Abstract
It is widely accepted that oxidative stress plays an important role in the pathophysiology of male infertility and that antioxidants could have a significant role in the treatment of male infertility. The main objectives of this study are: 1) to systematically review the current evidence for the utility of antioxidants in the treatment of male infertility; and 2) propose evidence-based clinical guidelines for the use of antioxidants in the treatment of male infertility. A systematic review of the available clinical evidence was performed, with articles published on Scopus being manually screened. Data extracted included the type of antioxidant used, the clinical conditions under investigation, the evaluation of semen parameters and reproductive outcomes. The adherence to the Cambridge Quality Checklist, Cochrane Risk of Bias for randomized controlled trials (RCTs), CONSORT guidelines and JADAD score were analyzed for each included study. Further, we provided a Strength Weakness Opportunity Threat (SWOT) analysis to analyze the current and future value of antioxidants in male infertility. Of the 1,978 articles identified, 97 articles were included in the study. Of these, 52 (53.6%) were uncontrolled (open label), 12 (12.4%) unblinded RCTs, and 33 (34.0%) blinded RCTs, whereas 44 (45.4%) articles tested individual antioxidants, 31 (32.0%) a combination of several products in variable dosages, and 22 (22.6%) registered antioxidant products. Based on the published evidence, we 1) critically examined the necessity of additional double-blind, randomized, placebo-controlled trials, and 2) proposed updated evidence-based clinical guidelines for antioxidant therapy in male infertility. The current systematic review on antioxidants and male infertility clearly shows that antioxidant supplementation improves semen parameters. In addition, it provides the indications for antioxidant treatment in specific clinical conditions, including varicocele, unexplained and idiopathic male infertility, as well as in cases of altered semen quality.
Keywords: Antioxidants, Oxidative stress, Practice guideline, Semen analysis, Sperm maturation
INTRODUCTION
Infertility affects approximately 15% of couples globally, with 2.5%–12% believed to be solely due to male factors. The incidence of male factor infertility varies according to the geographical location, ranging from 20% to 70% [1]. The causes of male infertility are diverse, including genetic causes, varicocele, reproductive tract infections, obstructive or non-obstructive azoospermia, male hypogonadism, and anti-sperm antibodies. However, a large proportion of cases remain unexplained (unexplained male infertility, UMI) (±15%) or idiopathic (idiopathic male infertility, IMI) (30%–50%) in the absence of identifiable female factors [2,3]. In addition, numerous environmental and lifestyle factors have been associated with poor reproductive outcomes in males [4]. Importantly, oxidative stress has been established as a significant mediator in many known causes and risk factors of male infertility, and has further been associated with 30% to 80% of IMI cases in a condition termed male oxidative stress infertility (MOSI) [2,5]. Therefore, the use of antioxidants to reduce oxidative stress across a range of etiologies and risk factors of male infertility has gained increasing attention. This is supported by the wide availability of oral antioxidants, excellent safety and bioavailability profiles, and that antioxidants are considered relatively cost effective [5,6]. Therefore, there is a growing trend of prescribing antioxidants to all males with infertility, even without complete evaluation or relevant guidelines [7].
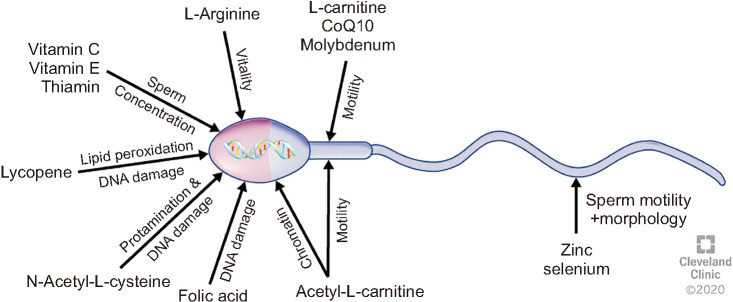
Exogenous administration of antioxidants has been explored for decades, and the effects of several antioxidants on male fertility have been extensively reported. Numerous trials of different qualities using various antioxidants as mono- or poly-formulations, which may include pharmacologically-active herbal extracts, have been reported [8,9,10,11]. The topic was first summarized by a Cochrane meta-analysis in 2011, with updated reviews in 2014 and 2019 [8,9,11]. These reviews investigated the therapeutic benefit of male antioxidant treatment for couples undergoing assisted reproductive technology (ART). Based on limited randomized controlled trials (RCTs), the reviews concluded that low level evidence supports antioxidant therapy in infertile males to increase pregnancy and live birth rates, with no evidence for increased risk of miscarriage [8,9,11]. Majzoub and Agarwal (2018) [10] conducted a systematic review on antioxidant treatment in infertile men, concluding that antioxidants have a positive effect on male fertility, including semen parameters and advanced sperm function, ART outcomes and live birth rates. Antioxidants that are commonly used clinically and investigated scientifically as either an individual application or in combination include vitamin A, vitamin C, vitamin E, carnitine, N-acetyl cysteine, coenzyme Q10 and lycopene, along with important antioxidant co-factors zinc, selenium, and folic acid, as these compounds are significantly involved in essential sperm functions (Fig. 1) [12,13,14,15]. However, the outcomes of clinical trials included in the systematic reviews are not consistent, ranging from clear benefit to no clinical effect of the treatment, or even having significant detrimental effects [16,17,18,19,20]. The reasons for this inconsistency are multifactorial and include: small numbers of participants in the studies, variable treatment regimens, dosages, treatment duration, and the lack of placebo-controlled studies. In addition, many of the trials did not evaluate final reproductive outcomes, such as live birth rate, but only certain specific aspects such as seminal volume, sperm concentration and motility, morphology, seminal levels of reactive oxygen species (ROS) or oxidative stress. Furthermore, these antioxidants were often given in non-proven dosages, thus neglecting the fine bodily redox balance that is necessary for normal physiology, including reproductive functions [21]. If the dosage is too low, the treatment might be ineffective; if it is too high, it can result in an excess of antioxidants causing ‘reductive stress’, which is as detrimental as oxidative stress [22,23]. Reductive stress due to inappropriate antioxidant dosage may lead to infertility [23,24]. In this regard, high dosages of vitamin E have been shown to have adverse effects [20]. Recently more balanced antioxidant formulations have shown promising results whereby seminal oxidative stress was reduced, sperm function improved and pregnancies achieved [25,26].
Fig. 1. Individual antioxidant compounds that have significant effects on sperm functions. CoQ: coenzyme Q.
With the current rationale and increased use of antioxidants to counteract male infertility, and the heterogeneous and inconsistent data currently available, this study aims to: 1) systematically review the current evidence for antioxidant use to ameliorate male infertility; and 2) propose updated evidence-based clinical guidelines for the use of antioxidants in male infertility.
MATERIALS AND METHODS
1. Literature search strategy
In order to support the development of clinical guidelines for antioxidant use in male infertility, a systematic review of the available clinical evidence was performed to systematically identify relevant clinical trials investigating the impact of antioxidant therapy on semen quality. A literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. The Scopus database was chosen as it currently includes over 1.4 billion cited references, with over 70,000 indexed articles (https://www.elsevier.com/solutions/scopus/how-scopus-works/content). The search was conducted on July 15th, 2020 to identify studies investigating the use of antioxidants in the treatment of male infertility, with no restriction on publication date.
The following keyword strings and Boolean Operators were used: TITLE-ABS-KEY (“antioxidant*”) AND TITLE-ABS-KEY (“male infertil*” OR “infertile male*” OR “infertile men” OR “male subfertil*” OR “male steril*” OR “sperm*” OR “seminal” OR “semen”). Further specifications of the search are presented in Supplement Table 1. Automatic filters were used in the database to specifically include only English original articles and exclude other types of publication such as book chapters, conference papers, editorials, notes, letters, short surveys, erratum, and books.
The articles identified through the keyword search were subsequently screened manually by title, keywords and abstract for eligibility. This screening was independently done by three researchers (RF; KL; MKPS), and the number of articles excluded through screening was recorded. Full text articles were then reviewed for eligibility using the inclusion and exclusion criteria provided in Table 1, and the number of articles excluded based on these criteria was recorded.
Table 1. Proposed inclusion and exclusion for article selection.
| Inclusion | Exclusion |
|---|---|
| Human participants | Animal and in vitro studies |
| Antioxidants used as intervention individually or combined | Intervention not clearly reported as an antioxidant |
| Open or controlled clinical trials | Abstracts only, conference abstracts, book chapters, case series, review articles |
| At least one semen parameter (sperm concentration, motility, morphology) and/or sperm function parameters (sperm DNA fragmentation, seminal oxidative stress markers, mitochondrial membrane integrity) reported after antioxidant treatment | Non-english studies |
Data was subsequently extracted from the eligible articles, including the clinical trial design, the type of antioxidant or antioxidant formulation used, the clinical condition under investigation, the evaluation of semen parameters and/or sperm function tests (i.e., sperm DNA fragmentation [SDF], oxidative stress markers, capacitation/acrosome reaction, and zona binding test) as well as reproductive outcomes (i.e., fertilization, implantation, pregnancy, miscarriage, and live birth rates).
2. Evaluation of study quality
The quality of all studies included was evaluated by applying the Cambridge Quality Checklist [28]. Moreover, the quality of RCT was further evaluated by using the Cochrane Risk of Bias [29] and the JADAD score [30], as well as by evaluating the adherence to CONSORT guidelines [31]. Based on a combination of these quality evaluation tools, the studies were categorized into “low” (0) and “high” (1) quality. All uncontrolled studies were considered “low-quality”, in comparison with controlled studies, which were evaluated according to the criteria reported in Supplement Table 2. We have created this scoring system, as no such method was previously reported in the literature.
In addition, the most recent clinical trials reporting the effect of antioxidant treatment on male infertility, published from January 2019 to July 2020, were further ranked based on study design, the sample size analysed, the inclusion/exclusion criteria used for selecting the population, the antioxidant regimen used, the length of treatment, the assessment of oxidative stress markers, pregnancy and live birth rates. This range of time was specifically selected to gain an understanding of the most recent evidence on the antioxidant therapy and the quality of the studies currently conducted. The selection of these criteria was achieved through a consensus among the male infertility experts involved in this study. The system provides a total score of a maximum of 12 points and a classification of the articles in “low” (<6 points) and “high” (≥6 points) quality, as reported in Supplement Table 3.
