Abstract
Purpose
Sesquiterpene lactones, which are found in plants of the Asteraceae family, contain costunolide (CO) and dehydrocostus lactone (DCL) as indicator material. CO, in particular, has been reported to possess varied pharmacological activity, including anti-inflammatory, antibacterial, and antioxidant effects. This study was designed to characterize the effects of CO and DCL on benign prostate hyperplasia (BPH).
Materials and Methods
Rats were injected subcutaneously daily for 8 weeks with 5 mg/kg testosterone to induce prostatic hyperplasia. Wistar rats were randomly divided into 5 groups of 10 animals each and received the following treatment: I. Normal control group; II. BPH-induced group; III. CO group (0.075 mg/kg); IV. DCL group (0.075 mg/kg); and V. Finasteride group (0.8 mg/kg). After treatment, changes in prostate weight and serum biochemical indices, serum dihydrotestosterone level, and mRNA levels of BCL2 were measured and histological examinations performed.
Results
Absolute and relative prostate weight in the indicator material treated groups, as well as prostate volume, decreased compared to those in the disease-induced group. Epithelial cell thickness increased significantly in the disease-induced group, with a significant decrease being observed in the CO group. The level of the anti-apoptotic protein BCL2 (B-cell lymphoma 2) tended to decrease to a greater extent in the DCL group than in the disease-induced group.
Conclusions
In this study, we confirmed that the indicator materials (CO and DCL) can help suppress the development of BPH.
Keywords: Benign prostatic hyperplasia, Costunolide, Dehydrocostus lactone, Dihydrotestosterone, Testosterone
INTRODUCTION
Benign prostatic hyperplasia (BPH), common in the elderly men patients with urinary diseases, is increasing gradually due to aging and the westernization of dietary patterns. The incidence of moderate to severe lower urinary tract symptoms is high [1,2,3] in men aged 40 or older and increases by 40% to 70% in men aged 60 to 70. Prostate hyperplasia is known to be closely related to male hormone levels and deterioration of endocrine function due to the aging process. Typical symptoms include delayed urination, urinary incontinence, nocturia, and lower urinary tract symptoms. Lower urinary tract symptoms resulting from prostate enlargement not only deteriorate male sexual function due to erectile dysfunction, but also lower their overall quality of life; consequently, lower urinary tract symptoms are closely related to depression [3]. Prostate hypertrophy, which affects both physical and mental functions in older men, has become an important medical problem [4]. As economic and social conditions have improved in recent years, there has been a growing public interest in and a study for BPH. Although the blood testosterone concentration decreases with age, the activity of intracellular steroid 5 alpha-reductase type 2 is maintained at a high level and testosterone conversion to its highly biologically active metabolite, dihydrotestosterone (DHT), is increased [5]. Increased DHT binds to the androgen receptor (AR), the male hormone receptor, which promotes the production of prostate-specific antigen (PSA) [6]. In other words, as the aging of the male progresses, the secretion of male hormones produced in the testis is reduced, and AR is increased to maintain the balance of the endocrine system, and DHT binds to more parts, resulting in enlargement of the prostate [7]. Increased levels of PSA, commonly observed in patients with prostate hypertrophy, are treated with 5 alpha-reductase inhibitors, such as finasteride and dutasteride. It is known that increased DHT reduces binding to AR and decreases PSA levels [8,9].
Apoptosis is one of the mechanisms that inhibits the proliferation of cancer cells in a way that cells are controlled by genes and die. Survivin, an apoptosis-suppressing gene, has been reported to be increased in patients with prostate cancer and prostatic hyperplasia [10]. It is known that BCL-2, which plays an important role in the apoptosis process, is expressed in the prostate epithelium.
Prostate hyperplasia is characterized histologically by progressive hyperplasia of the peri-urethral anastomosis and excessive proliferation of stromal tissue in the prostate transitional zone [11]. Additionally, there is evidence that specimens with induced prostate hypertrophy show inflammatory infiltration, suggesting that prostatectomy may be an inflammatory disease and that reducing inflammation may be important in BPH therapy [12].
Sesquiterpene lactones are found predominantly in plants of the Asteraceae family, and include costunolide (CO) and dehydrocostus lactone (DCL) as indicator materials [13,14,15]. CO, one of the major sesquiterpene lactones, has been shown to possess a variety of pharmacological properties, such as anti-inflammatory, antimicrobial, and antiviral activity [16,17,18], as well as being a strong antioxidant that prevents blood vessel oxidation [19]. Another study demonstrated that sesquiterpene lactone, including CO and DCL, strongly inhibited nitric oxide production in lipopolysaccharide-induced macrophages and inhibited inducible nitric oxide synthase and tumor necrosis factor-α expression by inhibiting nuclear factor-κB activation [18].
