, compared to placebo [28]. Interestingly, this effect was not observed when our rodent relatives where given alfuzosin [28], even though alfuzosin's selectivity for α1A-receptor compared to α1B is significantly lower than tamsulosin (0.31:1 versus 15.8:1, respectively) [26].
A differential central inhibitory effect of ejaculation may account for this discrepancy between uroselective alpha blockers. Unlike alfuzosin, tamsulosin readily crosses the blood-brain barrier and bind to dopaminergic and/or serotonergic receptors that are integrally involved in the central coordination of ejaculation [28]. In animal models, tamsulosin demonstrated a binding affinity for 5-HT1A and D2-like receptors almost 10,000 times greater than other ABs and significantly decreased bulbuspongiosus contractions in male rats [29]. While it is conceivable that alfuzosin has less sexual side effects due less central inhibition, the mechanism behind improved ejaculatory function is only speculative. In one study, alfuzosin was found to fully relax rodent cavernosal tissue in vitro [30], highlighting the potential link between EjD and ED. Other studies have also demonstrated IPSS-score improvements using tadalafil for men with BPH [31]. While the vasodilatory effect is similar between tadalafil's phosphodiesterase 5-inhibiting mechanism and alfuzosin's blockage of peripheral α1B-adrenoreptor, an explanation for the improvement in MSHQ-EjD scores remains elusive.
The strengths of the present review include its demonstration of homogenous results, showing similar trends in both IPSS and MSHQ-EjD-SF scores. The review was also based on a predefined and specific search strategy undertaken separately by two reviewers. Despite this, there are a number of limiting factors that this systematic review was unable to address. As there are no randomised control trials measuring ejaculatory function using validated scoring systems, all selected studies were open, non-comparator cohort studies with a single treatment arm. Meta-analysis was therefore unable to be performed and confounding factors for ejaculatory function, such as age and other comorbidities, could not be controlled for. It is also important to note that a proportion of patients lost to follow-up experienced adverse effects that lead to alfuzosin discontinuation. This may indicate that certain population groups may have different results with alfuzosin. Interpretation of the findings in publications of non-English language may also be affected by the use of an online translator rather than professional interpreter.
We were only able to quantify change of IPSS and MSHQ-EjD-SF after a certain duration of treatment, given that included trials report variable length of follow-up. As α1-blocker therapy is known to provide maximum symptom control anywhere from weeks to months after treatment initiation, shorter trials like Kim et al [17] may not accurately reflect true treatment effect. Conversely, Yoon et al [15] demonstrated continual improvement of LUTS and ejaculatory function from 6 months to 2 years of treatment.
Despite using a validated questionnaire, the review assumes that a given change in score represents a similar improvement in ejaculatory function. For example, a score from 1 to 3 is assumed to have the same impact for a patient with a score increase from 15 to 17. Unlike the IPSS which has 3 clinical categories of severity, the MSHQ-EjD-SF does not. Rather, it is a combination of different aspects of EjD with one bother item, Question 4: “If you have had any ejaculation difficulties or have been unable to ejaculate, have you been bothered by this?” Minimal clinically important difference (MCID) based on MSHQ-EjD-SF score changes have not been formally established or published and therefore must be interpreted with caution. Therefore, a median score increase of 1.9 in this review, may or may not correlate to clinical improvement. Furthermore, the studies inconsistently assessed ejaculatory bother with only Hwang et al [22] documenting changes in the different domains included within the MSQH-EjD-SF.
Despite this, we can consider total MSHQ-EjD-SF scores as an acceptable, albeit imperfect, quantification of an otherwise subjective experience. While the baseline severity of symptoms was variable in this patient group, influencing the outcome of MCID, none of the studies indicated a decrease in ejaculatory function. This result shows that alfuzosin is arguably superior to other α1-blockers by providing LUTS relief without any adverse sexual side effects.
CONCLUSIONS
This novel review of alfuzosin demonstrates a homogenous improvement of LUTS without a detrimental effect on ejaculatory function. While we lack a precise understanding of how LUTS is linked with ejaculatory function, alfuzosin appears to even ameliorate ejaculatory function. Although, the conclusions of this review should be interpreted with caution given the overall moderate risk of bias. Importantly, the present findings serve as a firm reminder that urologists should discuss the different sexual side effects of available α1-blocker therapy.
Appendix
Male Sexual Health Questionnaire Ejaculatory Function (MSHQ-EjD) Short Form

Footnotes
Conflict of Interest: Henry H. Woo has served on advisory boards for Astellas. The anothor authors have no potential conflicts of interest to disclose.
- Conceptualisation, data curation, formal analysis, investigation, methodology: HELY, SJS, HHW.
- Supervision: RJC, HHW.
- Writing — original draft: HELY, HHW.
- Writing — review and editing: all authors.