Clinical recommendations were proposed based on the quality of the evidence, classified as A, B, C, D (Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence; https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-Introduction-2.1.pdf and https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf).
3. Statistical analysis
Statistical analysis was performed using MedCalc statistical software version 19.5.3. (MedCalc Software bv, Ostend, Belgium). Chi-square test was used to evaluate the association between the quality of the study and the outcomes (positive or no/negative effect) due to antioxidant treatment on semen parameters and sperm function such as oxidative stress and SDF are presented in Table 2 [25,26,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121]. A p-value <0.05 was considered statistically significant. When the p-value was ≥0.05, a sample size calculation was carried out to predict the required sample size to attain a statistical significance of p<0.05.
Table 2. Articles investigating the impact of antioxidant treatment on reproductive outcomes.
| Clinical condition | SN | Reference | Clinical trial design | Antioxidant formulation dosage and length of treatment | Study population | Reproductive outcomes after antioxidant treatment | Cambridge Quality Checklist | Cochrane Risk of Bias for RCT | CONSORT Guidelines (out of 25) | JADAD (Oxford Quality) (out of 5) | Quality of evidence published | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Checklist for correlate (out of 5) | Checklist for risk factors (out of 3) | Checklist for causal risk factors (out of 7) | |||||||||||
| Infertile men | 1 | Kessopoulou et al (1995) [19] | RCT blinded | α-tocopheryl acetate (Ephynal, F. Hoffman-La Roche ltd) 300 mg/daily for 3 months | 30 infertile men | No difference in semen parameters before and after treatment | 2 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources and blinding (outcome assessment) | 20 | 4 | 1 |
| No difference in ROS levels | |||||||||||||
| Improved zona binding | |||||||||||||
| 2 | Roseff (2002) [32] | Uncontrolled (open label) | Pycnogenol (Horphag Research, Geneve, Switzerland) 200 mg daily for 3 months | 19 infertile men | No difference in semen parameters | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Improved sperm binding capacity | |||||||||||||
| 3 | Keskes-Ammar et al (2003) [18] | RCT unblinded | Vitamin E (400 mg) (Ephynal 100 mg, 2 tablets) or selenium (225 μg) for 3 months | 54 infertile men | Improved sperm motility | 1 | 3 | 4 | No risk of bias identified | 12 | 3 | 0 | |
| Reduced MDA levels | |||||||||||||
| 4 | Tremellen et al (2007) [33] | RCT blinded | Menevit (Bayer, Sydney, Australia) 1 capsule/day for 3 months | Men with evidence of seminal oxidative stress and SDF>25% by TUNEL. The total number of patients is not clearly reported. | No differences between treated and placebo for fertilization, implantation, pregnancy, and miscarriage rates | 0 | 3 | 7 | No risk of bias identified | 19 | 3 | 0 | |
| Live pregnancy rate higher in treated patients | |||||||||||||
| 5 | Ménézo et al (2007) [20] | Uncontrolled (open label) | Vitamins C and E (400 mg each), ß-carotene (18 mg), zinc (500 μmoL), selenium (1 μmoL) for 3 months | 58 patients experiencing 2 previous failures of IVF or ICSI, and DFI and chromatin decondensation>15% | Reduced SDF but higher sperm decondensation | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 6 | Tunc et al (2009) [34] | Uncontrolled (open label) | Menevit (Bayer Australia Ltd, Sydney, Australia) 1 capsule/daily for 3 months for a maximum of 3 months | 50 infertile men with high OS | No difference in semen parameters | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced SDF, ROS and apoptotic markers | |||||||||||||
| Improved DNA protamination | |||||||||||||
| 7 | Shukla et al (2010) [35] | Uncontrolled (open label) | Mucuna pruriens seed powder 5 g/daily for 3 months | 120 infertile men | Improved sperm count and motility, seminal plasma lipid peroxide levels, SOD, catalase, GSH and ascorbic acid | 1 | 3 | 6 | N/A | N/A | N/A | 0 | |
| 8 | da Silva et al (2013) [17] | RCT blinded | Folic acid 5 mg/daily for 3 months | 70 infertile men | No difference in semen parameters | 3 | 3 | 7 | No risk of bias identified | 15 | 5 | 1 | |
| 9 | Bejarano et al (2014) [36] | Uncontrolled (open label) | Melatonin 6 mg/daily for 45 days | 30 infertile men | Improved semen parameters, urinary and semen TAC | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced SDF | |||||||||||||
| Improved embryo quality | |||||||||||||
| 10 | Martínez-Soto et al (2016) [37] | Uncontrolled (open label) | 1.5 g capsules of docosahexaenoic acid oil/daily for 10 weeks | 57 infertile men | No changes in semen parameters | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Increased seminal TAC, reduced DNA damage | |||||||||||||
| 11 | Chattopadhyay et al (2016) [16] | Uncontrolled (open label) | L-Carnitine, Acetyl-L-Carnitine, CoQ10, Lycopene, Zinc, Folic acid, Vitamin B12, Selenium, Fructose, and citric acid (dosage not reported) for 6 months | 115 infertile men | Increased sperm count, motility, TAC | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced ROS levels | |||||||||||||
| 12 | Hosseini et al (2016) [38] | RCT blinded | Ginger powder 250 mg/daily for 3 months | 100 patients with SDF≥15% | No difference in semen parameters | 2 | 3 | 7 | Unclear risk of bias for other sources | 23 | 5 | 1 | |
| Decreased SDF | |||||||||||||
| 13 | Stenqvist et al (2018) [39] | RCT blinded | Vitamin C (30 mg), vitamin E (5 mg), vitamin B12 (0.5 ug), lcarnitine (750 mg), coenzyme Q10 (10 mg), folic acid (100 ug), zinc (5 mg), selenium (25 ug) with maltodextrin, calcium carbonate, citric acid, steviol glycoside, flavours, betacarotene, silicon dioxide/daily for 6 months | 77 infertile men with DFI ≥25% | Improved sperm concentration, no change in DNA damage | 4 | 3 | 7 | No risk of bias identified | 19 | 5 | 1 | |
| 14 | Ahmad et al (2008) [40] | Uncontrolled (open label) | Mucuna pruriens seed powder 5 g/daily for 3 months | 60 infertile men | Improved volume, sperm concentration, count, motility | 0 | 3 | 5 | N/A | N/A | N/A | 0 | |
| Reduced MDA levels | |||||||||||||
| 15 | Alizadeh et al (2018) [41] | RCT blinded | Curcumin 80 mg/daily for 10 weeks | 60 infertile men | Increased sperm count, concentration, total motility and vitality, TAC | 0 | 3 | 7 | Unclear risk of bias for other sources | 19 | 4 | 0 | |
| Reduced MDA and inflammatory biomarkers | |||||||||||||
| 16 | Salehi et al (2019) [42] | Uncontrolled (open label) | Vitamin E (50 mg), vitamin C (500 mg) and CoQ10 (100 mg) for 3 months | 485 infertile men with DFI>27% by SCSA | Improved semen parameters | 5 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced DNA damage | |||||||||||||
| Pregnancy rate=16.8% | |||||||||||||
| 17 | Hasoon (2019) [43] | Uncontrolled (open label) | L-arginine (1 g) and CoQ10 (200 mg) for 8 months | 24 infertile men | Improved volume, sperm count, motility, and normal morphology | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 18 | Nurmawati et al (2020) [44] | RCT blinded | Astaxanthin 8 mg/daily for 1 month | 25 infertile men | Improved sperm concentration, motility, and morphology | 4 | 3 | 7 | Unclear risk of bias for selective reporting and other sources; high risk of bias for random sequence generation, allocation concealment and blinding (outcome assessment) | 15 | 3 | 0 | |
| Reduced MDA and 8-OHdG levels | |||||||||||||
| 19 | Hadi et al (2020) [45] | Uncontrolled (open label) | L-carnitine 2 g/daily for 3 months | 58 infertile men | Improved sperm count, total motility, and normal morphology | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| In serum: reduced FSH and LH levels, increased testosterone, and inhibin levels | |||||||||||||
| 20 | Schisterman et al (2020) [46] | RCT blinded | Folic acid 5 mg/daily and 30 mg zinc for 6 months | 1,185 male partners of couples planning IVF for infertility treatment | No changes in semen parameters; improved SDF by Comet assay; no significant differences in β-HCG–detected pregnancy, clinical intrauterine pregnancy, ectopic pregnancy, pregnancy with multiple fetuses, live birth rate | 2 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, other sources, selective reporting and blinding | 14 | 3 | 0 | |
| Varicocele | 21 | Comhaire et al (2000) [47] | Uncontrolled (open label) | Acetylcysteine (600 mg) or capsules providing a daily amount of β-carotene (30 mg) and α-tocopherol (180 mg)/daily. In addition, capsules containing essential fatty acids for a daily intake of docosahexaenoic acid (1 g), gammalinolenic acid (0.25 g) and arachidonic acid (0.10 g) for 6 months | 7 idiopathic patients 11 varicocele patients History of cryptorchidism (n=2), patients with male accessory gland infection (n=7), immunological infertility (n=4), endocrine cause (n=1) | Improved sperm concentration and acrosome reaction | 2 | 3 | 3 | N/A | N/A | N/A | 0 |
| Reduced ROS levels and 8-OHdG levels | |||||||||||||
| 22 | Paradiso Galatioto et al (2008) [48] | RCT unblinded | Ccommercial preparation of: NAC (10 mg/kg/die), vitamin C (3 mg/kg/die), vitamin E (0.2 mg/kg/die), vitamin A (0.06 IU/kg/die), thiamine (0.4 mg/kg/die), riboXavin (0.1 mg/kg/die), piridoxin (0.2 mg/kg/die), nicotinamide (1 mg/kg/die), pantothenate (0.2 mg/kg/die), biotin (0.04 mg/kg/die), cyanocobalamin (0.1 mg/kg/die), ergocalciferol (8 IU/kg/die), calcium (1 mg/kg/die), magnesium (0.35 mg/kg/die), phosphate (0.45 mg/kg/die), iron (0.2 mg/kg/ die), manganese (0.01 mg/kg/die), copper (0.02 mg/kg/die), zinc (0.01 mg/kg/die) for a minimum of 90 days | 42 varicocele patients with persistent oligospermia 6 months after retrograde embolization | Improved semen parameters | 3 | 3 | 7 | No risk of bias identified | 16 | 5 | 1 | |