Although many studies on the efficacy of sequiterpene lactone have been reported, the efficacy of prostate hypertrophy is unknown. In this study, therefore, we investigated the potential of CO and DCL as treatments for BPH in a previous study on sesquiterpene lactones.
MATERIALS AND METHODS
1. Experimental material
CO was purchased from Selleckchem (S1319; Houston, TX, USA) and DCL was purchased from ChemFaces (CFN98720; Wuhan, China). Testosterone undecanoate was purchased from Bayer Co., Ltd (Nebido®; Seoul, Korea). Finasteride was purchased from MSD (Proscar®; Seoul, Korea). Specific pathogen-free Wistar rats (n=60) were purchased from Samtaco Bio Co. (Osan, Korea). Male Wistar rats weighing 320 to 350 g were used and housed in plastic cages containing two rats each. All test animals had ad libitum access to food and water for 16 weeks after an acclimatization period of 1 week prior to the start of the experiment. The housing room was maintained at 22℃±2℃, 50%±5% humidity, and a 12-hour light/dark cycle.
2. Ethics statement
All experimental animals were treated in accordance with ethical guidelines issued by the Institutional Animal Care and Use Committee (IACUC) of Sahmyook University for the care and use of laboratory animals (No. SYUIACUC 2016-007).
3. Design of a benign prostatic hyperplasia animal model
The animals in this study were randomly assigned to 5 groups of 10 animals each, as follows: group I, normal control group that received distilled water with corn oil; group II, BPH induced by testosterone undecanoate 5 mg/kg; group III, CO (0.075 mg/kg) treatment group; group IV, DCL (0.075 mg/kg) treatment group; and group V, finasteride (0.8 mg/kg) treatment group. As a preliminary experiment to determine the capacity of the group III and IV, the optimal dose was set based on the results of the anti-inflammatory efficacy test (nitric oxide assay) and the cytotoxicity test (MTT assay) (data not shown). Finasteride, a 5 alpha-reductase inhibitor, was used as a positive anti-BPH drug. All the animals, except for those in the normal control group, were castrated through aseptic removal of both testes. All groups except the group I were injected subcutaneously with 5 mg/kg testosterone daily for 8 weeks to induce prostate hyperplasia. During the experiment, testosterone was injected daily to maintain constant prostate hypertrophy, whereas the group I was injected with corn oil. Group III and group IV, suspended in physiological saline, were administered orally at predetermined doses; group V was orally administered finasteride at a dose of 0.8 mg/kg suspended in physiological saline. The group I was treated with distilled water in the same manner as the other experimental groups to exclude the variable due to oral administration stress. Oral administration was administered daily for a total of 8 weeks.
4. Benign prostatic hyperplasia markers
All the rats were euthanized by ether anesthesia after fasting. The prostate, liver, thymus, and spleen were weighed using an electronic scale (CUX220H; CAS Corporation, Seoul, Korea). The prostate index (PI) was calculated as ‘prostate weight/rat body weight×100’. The lengths of the long and short axes were measured using a digital caliper and the prostate volume was then calculated using the following equation: 1/2 (a×b2) where a and b indicate the long and short axes, respectively [20].
5. Serum biochemical indices
Blood for biochemical analysis was obtained via the abdominal vein. After incubation for 30 minutes at 20℃, serum was separated by centrifugation at 10,000 rpm for 5 minutes. The isolated serum was stored at −70℃ and measured for various parameters, namely, aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatinine, total cholesterol, high density lipoprotein (HDL), and low density lipoprotein (LDL) levels, using a blood biochemical analyzer (AU480; Beckman Coulter, Brea, CA, USA).
6. Measurement of serum dihydrotestosterone levels
Analysis of serum DHT levels was performed using the blood collected from the test animals after autopsy. The serum was separated by centrifugation at 10,000 rpm at 4℃ for 5 minutes and DHT levels were detected using a DHT ELISA kit (Rat DHT ELISA kit; Cusabio, Barksdale, DE, USA). All analyses were performed according to the manufacturer's protocol and the values were represented as pg/mL.