References
- 1.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol. 1999;162:1301–1306. [PubMed] [Google Scholar]
- 3.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, McConnell JD, Lieber M, Kaplan S, Geller J, Malek GH, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999;53:473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
- 5.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Vallancien G, Emberton M, Harving N, van Moorselaar RJ. Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol. 2003;169:2257–2261. doi: 10.1097/01.ju.0000067940.76090.73. [DOI] [PubMed] [Google Scholar]
- 7.Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005;96:1339–1354. doi: 10.1111/j.1464-410X.2005.05831.x. [DOI] [PubMed] [Google Scholar]
- 8.Fourcade RO, Théret N, Taïeb C. Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int. 2008;101:1111–1118. doi: 10.1111/j.1464-410X.2008.07498.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RC, Fitzpatrick JM. Ejaculatory dysfunction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. BJU Int. 2009;104:974–983. doi: 10.1111/j.1464-410X.2009.08503.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawabe K, Yoshida M, Homma Y Silodosin Clinical Study Group. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–1024. doi: 10.1111/j.1464-410X.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176:1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lefèvre-Borg F, O'Connor SE, Schoemaker H, Hicks PE, Lechaire J, Gautier E, et al. Alfuzosin, a selective alpha 1-adrenoceptor antagonist in the lower urinary tract. Br J Pharmacol. 1993;109:1282–1289. doi: 10.1111/j.1476-5381.1993.tb13762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology. 2007;69:805–809. doi: 10.1016/j.urology.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777–782. doi: 10.1016/j.urology.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 15.Yoon S, Choi JH, Lee SH, Choi SM, Jeh SU, Kam SC, et al. Efficacy of long-term daily dosage of alfuzosin 10 mg upon sexual function of benign prostatic hypertrophy patients: two-year prospective observational study. World J Mens Health. 2014;32:133–138. doi: 10.5534/wjmh.2014.32.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Rhouma S, H'sairi M, Adbi H, Binous MY, Nouira Y, Ben Raies N, et al. Impact of alfuzosin 10 mg once daily on quality of life in Tunisian patients with lower urinary symptoms suggestive of benign prostatic hyperplasia. Tunis Med. 2015;93:164–169. [PubMed] [Google Scholar]
- 17.Kim MK, Cheon J, Lee KS, Chung MK, Lee JY, Lee SW, et al. An open, non-comparative, multicentre study on the impact of alfuzosin on sexual function using the Male Sexual Health Questionnaire in patients with benign prostate hyperplasia. Int J Clin Pract. 2010;64:345–350. doi: 10.1111/j.1742-1241.2009.02247.x. [DOI] [PubMed] [Google Scholar]
- 18.Martín-Morales A, Meyer G, Ramírez E. Prevalence of ejaculatory dysfunction secondary to alpha-blocker therapy in patients with benign prostatic hyperplasia. Actas Urol Esp. 2008;32:705–712. doi: 10.1016/s0210-4806(08)73918-4. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leungwattanakij S, Watanachote D, Noppakulsatit P, Petchpaibuol T, Choeypunt N, Tongbai T, et al. Sexuality and management of benign prostatic hyperplasia with alfuzosin: SAMBA Thailand. J Sex Med. 2010;7:3115–3126. doi: 10.1111/j.1743-6109.2010.01743.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung BH, Lee JY, Kim CI, Kim CS, Oh CY, Lee SW, et al. Sexuality and the management of BPH with alfuzosin (SAMBA) trial. Int J Impot Res. 2009;21:68–73. doi: 10.1038/ijir.2008.62. [DOI] [PubMed] [Google Scholar]
- 22.Hwang TI, Chu SH, Lin MS, Chen CS, Lee LM, Chang HC, et al. Impact of alfuzosin on sexual function in Taiwanese men with benign prostatic hyperplasia. Kaohsiung J Med Sci. 2012;28:429–434. doi: 10.1016/j.kjms.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol. 2006;13:1311–1316. doi: 10.1111/j.1442-2042.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 24.Stojanović N, Ignjatović I, Djenić N, Bogdanović D. Adverse effects of pharmacological therapy of benign prostatic hyperplasia on sexual function in men. Srp Arh Celok Lek. 2015;143:284–289. doi: 10.2298/sarh1506284s. [DOI] [PubMed] [Google Scholar]
- 25.Henkel R, Bastiaan HS, Schüller S, Hoppe I, Starker W, Menkveld R. Leucocytes and intrinsic ROS production may be factors compromising sperm chromatin condensation status. Andrologia. 2010;42:69–75. doi: 10.1111/j.1439-0272.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin DJ, Lluel P, Guillot E, Coste A, Jammes D, Angel I. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J Pharmacol Exp Ther. 1997;282:228–235. [PubMed] [Google Scholar]
- 27.Mirone V, Sessa A, Giuliano F, Berges R, Kirby M, Moncada I. Current benign prostatic hyperplasia treatment: impact on sexual function and management of related sexual adverse events. Int J Clin Pract. 2011;65:1005–1013. doi: 10.1111/j.1742-1241.2011.02731.x. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano F, Bernabe J, Droupy S, Alexandre L, Allard J. A comparison of the effects of tamsulosin and alfuzosin on neurally evoked increases in bladder neck and seminal vesicle pressure in rats. BJU Int. 2004;93:605–608. doi: 10.1111/j.1464-410x.2003.04674.x. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano F. Impact of medical treatments for benign prostatic hyperplasia on sexual function. BJU Int. 2006;97 Suppl 2:34–38. doi: 10.1111/j.1464-410X.2006.06104.x. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 30.Barqawi AB, Myers JB, O'Donnell C, Crawford ED. The effect of alpha-blocker and 5alpha-reductase inhibitor intake on sexual health in men with lower urinary tract symptoms. BJU Int. 2007;100:853–857. doi: 10.1111/j.1464-410X.2007.07092.x. [DOI] [PubMed] [Google Scholar]
- 31.Oelke M, Shinghal R, Sontag A, Baygani SK, Donatucci CF. Time to onset of clinically meaningful improvement with tadalafil 5 mg once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: analysis of data pooled from 4 pivotal, double-blind, placebo controlled studies. J Urol. 2015;193:1581–1589. doi: 10.1016/j.juro.2014.11.094. [DOI] [PubMed] [Google Scholar]