| No change in pregnancy rate | |||||||||||||
| 23 | Oliva et al (2009) [49] | Uncontrolled (open label) | Pentoxifylline (1.2 g), folic acid (5 mg) and zinc sulfate (66 mg) for 3 months | 36 varicocele patients | Improved semen parameters | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 24 | Festa et al (2014) [50] | Uncontrolled (open label) | CoQ10 100 mg/daily for 3 months | 38 varicocele patients | Sperm concentration, progressive motility, and TAC | 0 | 3 | 4 | N/A | N/A | N/A | 0 | |
| 25 | Pourmand et al (2014) [51] | RCT unblinded | L-carnitine 750 mg/daily for 6 months | 100 varicocele patients | No changes in semen parameters, SDF and protamine damage assay | 0 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, and incomplete outcome data | 14 | 2 | 0 | |
| 26 | Nematollahi-Mahani et al (2014) [52] | RCT unblinded | A) Zinc sulphate/folic acid | 160 varicocele patients | No difference in TAC between the groups | 0 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources, and incomplete outcome data | 12 | 2 | 0 | |
| B) Folic acid (5 mg/daily) | |||||||||||||
| C) Zinc sulphate (66 mg/daily) | Increased SOD activity in group A and C | ||||||||||||
| D) Placebo | |||||||||||||
| For 6 months | |||||||||||||
| 27 | Cyrus et al (2015) [53] | RCT blinded | Vitamin C 250 mg/daily for 3 months | 115 varicocele patients | Improved semen parameters | 2 | 3 | 6 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources, blinding (participants and personnel, outcome assessment), and incomplete outcome data | 18 | 5 | 0 | |
| 28 | Gual-Frau et al (2015) [54] | Uncontrolled (open label) | L-Carnitine (1,500 mg), vitamin C (60 mg), CoQ10 (20 mg), vitamin E (10 mg), vitamin B9 (200 μg), vitamin B12 (1 μg), zinc (10 mg), selenium (50 μg) for 3 months | 20 varicocele patients | Improved total sperm count and reduced SDF | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 29 | Barekat et al (2016) [55] | RCT blinded | N-acetyl-L-cysteine (NAC) 200 mg/daily for 3 months | 35 varicocele patients | No changes for sperm concentration, motility, morphology, % of ROS negative sperm and intensity of sperm ROS Improved normal protamine content and DNA integrity | 1 | 3 | 4 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources and blinding (participants and personnel as well as outcome assessment) | 12 | 2 | 0 | |
| Pregnancy rate: NAC group=33.4%, control group=10%. No p-value reported | |||||||||||||
| 30 | Kızılay and Altay (2019) [56] | RCT unblinded | L-carnitine fumarate (2 g), Acetyl-L- carnitine HCl (1 g), fructose (2 g), citric acid (100 mg), vitamin C (180 mg), zinc (20 mg), folic acid (400 mg), selenium (100 mg), coenzyme Q-10 (40 mg), vitamin B12 (3 mg)/daily for 6 months | 90 varicocele patients | Improved semen parameters | 3 | 3 | 7 | High risk of bias for random sequence generation, allocation concealment, other sources, blinding (participants and personnel, outcome assessment), incomplete outcome data | 19 | 2 | 0 | |
| Higher pregnancy rate | |||||||||||||
| 31 | Ardestani Zadeh et al (2019) [57] | RCT unblinded | Folic acid (5 mg), Selenium (200 μg) and vitamin E (400 IU)/daily for 6 months | 60 varicocele patients | Improved sperm count and motility | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources; high risk of bias for blinding (participants and personnel, outcome assessment) | 24 | 4 | 1 | |
| Abnormal semen quality | 32 | Suleiman et al (1996) [58] | RCT blinded | Vitamin E 300 mg/daily for 6 months | Oligoastheno-(n=74), azoospermic (n=38), asthenospermic (n=94), oligospermic (n=30) patients High viscosity (n=22); oligospermic with high viscosity (n=6); asthenospermic with high viscosity (n=12); oligoasthenospermic with high viscosity (n=10) | Improved sperm motility | 4 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, other sources, blinding (participants and personnel, outcome assessment), incomplete outcome data | 12 | 3 | 0 |
| Reduced MDA levels | |||||||||||||
| Higher pregnancy and live birth rates | |||||||||||||
| 33 | Rolf et al (1999) [59] | RCT blinded | Vitamin C (1,000 mg) and Vitamin E (800 mg)/daily for 56 days | 31 asthenozoospermic patients | No changes in semen parameters | 0 | 3 | 7 | No risk of bias identified | 21 | 5 | 1 | |
| 34 | Vicari and Calogero (2001) [60] | Uncontrolled (open label) | Carnitene® (Sigma-Tau, Pomezia-Rome, Italy) Twice/day for3 months, followed by a treatment-free period of 3 months | 54 OAT patients with prostatovesiculo-epididymitis | Improved sperm progressive motility and viability | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced ROS production | |||||||||||||
| Higher pregnancy rate | |||||||||||||
| 35 | Suzuki et al (2003) [61] | Uncontrolled (open label) | Sairei-to 9.0 g/daily for 3 months | 16 healthy men 47 non-normozoospermic patients | Improved sperm concentration, and total motility | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| No change in SOD activity | |||||||||||||
| 36 | Balercia et al (2004) [62] | Uncontrolled (open label) | CoQ10 (PharmaNord, Veyle, Denmark) 400 mg/daily for 6 months | 22 asthenozoospermic patients | Improved progressive motility after treatment, which reduced after 6 months of washout | 4 | 3 | 4 | N/A | N/A | N/A | 0 | |
| Pregnancy rate=2.4%, with 3 out of 22 patients achieving a spontaneous pregnancy | |||||||||||||
| 37 | Piomboni et al (2008) [63] | Uncontrolled (open label) | Fattore M (Progine, Florence, Italy) 2 tables/day for 3 months | 51 asthenoteratozoospermic patients | Improved semen parameters and leukocytospermia | 1 | 3 | 6 | N/A | N/A | N/A | 0 | |
| Reduced SDF | |||||||||||||
| 38 | Ghanem et al (2010) [64] | RCT blinded | Clomiphene citrate (25 mg/day) and vitamin E (400 mg/day) for 6 months | 60 oligoasthenozoospermic patients | Increased sperm concentration and motility | 0 | 3 | 7 | Unclear risk of bias for other sources and blinding (participants and personnel, outcome assessment) | 17 | 4 | 0 | |
| Higher pregnancy rate | |||||||||||||
| 39 | Ahmad et al (2010) [65] | Uncontrolled (open label) | Withania somnifera 5 g/daily for 3 months | Oligo- (n=25), astheno- (n=25) and normozoospermic (n=25) patients | Improved sperm count and motility, SOD, catalase, and glutathione levels | 2 | 3 | 4 | N/A | N/A | N/A | 0 | |
| Decreased MDA and Protein Carbonyl levels | |||||||||||||
| 40 | Nadjarzadeh et al (2011) [66] | RCT blinded | CoQ10 capsules (Nutraceutical Science Institute, NC, USA) 200 mg/daily for 3 months | 60 OAT patients | No changes in semen parameters | 4 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, other sources and blinding (participants and personnel); high risk of bias for incomplete outcome data | 20 | 4 | 1 | |
| Reduced MDA and improved TAC | |||||||||||||
| 41 | Shukla et al (2011) [67] | Uncontrolled (open label) | Withania somnifera 5 g/daily for 3 months | Oligo- (n=25), astheno- (n=25) and normozoospermic (n=25) patients | Decreased intracellular ROS and apoptosis; increased levels of Cu2+, Zn2+, Fe2+ and Au2+ | 2 | 3 | 4 | N/A | N/A | N/A | 0 | |
| 42 | Safarinejad (2011) [68] | RCT blinded | Eicosapentanoic (1.12 g) and docosahexaenoic (0.72 g) acid/daily for 8 months | 211 OAT patients | Improved total sperm count, concentration, motility, normal morphology, seminal SOD, and catalase | 3 | 3 | 7 | Unclear risk of bias for other sources and incomplete outcome data | 21 | 5 | 1 | |
| 43 | Safarinejad (2011) [69] | RCT blinded | Pentoxifylline 800 mg/daily for 6 months | 278 OAT patients | No changes in semen parameters, seminal SOD, catalase, and reproductive hormones | 3 | 3 | 7 | Unclear risk of bias for other sources and incomplete outcome data | 19 | 5 | 0 | |
| 44 | Moslemi and Tavanbakhsh (2011) [70] | Uncontrolled (open label) | Selenium (200 μg), vitamin E (400 units)/daily for 100 days | 690 asthenoteratospermic patients | Improved semen parameters | 1 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Higher spontaneous pregnancy | |||||||||||||
| 45 | Safarinejad et al (2011) [71] | RCT blinded | Crocus sativus 60 mg/daily for 26 weeks | 260 OAT patients | No changes in semen parameters, SOD and catalase-like activity, LH, FSH, PRL, TSH, testicular volume | 2 | 3 | 7 | Unclear risk of bias for random sequence generation, selective reporting | 20 | 5 | 1 | |
| 46 | Safarinejad (2012) [72] | Uncontrolled (open label) | CoQ10 300 mg/daily for 12 months | 287 OAT patients | Improved semen parameters | 2 | 3 | 4 | N/A | N/A | N/A | 0 | |
| No change in pregnancy and miscarriage rates | |||||||||||||
| 47 | Abad et al (2013) [73] | Uncontrolled (open label) | Androferti (Q Pharma Laboratories, Alicante, Spain) 1 capsule/daily for 3 months | 20 asthenoteratozoospermic patients | Improved sperm concentration, motility, vitality, morphology, DNA integrity | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Pregnancy rate=5% | |||||||||||||
| 48 | Ajayi et al (2013) [74] | Uncontrolled (open label) | Vitamin C (200 mg), vitamin E (200 mg), folic acid (1 mg), zinc (50 mg), selenium (200 μg), nacetyl-L-cysteine (100 mg), Lcarnitine (600 mg), citrulline (600 mg), glutathione red. (100 mg), lycopene (8 mg), CoQ10 (30 mg)/daily for at least 2 months | Oligo- (n=20), astheno- (n=33), OAT (n=42) patients 65 healthy men | Improved semen parameters | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 49 | Nadjarzadeh et al (2014) [75] | RCT blinded | CoQ10 (Nutraceutical Science Institute, NC, USA) 200 mg/daily for 3 months | 60 OAT patients | No changes in semen parameters | 4 | 3 | 7 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources, blinding (participants and personnel, outcome assessment), and incomplete outcome data | 18 | 3 | 0 | |