7. RNA isolation and real-time polymerase chain reaction
Total RNA (a total of 200 ng) was isolated from prostate tissues using a total RNA purification kit (GeneAll Hybrid-R™; GeneAll, Seoul, Korea). For cDNA synthesis, the extracted 1 µg RNA was mixed with RT-premix (GeneAll HyperScript™, GeneAll) and oligo (dT) 16 primer. The conditions used were 42℃ for 5 minutes, 55℃ for 60 minutes, and 95℃ for 5 minutes. The primer sequences for GAPDH (glyceraldehyde 3-phosphate dehydrogenase) used as housekeeping gene and BCL-2 are shown in Table 1. Real-time polymerase chain reaction (PCR) (AB StepOnePlus™ System, Waltham, MA, USA) was performed using a PCR Master mix (AB Power SYBR® Green, Waltham, MA, USA), 0.5 µM of each primer, and synthesized cDNA. The results were analyzed using the StepOnePlus™ real-time PCR system software, with GAPDH as a control for the amplified genes.
Table 1. Primer sequences (5′-3′) for real-time PCR analysis.
| Primer name | Sequence of primers (5′–3′) | Annealing temperature (°C) | |
|---|---|---|---|
| BCL-2 | Forward | ACA AAG GCA TCC CAG CCT CC | 55 |
| Reverse | TGG TGG AGG TGC TCT TCA GG | ||
| GAPDH | Forward | CAA CTT TGG CAT TGT GGA AGG | 55 |
| Reverse | ATG GAA ATT GTG AGG GAG ATG C |
PCR: polymerase chain reaction, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
8. Histological examinations
The size and weight of rat prostate tissues were measured after the experiment, and the tissues were then fixed in a 10% formalin solution for histopathological evaluation. Each tissue was cut into 4- to 5-µm thick paraffin blocks. Histopathological slides were prepared and stained with H&E. Changes in prostate surface area and epithelial thickness were determined by optical microscopy (Olympus, Tokyo, Japan).
9. Statistical analysis
All statistical analyses were performed using GraphPad Prism® ver. 5.0 (GraphPad Software, San Diego, CA, USA). Significant differences between groups were verified by one-way analysis of variance (ANOVA) and Dunnett's multiple comparison tests. All data are presented as the mean±standard error of the mean. Values of p<0.05 were considered statistically significant.
RESULTS
1. Body weight change
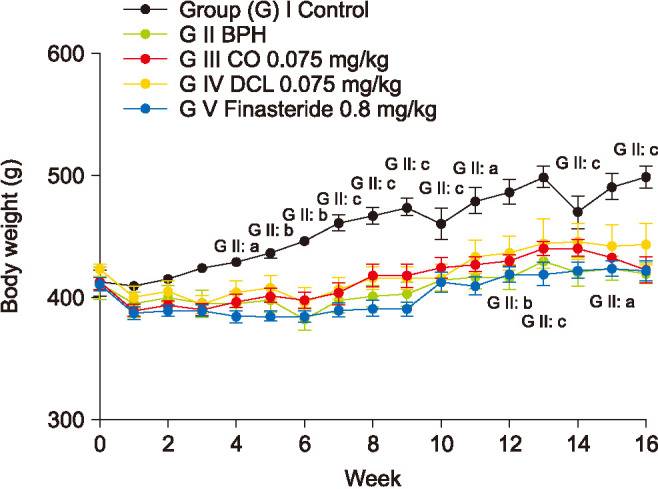
The body weight of rats in each group was measured every week during the experimental period. Body weight in the disease-induced group showed a gradual decrease compared to the normal control group (Fig. 1). There was no significant difference in body weight between the indicator material-treated groups and the finasteride-treated group.
Fig. 1. A rat model of benign prostate hyperplasia (BPH) was induced by daily injections of 5 mg/kg testosterone for 16 weeks after castration. Then, 0.075 mg/kg costunolide (CO), 0.075 mg/kg dehydrocostus lactone (DCL), or 0.8 mg/kg finasteride was administered orally to the rats for 8 weeks after induction. Values are expressed as mean±standard error of the mean (n=6). ap<0.05, bp<0.01, cp<0.001 compared to the control group. Statistical analyses were performed by ANOVA and Dunnett's multiple comparison test.
2. Inhibitory effect of indicator material on prostate hyperplasia
Prostate weight, PI, and prostate volume were significantly increased in the disease-induced group (1.87±0.17 g, 0.45%±0.04%, 6,471±456 mm3, respectively) compared to the normal control group (1.09±0.10 g, 0.22%±0.02%, 2,589±576 mm3, respectively). The values for the three parameters were significantly reduced in both the CO and finasteride groups compared to the disease-induced group (p<0.001, in each group) (Table 2).