| Increased seminal level of CoQ10, catalase and SOD activity; reduced level of seminal plasma 8-isoprostane | |||||||||||||
| 50 | Raigani et al (2014) [76] | RCT blinded | Folic acid (5 mg) and zinc sulphate (220 mg)/daily for 4 months | 83 OAT patients | No difference in semen parameters | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources | 20 | 4 | 1 | |
| Increased sperm chromatin integrity | |||||||||||||
| 51 | Kobori et al (2014) [77] | Uncontrolled (open label) | CoQ10 (120 mg), vitamin C (80 mg), vitamin E (40 mg)/daily for 6 months | 169 OAT patients | Improved sperm concentration and motility | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 48 (28.4%) pregnancies achieved; of those, 16 were spontaneous and 32 by using ART | |||||||||||||
| 52 | Thakur et al (2015) [78] | Uncontrolled (open label) | Ubiquinol 150 mg/daily for 6 months | 60 OAT patients | Improved sperm concentration, total and progressive motility | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Testosterone unchanged | |||||||||||||
| 53 | Kobori et al (2015) [79] | Uncontrolled (open label) | Edicare (KOBAYASHI Pharmaceutical Co., Ltd, Japan) 6 pills/daily for 3 months | 47 OAT patients | Improved sperm concentration | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 54 | Hadwan et al (2015) [80] | Uncontrolled (open label) | Zinc sulphate 440 mg/daily for 3 months | 60 asthenozoospermic patients 60 healthy men | Improved volume, progressive motility, total sperm count, and catalase activity | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 55 | Al-Hilli et al (2009) [81] | Uncontrolled (open label) | Simvastation tablet 40 mg/daily for 3 months | Astheno- (n=1), oligoastheno-(n=2), OAT (n=7), terato- (n=7), asthenonecro-(n=2), asthenoterato-(n=20), asthenoteratonecro-(n=4), 0ligoasthenoteratonecrozoospermic (n=2) patients | Improved sperm motility and normal sperm morphology | 2 | 3 | 4 | N/A | N/A | N/A | 0 | |
| Decreased MDA level | |||||||||||||
| 56 | Martinez et al (2015) [82] | RCT blinded | Resveratrol 25 mg/daily SG1002 (Nuevas Alternativas Naturales Thermafat, S.A. de C.V., Monterrey, Mexico) 750 mg/daily for 75 days | 54 oligoasthenozoospermic patients | Improved sperm concentration and motility | 0 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources; high risk of bias for incomplete outcome data | 18 | 5 | 0 | |
| 57 | Gvozdjáková et al (2015) [83] | Uncontrolled (open label) | Carni-Q-Nol (Tishcon Corp., Westbury, NY, USA) 2 softsules for the first 3 months, 3 softsules for the last 3 months | 40 oligoasthenozoospermic patients | Improved sperm concentration | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced concentrations of α-tocopherol and γ-tocopherol in seminal fluid, as well as TBARS, a marker of lipid peroxidation | |||||||||||||
| Pregnancy in 45% of couples | |||||||||||||
| 58 | ElSheikh et al (2015) [84] | RCT unblinded | A) Vitamin E (400 mg/daily) | 90 oligoasthenozoospermic patients | Improved sperm concentration in group B and C, while total sperm motility improved in all groups | 0 | 3 | 7 | Unclear risk of bias for other sources | 15 | 3 | 0 | |
| B) Clomiphene citrate (25 mg/daily) | |||||||||||||
| C) Vitamin E+clomiphene citrate for 6 months | |||||||||||||
| 59 | Montanino Oliva et al (2016) [85] | Uncontrolled (open label) | (Andrositol, Lo.Li. Pharma s.r.l., Rome, Italy) 2 capsules/daily for 3 months | 45 asthenozoospermic patients | Improved concentration, motility, normal morphology | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 60 | Singh et al (2016) [86] | Uncontrolled (open label) | Tablet Fertisure M (Sun Pharma) Twice/day for 3 months | 7 oligozoospermic patients 31 oligoasthenozoospermic patients 2 OAT patients | Improved sperm count and motility, glutathione level | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced MDA level | |||||||||||||
| 61 | Alahmar (2017) [87] | Uncontrolled (open label) | Hansal A–Z Vital (Hansal Pharm GmbH, Germany) for 3 months | 32 oligoasthenozoospermic patients | Improved sperm concentration, total and progressive motility | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 62 | Yamamoto et al (2017) [88] | RCT unblinded | A) Natsushibori (Kagome Co., Ltd., Japan) | 54 oligoasthenozoospermic patients | Improved sperm motility | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources; high risk of bias for blinding (participants and personnel, outcome assessment) | 16 | 2 | 0 | |
| B) CINAL Combination Tablet (600 mg/day, Shionogi Pharmaceutical Co., Japan), Juvela N Soft Capsule (200 mg/day, Tanabe Seiyaku Hanbai Co., Japan), and Tathion Tablet (300 mg/day, Eisai Co., Japan) | |||||||||||||
| For 3 months | |||||||||||||
| 63 | Magdi et al (2017) [89] | Uncontrolled (open label) | Vitamin C (1 g), vitamin E (400 mg) and Lcarnitine (2 g)/daily for 6 months | 210 OAT patients | Improved sperm count, total and progressive motility, normal morphology after treatment | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 64 | Alsalman et al (2018) [90] | Uncontrolled (open label) | Zinc 220 mg/daily for 3 months | 60 asthenozoospermic patients | Improved volume, progressive motility, normal morphology, total thiol concentration, total disulfide linkage concentration, GPx levels | 3 | 3 | 6 | N/A | N/A | N/A | 0 | |
| 65 | Busetto et al 2018 [26] | RCT blinded | Proxeed Plus (Sigma-Tau HealthScience, Utrecht, the Netherlands) 2 sachets/daily for 6 months | 104 patients with semen abnormalities (of those, 52 with varicocele) | Increased semen parameters, except sperm morphology | 0 | 3 | 7 | Unclear risk of bias for other sources | 20 | 4 | 1 | |
| 10 spontaneous pregnancy in treated couples vs. 2 in the placebo | |||||||||||||
| 66 | Lu et al (2018) [91] | RCT blinded | Melatonin 400 mg/daily for 3 months | 54 oligozoospermic patients | Improved semen parameters | 1 | 3 | 6 | Unclear risk of bias for random sequence generation, allocation concealment, selective reporting, other sources, blinding (participants and personnel, outcome assessment), and incomplete outcome data | 15 | 5 | 0 | |
| Improved TAC | |||||||||||||
| 67 | Jannatifar et al (2019) [92] | Uncontrolled (open label) | N-acetylcysteine 600 mg/daily for 3 months | 50 asthenozoospermic patients | Improved volume, sperm concentration, total and progressive motility, normal morphology | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced MDA, SDF and protamine deficiency; improved TAC | |||||||||||||
| 68 | Gambera et al (2019) [93] | Uncontrolled (open label) | Arginine (3 g), CoQ10 (200 mg), vitamin C (240 mg), vitamin B3 (27 mg), Tribulus terrestris (60 mg), ginseng (12 mg), inositol (100 mg), vitamin E (36 mg) for 2 months | 32 OAT patients | Improved sperm concentration, sperm count, progressive motility, normal morphology and vitality after therapy | 0 | 3 | 2 | N/A | N/A | N/A | 0 | |
| Oxisperm; reduced seminal oxidative stress after therapy | |||||||||||||
| Unclear capacitation check | |||||||||||||
| 69 | Micic et al (2019) [94] | RCT blinded | Proxeed Plus, consisting of 1 g LC, 0.5 g ALC, 0.725 g fumarate, 1 g fructose, 50 mg citric acid, 10 mg zinc, 20 mg coenzyme Q10, 50 μg selenium, 90 mg vitamin C, 200 μg folic acid and 1.5 μg vitamin B12 for 6 months | 175 oligoasthenozoospermic patients | Improved semen parameters; increased seminal carnitine and α‐glucosidase activity; reduced SDF | 3 | 3 | 7 | Unclear risk of bias for selection, other sources of bias, blinding of participants and personnel, and outcome assessment | 16 | 3 | 0 | |
| 70 | Nouri et al (2019) [95] | RCT blinded | Lycopene 25 mg/daily for 3 months | 44 oligozoospermic patients | Improved volume, total sperm count, concentration, total motility, TAC | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources | 18 | 4 | 0 | |
| 71 | Busetto et al (2020) [96] | RCT blinded | L-carnitine (1 g), fumarate (725 mg), acetyl-Lcarnitine (500 mg), fructose (1 g), citric acid (50 mg), selenium (50 μg), coenzyme Q10 (20 mg), vitamin C (90 mg), zinc (10 mg), folic acid (200 μg), vitamin B12 (1.5 μg)/daily for 6 months | 104 patients with altered semen quality. Of those, 52 showed grade I-III varicoceles | Improved total sperm count, total and progressive motility | 4 | 3 | 7 | No risk of bias identified | 22 | 5 | 1 | |
| Higher pregnancy rate | |||||||||||||
| 72 | Alahmar et al (2020) [97] | Uncontrolled (open label) | CoQ10 200 mg/daily for 3 months | 65 oligoasthenozoospermic patients | Improved sperm concentration, progressive and total motility, CoQ 10 level, TAC and GPx | 4 | 2 | 4 | N/A | N/A | N/A | 0 | |
| Reduced ROS levels and SDF | |||||||||||||
| 73 | Terai et al (2020) [98] | RCT unblinded | A) L-carnitine (750.1 mg), zinc (30 mg), astaxanthin (16.05 mg), CoQ10 (90.26 mg), vitamin C (1 g), vitamin B12 (60.1 μg), vitamin E (150 mg) | 31 oligoasthenozoospermic patients | Increased total motile sperm count after treatment in group A | 0 | 3 | 3 | Unclear risk of allocation concealment, selective reporting, other sources; no blindness of participants and personnel | 16 | 3 | 0 | |
| B) Hochu-ekkito (dosage not reported) | |||||||||||||
| For 3 months | |||||||||||||
| 74 | Steiner et al (2020) [99] | RCT blinded | Vitamin C (500 mg), vitamin E (400 mg), selenium (0.20 mg), L-carnitine (1 g), zinc (20 mg), folic acid (1 g), lycopene (10 mg), and vitamin D (2,000 IU)/daily for a maximum of 6 months | 174 oligozoospermic patients | Improved sperm concentration No change in SDF | 2 | 3 | 7 | No risk of bias identified | 20 | 5 | 1 | |