Table 2. Prostate profile.
| Variable | Control | Testosterone treatment | |||
|---|---|---|---|---|---|
| BPH | CO (0.075 mg/kg) | DCL (0.075 mg/kg) | Finasteride (0.8 mg/kg) | ||
| Prostate weight (g) | 1.09±0.10 | 1.87±0.17a | 1.40±0.22b | 1.66±0.16 | 1.42±0.07b |
| Prostate index (%) | 0.22±0.02 | 0.45±0.04a | 0.32±0.05b | 0.40±0.05 | 0.34±0.02b |
| Prostate volume (mm3) | 2,589±576 | 6,471±456a | 4,135±679b | 5,399±790 | 4,084±437b |
Values are presented as mean±standard error of the mean (n=6). A rat model of benign prostate hyperplasia (BPH) was induced with daily injections of 5 mg/kg testosterone for 16 weeks after castration; then, 0.075 mg/kg costunolide (CO), 0.075 mg/kg dehydrocostus lactone (DCL), or 0.8 mg/kg finasteride was administered orally to the rats for 8 weeks after induction. Prostate weight and prostate index ‘prostate weight (g)/body weight (g)×100’ were calculated, and prostate volume was measured using a digital caliper (mm3).
ap<0.001 compared to the control group; bp<0.001 compared to the BPH group. Statistical analyses were conducted using ANOVA and Dunnett's multiple comparison test.
3. Comparison of changes in organ weights
Liver weight decreased significantly in the disease-induced group (9.57±0.49 g) compared to the normal control group (11.28±0.87 g). Liver weight in the CO- and DCL-treated groups also decreased compared to the normal control group, but with no significant difference. Spleen weights were similar in all groups (Table 3).
Table 3. Liver and spleen weights.
| Variable | Control | Testosterone treatment | |||
|---|---|---|---|---|---|
| BPH | CO (0.075 mg/kg) | DCL (0.075 mg/kg) | Finasteride (0.8 mg/kg) | ||
| Liver weight (g) | 11.28±0.87 | 9.57±0.49a | 10.72±1.71 | 9.38±0.73 | 9.46±0.79 |
| Spleen weight (g) | 0.72±0.10 | 0.62±0.04 | 0.62±0.14 | 0.67±0.09 | 0.70±0.12 |
Values are presented as mean±standard error of the mean (n=6). A rat model of benign prostate hyperplasia (BPH) was induced with daily injections of 5 mg/kg testosterone for 16 weeks after castration. Then, 0.075 mg/kg costunolide (CO), 0.075 mg/kg dehydrocostus lactone (DCL), or 0.8 mg/kg finasteride was administered orally to the rats for 8 weeks after induction.
ap<0.05 compared to the control group. Statistical analyses were conducted using ANOVA and Dunnett's multiple comparison test.
4. Biochemical index
The levels of AST, ALP, and creatinine were measured to determine the toxicity of the tested substances for liver and kidney function. Total cholesterol, HDL, and LDL levels were also evaluated. CO and DCL did not promote the activity of the serum toxicity marker enzymes, such as AST and ALP. It is indicated that rats of each group had a normal function of livers. In addition, creatinine levels did not increase, so there was no problem in nephrotoxicity. Total cholesterol levels were similar in all the groups. The levels of HDL were similar in all groups, except for the finasteride-treated group where a significant difference was recorded compared to the disease-induced group (p<0.05). The levels of LDL showed a similar tendency in all groups (Table 4).
Table 4. Biochemical index.
| Variable | Control | Testosterone treatment | |||
|---|---|---|---|---|---|
| BPH | CO (0.075 mg/kg) | DCL (0.075 mg/kg) | Finasteride (0.8 mg/kg) | ||
| AST (U/L) | 96.0±18.5 | 125.2±46 | 78.3±21.3a | 89.5±9.3 | 80.7±15.3a |
| ALP (U/L) | 91.5±21.8 | 85.7±22.2 | 61.2±12.1 | 77.5±13.7 | 81.5±17.7 |
| Creatinine (mg/dL) | 0.4±0.1 | 0.4±0.1 | 0.3±0.1a | 0.3±0 | 0.3±0.1a |
| Total cholesterol (mg/dL) | 93.3±10.4 | 88.2±11.1 | 79.7±14.9 | 76.8±17.6 | 70.8±9.7 |
| HDL (mg/dL) | 60.0±5.2 | 56.2±5.4 | 51.5±8.3 | 49.0±10.3 | 43.8±6.7a |
| LDL (mg/dL) | 12.7±1.6 | 13.5±1.9 | 13.2±2.6 | 14.7±4.3 | 13.7±2.5 |
Values are expressed as the mean±standard error of the mean (n=6). Serum biochemical indexes were analyzed using a biochemical analyzer.