| No change in pregnancy and live birth rates | |||||||||||||
| 75 | Alkumait et al (2020) [100] | RCT unblinded | A) Glutathione (250 mg sachets) | 51 OAT patients | Improved semen parameters | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources; high risk of bias for blinding (participants and personnel, outcome assessment) | 13 | 3 | 0 | |
| B) CoQ10 (200 mg sachets) | |||||||||||||
| For 6 months | |||||||||||||
| 76 | Nazari et al (2020) [101] | Prospective study | Androferti supplement (Daru Darman Parmida, Iran) twice daily for 3 months | 59 patients with idiopathic OAT | Improved semen parameters | 2 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Healthy men | 77 | Goyal et al (2007) [102] | Uncontrolled (open label) | Lycopene 22.8 mg/daily for 2 weeks | 6 healthy men | Increased seminal lycopene. No increase in TAC levels | 2 | 3 | 3 | N/A | N/A | N/A | 0 |
| 78 | Tartibian and Maleki (2012) [103] | RCT blinded | Honey dissolved in water (70 g) | 39 healthy men | Decreased ROS, MDA | 2 | 3 | 7 | Unclear risk of bias for allocation concealment, other sources | 18 | 4 | 0 | |
| Increased SOD, Catalase, TAC and decreased IL-1b, IL-6, IL-8, TNF-a | |||||||||||||
| 79 | Williams et al (2020) [104] | RCT blinded | Lactolycopene 14 mg/daily for 3 months | 60 healthy men | Improved % of fast progressive and normal morphology | 0 | 3 | 7 | No risk of bias identified | 25 | 5 | 1 | |
| No difference in SDF% | |||||||||||||
| Urogenital inflammation | 80 | Vicari et al (2002) [105] | RCT unblinded | A) Carnitines (Carnitene 2 g/daily+Nicetile 1 g/daily) | 98 patients with abacterial prostatovesiculoepididymitis and high seminal leukocytes (>1 million cells/ml) | Group C showed increased forward motility and vitality | 0 | 3 | 4 | Unclear risk of bias for allocation concealment, other sources; high risk of bias for selective reporting and incomplete outcome data | 16 | 3 | 0 |
| B) Nonsteroidal anti-inflammatory drugs (NSAID) (nimesulide 200 mg/daily+ serratiopeptidase 10 mg/daily) | Reduced leukocyte count in all groups | ||||||||||||
| C) NSAID+ carnitines (2 months each) | Groups B and C showed reduced ROS | ||||||||||||
| D) Carnitines+NSAID (2 months each) | Spontaneous pregnancy rate=8.2% (6 in group C, 1 in groups B and D) | ||||||||||||
| For 4 months | |||||||||||||
| 81 | Yang et al (2003) [106] | Uncontrolled (open label) | A) Dang Gui (Angelica Sinensis), Chuan Xiong (Ligusticum Chuanxiong Hort), Chi Shao (Paeonia Veitchii Lynch), Wu Ling Zhi (Trogopterus Xanthipes Miline-Edwards), Pu Huang (Typha Angustata Linne), My Yao (Commiphora Molmol Engler), Yuan Hu (Corydalis Yanhusuo), Gan Jiang (Zingiber Officinale Rosecoe), Guan Gui (Cinnamomum Cassia Presl), and Hui Xiang (Foenicunum Vulgare Miller) | Chronic prostatitis (n=36) | Improved semen parameters and acrosin activity | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| B) Shao-Fu-Zhu-Yu-Tang, Sun-Ten Pharmaceutical Company, Taichung, Taiwan | |||||||||||||
| For 2 months | |||||||||||||
| 82 | Chayachinda et al (2020) [107] | RCT blinded | CoQ10 200 mg/day for 1 month | Leukocytospermia (n=84) | No difference in sperm concentration, motility, normal morphology | 0 | 3 | 3 | No risk of bias identified | 22 | 5 | 1 | |
| IMI | 83 | Comhaire et al (2000) [47] | Uncontrolled (open label) | Acetylcysteine (600 mg) or capsules providing a daily amount of β-carotene (30 mg) and α-tocopherol (180 mg)/daily. In addition, capsules containing essential fatty acids for a daily intake of docosahexaenoic acid (1 g), gammalinolenic acid (0.25 g) and arachidonic acid (0.10 g) for 6 months | 7 idiopathic patients 11 varicocele patients History of cryptorchidism (n=2), patients with male accessory gland infection (n=7), immunological infertility (n=4), endocrine cause (n=1) | Improved sperm concentration and acrosome reaction | 2 | 3 | 3 | N/A | N/A | N/A | 0 |
| Reduced ROS levels and 8-OHdG levels | |||||||||||||
| 84 | Gupta and Kumar (2002) [108] | Uncontrolled (open label) | Lycopene 4 mg/daily for 3 months | 30 idiopathic patients | Improved sperm concentration and motility | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Higher pregnancy rate | |||||||||||||
| 85 | Balercia et al (2005) [109] | RCT blinded | A) Carnitene (Sigma Tau, Pomezia, Italy) | 60 idiopathic patients | Improved sperm motility, total oxyradical scavenging capacity of the semen | 2 | 3 | 7 | No risk of bias identified | 18 | 5 | 1 | |
| B) Zibren (Sigma Tau) | |||||||||||||
| C) A combination of carnitene and zibren | |||||||||||||
| For 6 months | |||||||||||||
| 86 | Heidary et al (2008) [110] | Uncontrolled (open label) | Saffron 50 mg, 3 times weekly for 3 months | 52 idiopathic patients | Improved normal morphology, total and progressive motility | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| 87 | Ciftci et al (2009) [111] | RCT unblinded | N-acetylcysteine 600 mg/daily for 3 months | 120 idiopathic patients | Improved volume, motility, semen viscosity, semen and serum oxidative stress (TAC, total peroxide, oxidative stress index) | 2 | 3 | 6 | Unclear risk of bias for blinding (participants and personnel); high risk of bias for other sources | 12 | 3 | 0 | |
| 88 | Haghighian et al (2015) [112] | RCT blinded | α-lipoic acid 600 mg/daily for 3 months | 44 idiopathic patients | Improved sperm concentration and motility, TAC; reduced MDA levels | 2 | 3 | 7 | No risk of bias identified | 20 | 5 | 1 | |
| 89 | Soleimani and Masoumi (2017) [113] | Uncontrolled (open label) | Grape seed extract 600 mg/daily for 3 months | 29 idiopathic patients | Increased catalase, reduced MDA | 2 | 1 | 3 | N/A | N/A | N/A | 0 | |
| 90 | Negri et al (2017) [114] | Uncontrolled (open label) | FertiPlus SOD (α-lipoic acid, glutathione, folic acid, zinc, and vitamins B2, B3, B6, B12) Dosage not specified for single component, length of treatment not reported | 55 idiopathic patients | No changes in semen parameters and SDF | 0 | 2 | 3 | N/A | N/A | N/A | 0 | |
| 91 | Kopets et al (2020) [115] | RCT blinded | L-carnitine/lacetyl-carnitine (1,990 mg), larginine (250 mg), glutathione (100 mg), co-enzyme Q10 (40 mg), zinc (7.5 mg), vitamin B9 (234 mcg), vitamin B12 (2 mcg), selenium (50 mcg)/daily for 6 months | 83 idiopathic patients | Increased % of normozoospermia in treated patients after 2 and 4 months in comparison with placebo | 0 | 3 | 7 | No risk of bias identified | 24 | 5 | 1 | |
| Higher pregnancy rate | |||||||||||||
| 92 | Arafa et al (2020) [25] | Uncontrolled (open label) | FH PRO for Men (Fairhaven Health LLC, Bellingham, WA, USA) Twice/day for 3 months | 119 idiopathic patients 29 unexplained infertile men | Improved progressive motility and seminal oxidation reduction potential | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced SDF | |||||||||||||
| UMI | 93 | Greco et al (2005) [116] | Uncontrolled (open label) | Vitamin C (1 g) and vitamin E (1 g)/daily for 2 months | Oligoterato-(n=6), OAT (n=26) patients, 6 unexplained infertile men | Improved semen parameters and SDF | 2 | 3 | 3 | N/A | N/A | N/A | 0 |
| No change in fertilization and cleavage rates after treatment | |||||||||||||
| Higher implantation and pregnancy rates | |||||||||||||
| 94 | Greco et al (2005) [117] | Uncontrolled (open label) | Vitamin C and E 1 g/daily for 2 months | 64 unexplained infertile men | No difference in semen parameters | 1 | 3 | 7 | N/A | N/A | N/A | 0 | |
| Reduced SDF | |||||||||||||
| 95 | Safarinejad et al (2012) [118] | RCT blinded | CoQ10 200 mg/daily for 26 week, followed by a treatment-free period of 12-week | 228 unexplained infertile men | Improved semen parameters, seminal catalase, and SOD | 4 | 3 | 7 | No risk of bias identified | 18 | 5 | 1 | |
| 96 | Khani et al (2013) [119] | Uncontrolled (open label) | Sesame 0.5 mg/kg body weight for 3 months | 25 unexplained infertile men | Improved sperm concentration, motility | 0 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Pregnancy: 3 out of 25 patients | |||||||||||||
| Live birth rate: 3 out of 25 patients | |||||||||||||
| 97 | Arafa et al (2020) [25] | Uncontrolled (open label) | FH PRO for Men (Fairhaven Health LLC, Bellingham, WA, USA) Twice/day for 3 months | 119 idiopathic patients 29 unexplained infertile men | Improved progressive motility and seminal oxidation reduction potential | 3 | 3 | 3 | N/A | N/A | N/A | 0 | |
| Reduced SDF | |||||||||||||
| Hyperinsulinaemic male patients | 98 | Bosman et al (2015) [120] | Uncontrolled (open label) | A) metformin (500–2,000 mg daily | 34 hyperinsulinaemic male patients | Improved sperm morphology in both groups | 0 | 3 | 6 | N/A | N/A | N/A | 0 |
| B) Metformin+ StaminoGro (Georen Pharmacuticals PTY LTD, Fontainebleau, South Africa) | Decreased CMA3 assay results in both groups after treatment | ||||||||||||
| For 3 months | |||||||||||||
| RPL | 99 | Hamidian et al (2020) [121] | Uncontrolled (open label) | Vitamin C 250 mg/daily for 3 months | 20 patients with recurrent pregnancy loss | Improved sperm morphology | 2 | 3 | 4 | N/A | N/A | N/A | 0 |
| Reduced SDF | |||||||||||||
| Changes in mRNA levels of PRM1, PRM2, and the PRM1/PRM2 ratio after treatment | |||||||||||||
Data is summarized based on the clinical trial design, the antioxidant formulation and the study population tested as well as the impact on reproductive outcomes. The quality and the risk of bias have been determined for each study by applying the Cambridge Quality Checklist, the Cochrane Risk of Bias for RCTs, CONSORT guidelines, and JADAD score.
SN: serial number, RCT: randomized controlled trial, 8-OHdG: 8-hydroxy-2′ -deoxyguanosine, IMI: iopathic male infertility, UMI: unexplained male infertility, RPL: recurrent pregnancy loss, ROS: reactive oxygen species, N/A: not available, MDA: malondialdehyde, TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labelling, SDF: sperm DNA fragmentation, ICSI: intracytoplasmic sperm injection, DFI: DNA fragmentation index, OS: oxidative stress, SOD: superoxide dismutase, GSH: gluthatione, TAC: total antioxidant capacity, SCSA: sperm chromatin structure assay, CoQ: coenzyme Q, FSH: follicle-stimulating hormone, LH: luteinizing hormone, IVF: in vitro fertilization, β-HCG: beta-uman chorionic gonadotropin, OAT: oliasthenoteratozoospermia, PRL: prolactin, TSH: thyroid-stimulating hormone, ART: assisted reproductive techniques, TBARS: thiobarbituric acid reactive substances, GPx: glutathione peroxidase, PRM: protamine, CMA3: chromomycin A3.
RESULTS
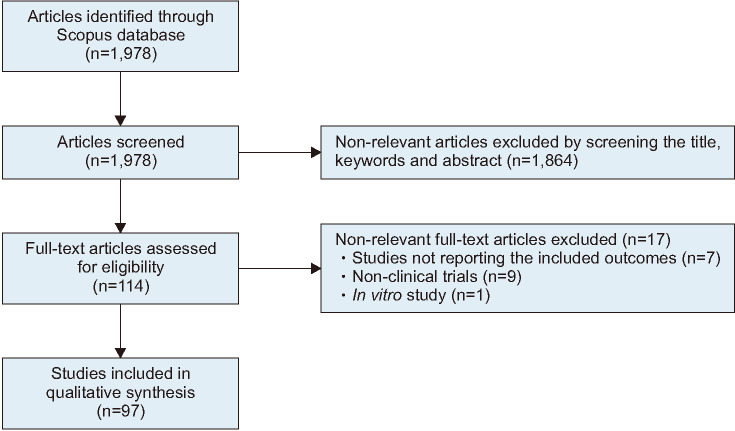
A total number of 1,978 articles were identified through the application of the keyword search strategy. Through manual screening of the title, keywords and abstract, non-relevant articles (n=1,864) were excluded (Fig. 2).
Fig. 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) workflow reporting the literature search strategy.
Following full-text review for eligibility using the inclusion and exclusion criteria, 17 articles were further excluded, resulting in 97 articles that were eligible for inclusion (Fig. 2). Two of the studies are each repeated as they included both IMI and UMI participants respectively, resulting in a total of 99 studies included in Table 2. Relevant data were extracted from the articles and summarized in Table 2, including the studied population, reported impact of treatment on reproductive outcomes, evaluation of quality, and risk of bias.
Of the 97 articles collected, 52 (53.6%) were uncontrolled (open label) clinical trials, 12 (12.4%) were unblinded RCTs and 33 (34.0%) were blinded RCTs. Based on the type of antioxidants investigated, 44 (45.4%) of the articles tested individual antioxidants, 31 (32.0%) tested a combination of several products in variable dosages, and 22 (22.7%) used registered antioxidants products. Semen parameters were evaluated after antioxidant treatment in 92.8% (n=90 out of 97) of the included publications, while the remaining 7 studies evaluated markers of sperm function.
Based on the statistical analysis, it is reported that 85.7% and 89.6% of the low-quality studies showed significant improvement (p<0.0001) in semen and sperm function parameters, respectively, in infertile men after antioxidant supplementation, whereas 65.0% and 58.3% of the high-quality studies, respectively, reported positive effect of antioxidant treatment on semen and sperm function parameters (Table 3). However, these results were not significant due to the availability of a small number of studies in the literature reporting semen parameters (n=20) and those reporting sperm functions (n=12) and this has led to the underpowering of statistical analysis. Sample size calculation predicted that a total number of 95 and 292 studies reporting the outcome of semen parameters and sperm functions, respectively, will allow it to gain a statistical significance of p<0.05. Furthermore, statistical analysis revealed that 78.6% (p=0.0733) and 60% (p=0.6949) of low and high-quality studies, respectively, reported a positive effect of antioxidant treatment on reproductive outcomes. However, these values were not significant (p≥0.05) due to the availability of very few studies (n=14 for low-quality and n=5 for high-quality) in the literature. Sample size calculation predicted that a total number of 33 low and 202 high-quality studies are required to attain a statistical significance of p<0.05 for reproductive outcomes.
Table 3. Number of low and high-quality studies analysing semen parameters and/or sperm function after antioxidant treatment, overall as well as in each clinical condition.
| Group | Category | Report of semen parameters | Report of sperm function | ||
|---|---|---|---|---|---|
| Number of articles on the total of studies | % of studies reporting an improvement after AOX treatment | Number of articles on the total of studies | % of studies reporting an improvement after AOX treatment | ||
| Overall (n=97) | Low quality | 70/90 (77.8) | 85.7*** | 50/60 (83.3) | 89.6*** |
| High quality | 20/90 (22.2) | 65.0 | 12/60 (20.0) | 58.3 | |
| Varicocele (n=11) | Low quality | 9/11 (81.8) | 75.0 | 6/11 (54.5) | 83.0 |
| High quality | 2/11 (18.2) | - | 0/11 (0) | - | |
| Abnormal semen quality (n=45) | Low quality | 36/44 (81.8) | 94.4*** | 20/25 (80.0) | 90.0** |
| High quality | 8/44 (18.2) | 50.0 | 5/25 (20.0) | 60.0 | |
| Idiopathic male infertility (n=10) | Low quality | 6/9 (66.7) | 83.0 | 5/7 (71.4) | 80.0 |
| High quality | 3/9 (33.3) | 100*** | 2/7 (28.6) | 100*** | |
| Unexplained male infertility (n=5) | Low quality | 4/5 (80.0) | 83.3 | 3/4 (75.0) | 100*** |
| High quality | 1/5 (20.0) | 100*** | 1/4 (25.0) | 100*** | |
Values are presented as number (%) or percentage only.
AOX: antioxidant, -: not available.
Chi-square test: **p<0.01, ***p<0.0001.
1. Varicocele
A total of 11 studies investigated a male population affected by varicocele (Table 2). Of those, semen parameters after antioxidant treatment were reported in 90.9% (n=10 out of 11) of the included publications. Based on these studies, antioxidant supplementation seems to be beneficial in varicocele patients as 75.0% and 83.0% of low-quality studies, respectively, available in the literature, reported positive effect of antioxidant treatment on semen and sperm function parameters (Table 3). However, these values were not significant. Sample size calculation predicted that a total number of 41 and 24 studies reporting the outcome of semen parameters and sperm function, respectively are needed to reach a statistical significance of p<0.05.
2. Abnormal semen quality
A total of 45 studies investigated a male population with abnormal semen quality (Table 2). Of those, semen parameters after antioxidant treatment were reported in 97.8% (n=44 out of 45) of the included publications, whereas sperm function biomarkers were reported in 25 out of 45 studies (55.6%) (Table 3). The majority of the studies showed significant improvement in semen and sperm function parameters of men with abnormal semen quality after antioxidant supplementation, although these results were not statistically significant in case of the high-quality studies (Table 3). Sample size calculation predicted that a total number of 204 studies reporting the outcome of sperm function are required to reach a statistical significance of p<0.05.