CO: costunolide, DCL: dehydrocostus lactone, AST: aspartate aminotransferase, ALP: alkaline phosphatase, HDL: high density lipoprotein, LDL: low density lipoprotein.
ap<0.05 compared to the benign prostate hyperplasia (BPH) group. Statistical analyses were performed using ANOVA and Dunnett's multiple comparison test.
5. Determination of dihydrotestosterone levels
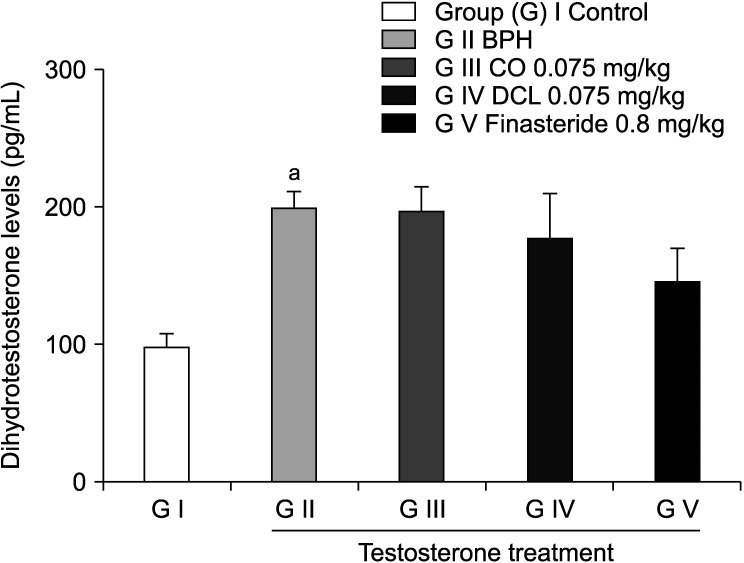
The serum DHT level increased in the disease-induced group compared to the normal control group, but with no statistically significant difference. DHT levels in the CO and DCL treated groups also did not show a statistically significant trend compared to those in the disease-induced group (Fig. 2).
Fig. 2. Serum levels of dihydrotestosterone. Dihydrotestosterone levels were measured using a commercial ELISA kit. Values are expressed as mean±standard error of the mean (n=6). ap<0.001 compared to the control group. Statistical analyses were performed using ANOVA and Dunnett's multiple comparison test. BPH: benign prostate hyperplasia, CO: costunolide, DCL: dehydrocostus lactone.
6. Influence on the expression of apoptosis factors
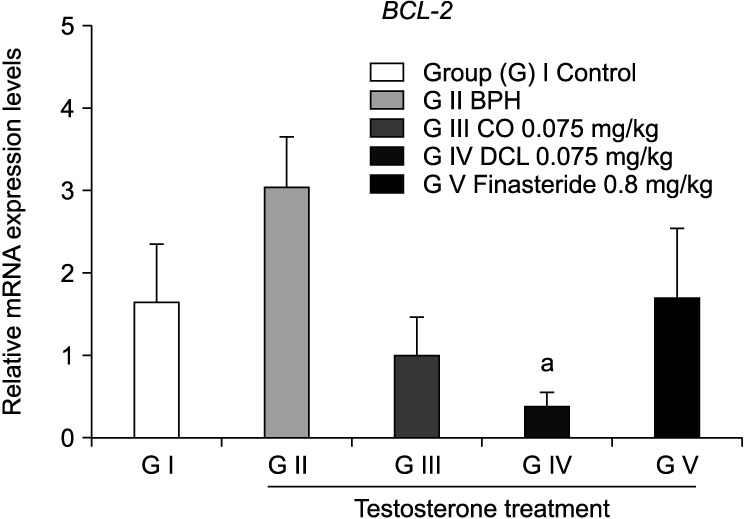
The mRNA expression level of BCL-2 was confirmed by quantitative real-time PCR (Fig. 3). The BCL-2 gene is an oncogene that suppresses the expression of apoptosis factors [21]. Our results showed that the BCL-2 mRNA expression level tended to increase in the disease induced group compared to the normal control group, but with no significant difference; however, the level of BCL-2 mRNA in the DCL group was significantly different from that in the disease-induced group.
Fig. 3. Effects of indicator materials (costunolide [CO] and dehydrocostus lactone [DCL]) on the mRNA levels of the apoptosis-related gene BCL-2 in prostate tissues. The mRNA expression levels of the apoptosis-related gene BCL2 in prostate tissues were measured by real-time polymerase chain reaction. Values are expressed as mean±standard error of the mean (n=6). ap<0.05 compared to the benign prostate hyperplasia (BPH) group. Statistical analyses were performed by ANOVA and Dunnett's multiple comparison test.