3. Idiopathic male infertility
A total of 10 studies investigated idiopathic infertile men (Table 2). Of those, semen parameters after antioxidant treatment were reported in 90.0% (n=9 out of 10) of the included publications, whereas sperm function biomarkers were reported in 7 out of 10 studies (70.0%) (Table 3). Our statistical analysis showed that all the high-quality studies reported improvement in the semen and sperm function parameters (p<0.0001) after antioxidant treatment in men with IMI. Although a high percentage of low-quality studies showed improvement in semen and sperm function parameters in men with IMI after antioxidant supplementation, these values were not significant. Sample size calculation revealed that a total number of 24 and 30 studies, respectively, reporting the outcome of semen parameters and sperm function, may allow to reach statistical significance.
4. Unexplained male infertility
A total of 5 studies investigated the effect of antioxidant therapy in unexplained infertile men (Table 2). All of those studies reported semen parameters after antioxidant treatment (100%), whereas sperm function biomarkers were reported in 4 out of 5 studies (80.0%) (Table 3). All the low-quality studies showed improvement in sperm function, while sample size calculation predicted that a total number of 41 low-quality studies reporting the outcome of semen parameters would allow to attain a statistical significance of p<0.05. Furthermore, all the high-quality studies reported a significant improvement in the semen and sperm function parameters after antioxidant treatment in men with UMI.
5. Analysis of the most recent publications
A total of 21 articles published between January 2019 and July 2020 investigated the effects of antioxidant treatment on semen quality (Table 4) [25,42,43,44,45,46,56,57,92,93,94,95,96,97,98,99,100,101,104,115,121]. Based on our analysis, 13 and 8 studies were ranked as low and high-quality, respectively. Of these, 19 out of 21 (90.5%) showed improvement in semen parameters, while 4 out of 6 (66.7%) reported a significant improvement in sperm function. The number of studies investigating reproductive outcomes after antioxidant treatment was very limited, with only 3 out of 5 (60.0%) reporting an improvement in pregnancy rate, while birth rate showed no variation in the two studies reporting its evaluation.
Table 4. Articles published between January 2019 and July 2020 investigating the impact of antioxidant treatment on reproductive outcomes.
| SN | Reference | Study design | Study population/sample size | Inclusion criteria | Exclusion criteria | Strict male inclusion/exclusion | Female factor | Main outcomes reported | Power of statisticalanalysis | Study quality score (out of 4) | Study outcome (out of 3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Terai et al (2020) [98] | RCT unblinded | 31 oligoasthenozoospermic patients | Age: 20–60 years old; presence of oligozoospermia and/or asthenozoospermia | Azoospermia | 0 | N/A | Improved TMSC (p=0.04) | N/A | 0 | 1 |
| Sperm concentration <5×106/mL | |||||||||||
| Sperm motility<5% | |||||||||||
| TMSC>30×106 | |||||||||||
| Clinical conditions resulting in infertility | |||||||||||
| History of cancer, chemotherapy, drug abuse | |||||||||||
| Administration of androgens, anti-androgens, and immunosuppressants | |||||||||||
| 2 | Schisterman et al (2020) [46] | Double-blind RCT | Treatment (n=1,185) vs. placebo (n=1,185) | Male partners of couples planning IVF for infertility treatment | Planning of donor sperm use or a gestational surrogate | 0 | N/A | No difference in semen parameters between both groups. | 90% power at a 2-sided α level of 0.05 to detect a risk difference of 7% in LBR (implying a risk ratio of 1.10), with continuity correction and allowing for a dropout rate of 15% | 2 | 0 |
| Pregnancy at enrollment | Increase in SDF by Comet assay in treatment group vs. placebo group (Adjusted MD 2.4, 95% CI 0.5–4.4) | ||||||||||
| Obstructive azoospermia | No significant differences in β-HCG–detected pregnancy, clinical intrauterine pregnancy, ectopic pregnancy, pregnancy with multiple fetuses | Esteem of risk differences and risk ratios Sequential approach of Lan and DeMets with Bonferroni adjustment to distribute the 1-sided type I error rate among 3 continuous semen quality parameters Post hoc sensitivity analyses | |||||||||
| Chronic diseases | LBR: Treatment group 404 (34%) vs. placebo group 416 (35%) (ns) | ||||||||||
| 3 | Steiner et al (2020) [99] | Double-blind RCT | Treated (n=85) vs. placebo (n=86) | Infertile men with abnormal semen analysis in the last 6 months or DFI≥25% | Sperm concentration <5×106/mL | 0 | Yes | No difference in semen parameters, DFI by SCSA and PR | Sample size calculation, assuming a 20% dropout rate, ≥80% power at α=0.05 | 3 | 0 |
| Consumption of fertility medication or testosterone | LBR: 15% AOX vs. 24% placebo (ns) | ||||||||||
| LBR=35% in the treated group and 25% in the placebo group with a 17% dropout | |||||||||||
| 4 | Kopets et al (2020) [115] | Double-blind RCT | Treated (n=42) vs. placebo (n=41) | Age: 21–50 years, with IMI | Allergy to any component | 1 | Yes | Significant difference between both groups as regards normalization of semen parameters at 2 months (26/42 [61.9%]) males in treatment group vs. 8/41 [19.5%] males in placebo group) and at 4 months (29/42 [69.0%] vs. 9/41 [22.0%]). | Sample size calculation assuming 1-beta error 0.80 and type I error alpha 5% | 2 | 1 |
| Any clinical cause of male or female infertility | Significant change from baseline in mean values for all main semen parameters at 2 and 4 months, except for sperm morphology | Control for confounders by ANCOVA analysis | |||||||||
| Alcohol or drug addiction | At 6 months higher PR in treatment than placebo group (10/42 [23.8%] vs. 2/41 [4.9%]) | ||||||||||
| Use of any investigational product within the previous 3 months | |||||||||||
| 5 | Arafa et al (2020) [25] | Prospective study | Idiopathic (n=119) and unexplained male infertility (n=29) | Infertile men (20–50 years) with unknown etiology and female infertility factor | Azoospermia | 1 | Yes | IMI: significant improvement in sperm concentration (p<0.001), total motility (p=0.001), normal morphology (p<0.001), ORP (p<0.001), SDF (p=0.001) by Halosperm | N/A | 3 | 3 |
| Sperm concentration <1×106/mL | |||||||||||
| Leucocytospermia | |||||||||||
| Any cause for infertility | |||||||||||
| Chemotherapy | |||||||||||
| Clinical endocrinopathy | |||||||||||
| Abnormal hormonal profile | |||||||||||
| AOXs in the past 6 months | |||||||||||
| Dietary, social habits or medical conditions which may impact on oxidative stress | UMI: significant improvement in progressive motility (p=0.002), ORP (p=0.03), SDF (p=0.02) | ||||||||||
| Use of drugs | |||||||||||
| 6 | Nazari et al (2020) [101] | Prospective study | 59 patients with idiopathic OAT | Infertile patients with at least 1 abnormal semen parameter; age<45 years, BMI<30 | Azoospermia | 1 | No | Significant improvements in sperm concentration (p=0.004) and normal morphology (p=0.01) | N/A | 1 | 1 |
| Prostatitis | |||||||||||
| Any clinical condition causing infertility History of hormonal therapy, drug addition, alcohol abuse, smoking, exposure to potential reproductive toxins | |||||||||||
| 7 | Nurmawati et al (2020) [44] | Single-blinded RCT | 25 infertile men | Inclusion criteria not clearly stated | Exclusion criteria not clearly stated | 0 | No | Improved sperm concentration, motility, and morphology (p<0.05) | Sample size calculation assuming that the prevalence of male infertile couples with idiopathic causes in the world is 15% and in Indonesia 1.11% | 2 | 2 |
| Reduced levels of 8-OHdG levels (p<0.01) and MDA, with the value< 1.98 being able to predict 100% of the normal sperm motility level (>40) | |||||||||||
| 8 | Hadi et al (2020) [45] | Uncontrolled (open label) | 58 infertile men | Inclusion criteria not clearly stated | Presence of varicocele, orchitis, cryptorchidism | 0 | No | Improved sperm volume, count, total motility, and normal morphology (p<0.05) | N/A | 1 | 1 |
| Consumption of herbals or medications that might affect seminal parameters in the last 3 months prior to the study | |||||||||||
| 9 | Busetto et al (2020) [96] | Double-blinded RCT | 104 patients with altered semen quality. Of those, 52 showed grade I–III varicoceles | Oligo- and/or astheno- and/or teratozoospermia, with or without varicocele (not surgically treated) and men from infertile couples | Known hypersensitivity to any of the compound | 0 | Yes | Improved total sperm count (p<0.0001), total (p<0.0001) and progressive motility (p=0.0012) | Sample size calculation assuming α=0.05 (significance), β=0.20 (power of 80%), and up to 15% of patients dropping out of the study esteemed | 3 | 1 |
| History of undescended testes or cancer, endocrine disorders, post-pubertal mumps, genitourinary surgery, obstructive azoospermia or obstructive pathology of the urogenital system, autoimmune disease, cystic fibrosis | |||||||||||
| History of taking any therapy affecting fertility, alcohol or drug abuse | Higher PR in treated group vs. placebo (10 vs. 2 pregnancies, respectively; p=0.0141) | ||||||||||
| Subjects following any special diet or taking AOXs | |||||||||||
| Involvement in any other clinical trials | |||||||||||
| 10 | Alahmar et al (2020) [97] | Uncontrolled (open label) | 65 oligoasthenozoospermic patients | Infertile patients showing oligoasthenozoospermia | Azoospermia | 1 | No | Improved sperm concentration, progressive and total motility (p<0.05), levels of CoQ 10 (p<0.001), TAC (p<0.01) and GPx (p<0.001) | N/A | 2 | 2 |
| Anatomical abnormalities of genital tract, varicocele, genital infection, scrotal surgery, systemic diseases | |||||||||||
| Smoking | |||||||||||
| Female factor | |||||||||||
| Consumption of ntioxidant and selective serotonin reuptake inhibitors intake in the last 6 months | Reduced ROS levels (p<0.05) and SDF by SCD assay (p<0.01) | ||||||||||