7. Prostate tissue morphology
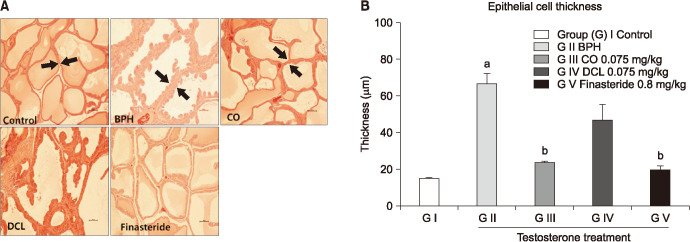
Prostate hypertrophy is characterized by proliferation of epithelial cells and increased area of prostate vesicles [22,23]. The mean epithelial thickness in the disease-induced group was 66.7±11.21 µm, which was significantly higher by approximately four-fold than that in the normal control group (15.8±2.32 µm). The mean epithelial thickness decreased significantly in the CO group compared to the disease-induced group, and decreased insignificantly in the DCL group. The mean epithelial thickness in the finasteride group was 19.84±4.55 µm, representing a significant decrease compared to that recorded for the disease-induced group (Fig. 4).
Fig. 4. Histomorphological changes in the prostate. (A) Representative prostate tissue samples of each group were selected and stained with H&E. Shown are microscopy images of prostate samples obtained at ×200 magnification. (B) Epithelial thickness values are expressed as mean±standard error of the mean (n=6). ap<0.001 compared to the control group. bp<0.001 compared to the benign prostate hyperplasia (BPH) group. Statistical analyses were performed by ANOVA and Dunnett's multiple comparison test. CO: costunolide, DCL: dehydrocostus lactone.
DISCUSSION
In this study, we examined the efficacy of CO and DCL as a potential treatment for prostatic hyperplasia. Both CO and DCL are sesquiterpene lactones that can be extracted as indicator materials from Asteraceae plants. The major efficacies of these materials include anti-angiogenic [13], antimicrobial [16], and anti-inflammatory activity that strongly inhibit nitric oxide production by lipopolysaccharides [17]. CO, in particular, has been shown to inhibit cancer cells through apoptosis in human leukemia cells and rat colorectal cancer [24,25].
In this study, we measured the levels of factors that contribute to prostate hypertrophy and evaluated the effects of key indicators on prostate hyperplasia. Absolute and relative prostate weight and volume decreased in the CO- and DCL-treated groups compared to the disease-induced group, with the CO group showing a statistically significant decrease. Organ weights tended to decrease slightly in all treatment groups compared to the normal group, but with no significant difference. The creatinine levels were measured to identify renal toxicity due to diseases such as lower urinary tract symptoms [4]. The AST and ALP levels were measured to confirm liver toxicity. Total cholesterol, and HDL and LDL levels were also measured. Toxicity tests values outside the normal range were not observed and that liver and kidney functions were normal. Because prostate enlargement is characterized by proliferation of epithelial cells and increased area of prostate vesicles [22,23], we measured changes in epithelial thickness. Epithelial thickness increased significantly in the disease-induced group compared to the normal group, whereas the CO group showed a statistically significant decrease.
The higher the testosterone concentration in the blood of castrated rats, the more DHT is synthesized by 5 alpha-reductase enzymes in the prostate; the synthesized DHT binds to the AR on prostate cells, resulting in prostate enlargement [4]. In this study, therefore, we first measured the levels of DHT in the different treatment groups. In the group treated with finasteride, a 5 alpha-reductase inhibitor, the level of DHT tended to decrease, but no difference in DHT levels was observed in the CO and DCL groups. In this study, DHT level confirmation was analyzed by serum with reference to several other studies [6,20], but it is considered that future studies should be analyzed as prostate tissue. The mRNA expression level of BCL-2, another mechanism, was evaluated by quantitative real-time PCR. After the prostate has grown to adult size, it enters a maintenance phase where proliferation of the prostate cells and death of the cells are balanced by an equal ratio. The pro-apoptotic BCL-2-associated X, apoptosis regulator (BAX) protein migrates to the mitochondrial membrane and inhibits the regulation of anti-apoptotic proteins, thereby depolarizing the mitochondrial membrane and releasing cytochrome c [26,27]. In contrast, BCL-2, an anti-apoptotic protein, is overexpressed in basal and luminal epithelial cells of BPH compared with normal prostate tissue, and deregulation of normal apoptotic cell death mechanisms in prostate. Eventually, cell proliferation causes growth imbalance, resulting in prostatic hyperplasia. Also, BCL-2, an anti-apoptotic protein, plays an important role in regulating apoptosis by inhibiting the depolarization of mitochondrial membranes and increasing membrane oxidative phosphorylation, thereby suppressing mitochondrial permeability. It was also confirmed that CO inhibits BCL-2 expression and plays a role in apoptosis [28]. The BCL-2 protein inhibits apoptosis by blocking permeable transition pores and limiting the release of cytochrome c present in the mitochondria [29]. The ratio of BCL-2 to BAX is important in determining cellular fate, and an increase in the ratio results in abnormal expression of proteins that is commonly observed in patients with BPH [30]. The BCL-2 mRNA expression level was not different in the finasteride group and showed a greater tendency to decrease in the CO and DCL groups than in the disease-induced group. In addition, reports that two major sesquiterpene lactones, CO and DCL, induce apoptosis, suggest that these two substances may reduce the expression of apoptosis-related genes [21].
The BCL-2 mRNA expression level was not different in the finasteride group and showed a greater tendency to decrease in the CO and DCL groups than in the disease-induced group.
In this study, we examined the effect of the sesquiterpene lactones, CO and DCL, on prostate hypertrophy. Both lactones not only reduced prostate weight, index, and volume, but also affected BCL-2 expression. CO, in particular, is thought to play a role in inhibiting the development of prostate hyperplasia as it was shown to have anti-inflammatory effects [17]. In the present study, we concluded that the functions of BCL-2 in apoptosis can ameliorate the symptoms of prostatic hyperplasia through mechanisms other than 5 alpha-reductase inhibition. Nevertheless, further experiments with BAX to confirm the increase of pro-apoptotic protein are needed and establishment of a detailed mechanism of apoptosis induction is necessary.
CONCLUSIONS
In summary, CO and DCL were significantly related to BPH. Prostate hypertrophy was significantly decreased in the CO and DCL treatment groups. Oral administration of the sesquiterpene lactones, CO and DCL, in a rat model of BPH elicited marked reductions in the prostatic index, prostate weight, and epithelial hyperplasia in the serum and prostate, as well as in BCL-2 serum. However, further studies using different solvents as well as treatment concentrations after addition will be necessary for a complete understanding of the effects of CO and DCL on BPH.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: YS, HK.
- Data curation: YS, HK.
- Formal analysis: JA.
- Experimental performance: JK, SH.
- Writing—original draft: DC.
- Writing—review & editing: HK.
Data Sharing Statement: The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/JSSB8L.
References
- 1.Oesterling JE. Benign prostatic hyperplasia: a review of its histogenesis and natural history. Prostate Suppl. 1996;6:67–73. [PubMed] [Google Scholar]
- 2.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim JS, Moon VN. Factors influencing health-related quality of life in patients with benign prostatic hyperplasia. J Korean Acad Nurs. 2010;40:287–297. doi: 10.4040/jkan.2010.40.2.287. [DOI] [PubMed] [Google Scholar]
- 4.Salinas-Sánchez AS, Hernández-Millán I, Lorenzo-Romero JG, Segura-Martin M, Fernández-Olano C, Virseda-Rodriguez JA. Quality of life of patients on the waiting list for benign prostatic hyperplasia surgery. Qual Life Res. 2001;10:543–553. doi: 10.1023/a:1013004602682. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Choi HR, Lee JH, Kwon JW, Lee HK, Jeong JT, et al. Effects of unripe black raspberry extracts on prostate cancer cell line and rat model of benign prostatic hyperplasia. J Korean Soc Food Sci Nutr. 2014;43:507–515. [Google Scholar]
- 7.Schubert M, Jockenhövel F. Late-onset hypogonadism in the aging male (LOH): definition, diagnostic and clinical aspects. J Endocrinol Invest. 2005;28(3 Suppl):23–27. [PubMed] [Google Scholar]
- 8.Lazier CB, Thomas LN, Douglas RC, Vessey JP, Rittmaster RS. Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004;58:130–144. doi: 10.1002/pros.10340. [DOI] [PubMed] [Google Scholar]
- 9.Angwafor F, 3rd, Anderson ML. An open label, dose response study to determine the effect of a dietary supplement on dihydrotestosterone, testosterone and estradiol levels in healthy males. J Int Soc Sports Nutr. 2008;5:12. doi: 10.1186/1550-2783-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shariat SF, Ashfaq R, Roehrborn CG, Slawin KM, Lotan Y. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J Urol. 2005;174:2046–2050. doi: 10.1097/01.ju.0000176459.79180.d1. [DOI] [PubMed] [Google Scholar]
- 11.Hong SJ. Benign prostatic hyperplasia: multiple factors for prostate tissue change with aging. Korean J Urol. 2005;46:547–554. [Google Scholar]
- 12.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Jeong SJ, Itokawa T, Shibuya M, Kuwano M, Ono M, Higuchi R, et al. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002;187:129–133. doi: 10.1016/s0304-3835(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 14.Jeon YJ, Lee HS, Yeon SW, Ko JH, An KM, Yu SW, et al. Inhibitory effects of dehydrocostuslactone isolated from Saussureae radix on CDK2 activity. Korean J Pharmacogn. 2005;36:97–101. [Google Scholar]
- 15.Choe IH, Lee HJ, Choe DH, Park JI, Choe TH. Extractives from Magnolia sieboldii. J Korean Wood Sci Technol. 2004;32:33–39. [Google Scholar]
- 16.Wedge DE, Galindo JC, Macías FA. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry. 2000;53:747–757. doi: 10.1016/s0031-9422(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Jung WT, Basnet P, Kadota S, Namba T. Syringin 4-O-beta-glucoside, a new phenylpropanoid glycoside, and costunolide, a nitric oxide synthase inhibitor, from the stem bark of Magnolia sieboldii. J Nat Prod. 1996;59:1128–1130. doi: 10.1021/np960452i. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H, Toguchida I, Ninomiya K, Kageura T, Morikawa T, Yoshikawa M. Effects of sesquiterpenes and amino acid-sesquiterpene conjugates from the roots of Saussurea lappa on inducible nitric oxide synthase and heat shock protein in lipopolysaccharide-activated macrophages. Bioorg Med Chem. 2003;11:709–715. doi: 10.1016/s0968-0896(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 19.Song JW, Min KJ, Cha CG. Antioxidative and antitumor activity of extracts from Saussurea lappa. J Environ Health Sci. 2008;34:55–61. [Google Scholar]
- 20.Kim YN, Kim MS, Chun SS, Choi JH. Effect of Phellius linteus water extract on benign prostatic hyperplasia. Nutr Res Pract. 2013;7:172–177. doi: 10.4162/nrp.2013.7.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DS, Lee SJ, Lee JC, Woo WH, Lim KS, Mun YJ. Ethanol extract of Saussurea lappa root induces apoptosis through an ROS-MAPKs-Linked cascade. Yakhak Hoeji. 2012;56:173–179. [Google Scholar]
- 22.Shin IS, Lee MY, Jung DY, Seo CS, Ha HK, Shin HK. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem Toxicol. 2012;50:884–888. doi: 10.1016/j.fct.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Babinski MA, Chagas MA, Costa WS, Sampaio FJ. Prostatic epithelial and luminal area in the transition zone acini: morphometric analysis in normal and hyperplastic human prostate. BJU Int. 2003;92:592–596. doi: 10.1046/j.1464-410x.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee MG, Lee KT, Chi SG, Park JH. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol Pharm Bull. 2001;24:303–306. doi: 10.1248/bpb.24.303. [DOI] [PubMed] [Google Scholar]
- 25.Mori H, Kawamori T, Tanaka T, Ohnishi M, Yamahara J. Chemopreventive effect of costunolide, a constituent of oriental medicine, on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1994;83:171–175. doi: 10.1016/0304-3835(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 26.Ju JY, Chung KS, Cheon SY, An HJ. Musulju improves benign prostatic hyperplasia by regulating inflammatory and apoptotic proteins. Mol Med Rep. 2016;14:4692–4698. doi: 10.3892/mmr.2016.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H, Xu W, Lin J, Peng J, Hong Z. Qianliening capsule treats benign prostatic hyperplasia via induction of prostatic cell apoptosis. Mol Med Rep. 2013;7:848–854. doi: 10.3892/mmr.2013.1265. [DOI] [PubMed] [Google Scholar]
- 28.Park HJ, Kwon SH, Han YN, Choi JW, Miyamoto K, Lee SH, et al. Apoptosis-inducing costunolide and a novel acyclic monoterpene from the stem bark of Magnolia sieboldii. Arch Pharm Res. 2001;24:342–348. doi: 10.1007/BF02975104. [DOI] [PubMed] [Google Scholar]
- 29.Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, et al. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999;31:305–319. doi: 10.1023/a:1005419617371. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Kim EJ, Lim DY, Kim JS, Lim SS, Shin HK, et al. Inhibitory effect of the Hexane extract of Saussurea lappa on the growth of LNCaP human prostate cancer cells. J Korean Soc Food Sci Nutr. 2008;37:8–15. [Google Scholar]