| 11 | Alkumait et al (2020) [100] | RCT unblinded | 51 OAT patients | Normal female factor with idiopathic OAT | Presence of chronic diseases, neoplasm, trauma, hypospadias, vas deference obstruction, varicocele, and genital tract infection | 1 | No | Improved sperm concentration, motility (p=0.01) and morphology (p=0.03) | N/A | 1 | 1 |
| Receiving treatment recently | |||||||||||
| 12 | Williams et al (2020) [104] | Double-blinded RCT | 60 healthy men | Healthy male volunteers, aged 18–30 years, lived within 1 h of the clinic or planning to live in the region for the duration of the study | Previous testicular surgery | 0 | No | Improved % of fast progressive (p=0.006) and normal morphology (p<0.001) | N/A | 3 | 1 |
| Existing or previous cancer | |||||||||||
| Allergy to tomato, whey protein or soy derivatives | No difference in SDF by TUNEL | ||||||||||
| 13 | Hamidian et al (2020) [121] | Uncontrolled (open label) | 20 patients with recurrent pregnancy loss | Recurrency of pregnancy loss, age<40 years, no history of alcohol/drug abuse or smoking, altered semen quality | Obesity, diabetes, and varicocele | 1 | Yes | Improved sperm morphology (p=0.000) | N/A | 2 | 3 |
| Previous treatments with AOXs or other medications | Reduced SDF by TUNEL (p=0.00) | ||||||||||
| For the female partners, the presence of hormonal imbalance, chromosomal alterations, tubal obstruction, and bacterial or viral infections | Reduced sperm protamine deficiency assessed by CMA3-based assay (p=0.00) | ||||||||||
| 14 | Salehi et al (2019) [42] | Uncontrolled (open label) | 485 infertile men with DFI>27% by SCSA | Aged 20–40 years | History of varicocele, surgery, and inflammation | 1 | No | Improved sperm concentration (p=0.003), total motility (p=0.001). Reduced DFI by SCSA (p=0.001) | N/A | 2 | 2 |
| PR=16.8% for AOX treated patients | |||||||||||
| 15 | Hasoon (2019) [43] | Uncontrolled (open label) | 24 infertile men | Unexplained subfertility | Presence of organic or obstructive infertility | 1 | No | Improved volume, sperm count, motility, and normal morphology (p<0.005) | N/A | 0 | 1 |
| 16 | Ardestani Zadeh et al (2019) [57] | Single blind RCT | 60 varicocele patients | Varicocele patients who underwent sub-inguinal varicocelectomy | Usage of supplements | 0 | No | Improved sperm count (p=0.021) and motility (p=0.003) | N/A | 2 | 1 |
| Alcohol and/or drug addiction, smoking | |||||||||||
| Diabetes mellitus, hormonal disorders, chronic or active infections | |||||||||||
| Presenting side effects, and delayed complications of varicocelectomy | |||||||||||
| 17 | Kızılay and Altay (2019) [56] | RCT unblinded | 90 varicocele patients | Varicocele patients treated with varicocelectomy, with spouses<35 years old, regular hormone profiles and menstrual cycles and no identified cause of infertility | Previous genitourinary system and/or varicocele surgery | 0 | Yes | Improved TSC, sperm concentration, sperm count in normal morphology, and total and progressive motile sperm count (p<0.05) | Study powered to detect an effect size of d≥0.70 as statistically significant in a two-tailed test with α=0.05 and power of 0.80 with n=24 per condition. | 3 | 1 |
| IMI | |||||||||||
| Any clinical condition affecting fertility for the previous 3 months | |||||||||||
| Patients following a fertility specific diet | Higher PR in AOX treated patients than placebo group (29% vs. 17.9%, respectively; p=0.029) | ||||||||||
| Alcohol or drug abuse, smoking | |||||||||||
| 18 | Gambera et al (2019) [93] | Uncontrolled (open label) | 32 OAT patients | Infertile patients with normal sexual development, medical history, serum hormone levels and physical examination | Azoospermia and infertility due to the female factor | 0 | Yes | Improved sperm concentration, sperm count, progressive motility, normal morphology, and vitality | N/A | 2 | 2 |
| Oxisperm test: reduced seminal oxidative stress after therapy (no pvalues reported) | |||||||||||
| 19 | Jannatifar et al (2019) [92] | Uncontrolled (open label) | 50 asthenozoospermic patients | Infertile couples with no previous report of pregnancy, normal female and male partners | Varicocele, leukospermia, hormonal abnormalities, and/or obstruction, cryptorchidism, vasectomy, abnormal liver function | 1 | Yes | Improved sperm concentration (p=0.02), total (p=0.01) and progressive motility (p=0.001), normal morphology (p=0.001), TAC (p=0.01) | N/A | 1 | 3 |
| Smoking, alcohol consumption | Reduced levels of MDA (p=0.01), SDF by TUNEL (p=0.001), % of sperm showing protamine deficiency by CMA3-based assay (p=0.009) | ||||||||||
| Anatomical disorders, Klinefelter's syndrome, cancer, fever in the 90 days prior to sperm analysis, seminal sperm antibodies | |||||||||||
| 20 | Nouri et al (2019) [95] | Double-blind RCT | 44 oligozoospermic patients | Infertile men (25–45 years), sperm count<20×106/mL, normal sperm <65% and average motility <60% | History of anatomical disorders, endocrinopathy, previous hormonal therapy, use of androgens, antiandrogens, anticoagulants, cytotoxic drugs, or immunosuppressants | 1 | No | Improved volume, TSC, concentration, total motility, TAC (p<0.05) | N/A | 2 | 2 |
| Alcohol and drug abuse | |||||||||||
| BMI≥30 kg/m2 | |||||||||||
| 21 | Micic et al (2019) [94] | Double-blind RCT | Treatment (n=125) vs. placebo (n=50) | Total sperm number ≤15×106/ mL; progressive motility <32%; normal viscosity and normal leucocytes number (<1×106/mL); sperm vitality ≤58%; normal sperm morphology <4% | Motility<5% | 0 | Yes | Improved ejaculated volume (p=0.001), progressive motility (p<0.001), vitality (p=0.002) after treatment | N/A | 4 | 3 |
| Sperm concentration <1×106/mL | |||||||||||
| History of therapy for infertility within the last 2 months | |||||||||||
| Alcohol consumption | |||||||||||
| Undescended testes, post‐pubertal mumps, endocrine and autoimmune diseases, cystic fibrosis, or testicular cancer | Reduced SDF by Halosperm test | ||||||||||
| Hypersensitivity to ingredients in Proxeed Plus | Increased seminal carnitine and α‐glucosidase activity, positively correlated with improved progressive motility | ||||||||||
| Presence of endocrine disorders, anti-sperm antibodies, leukocytospermia | |||||||||||
| Use of antioxidant agents or vitamins | |||||||||||
| Involvement in other clinical trials |
Data are summarized and ranked based on the study design, the population investigated, the inclusion/exclusion criteria, the analysis of the female partner, the main outcomes reported, and the power of the statistical analysis.
SN: serial number, RCT: randomized controlled trial, TMSC: total motile sperm count, N/A: not available, IVF: in vitro fertilization, SDF: sperm DNA fragmentation, MD: median, CI: confidence interval, β-HCG: beta-human chorionic gonadotropin, LBR: live birth rate, ns: non-significant, DFI: DNA fragmentation index, SCSA: sperm chromatin structure assay, AOX: antioxidant, IMI: idiopathic male infertility, ORP: oxidation reduction potential, UMI: unexplained male infertility, BMI: body mass index, 8-OHdG: 8-hydroxy-2′ -deoxyguanosine, MDA: malondialdehyde, PR: pregnancy rate, CoQ: coenzyme Q, TAC: total antioxidant capacity, GPx: glutathione peroxidase, ROS: reactive oxygen species, SCD: sperm chromatin dispersion, OAT: oligoasthenoteratozoospermia, TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling, CMA3: chromomycin A3, TSC: total sperm count.
DISCUSSION
Male infertility is a relatively common concern, contributing significantly to poor reproductive outcomes in couples. Oxidative stress has been increasingly identified as a common mechanism that mediates not only the pathophysiology, but also the many etiologies and risk factors associated with male infertility [1,2,5,6]. Within this context, there is increased use of antioxidants as a therapeutic option in male infertility, however, there remains no consensus on the efficacy, indications, dosage or length of treatment [8,9,10,11]. Therefore, the objective of this study was to systematically review the literature of trials investigating antioxidant use in male infertility, and to propose some broad guidelines for the practicing clinicians based on the currently available evidence. The results (Table 2) were stratified based on the currently available evidence on the clinical conditions investigated and the data were further analysed. Most studies reported men with abnormal semen quality (n=45) and infertile men (n=20) as well as male infertility conditions such as varicocele (n=11), IMI (n=10), UMI (n=5), and urogenital inflammation (n=3). Although there is no doubt that assessing sperm quality is just a first approach to a diagnosis and that evaluation of it as a predictor of fecundity or a couple's fertility success may lead to imprudent conclusions, there is no consensus whether the intake of exogenous antioxidants should be routinely done in clinical practice.
Majzoub and Agarwal (2018) [10] performed a systematic review and identified 26 studies showing positive effects of exogenous antioxidant intake on sperm quality and relevant outcomes of assisted reproduction such as live birth rates. The authors critically discussed the studies and highlighted that the treatment was given only for a short period to a small number of men. In addition, the lack of a standardized test to estimate oxidative stress levels in sperm and seminal fluid was another flaw in these studies, while the heterogeneity of the study designs made it particularly challenging to compare the effects and reach a robust conclusion. This has also been observed in our study, where 60 studies (61.9%) analyzed a variety of seminal oxidative stress markers, including the levels of seminal ROS and/or several endogenous antioxidants (i.e., total antioxidant capacity assay, superoxide dismutase, catalase, glutathione), markers of lipid peroxidation (i.e., malondialdehyde), oxidative DNA damage (8-hydroxy-2′ -deoxyguanosine), and oxidation-reduction potential (ORP) (Table 2). The lack of standardization in the evaluation of oxidative stress in seminal fluid before and after therapy hinders a definitive conclusion regarding the implementation of oral antioxidant supplementation for infertile men in the clinical practice. Moreover, the lack of detailed methodological descriptions in most articles testing oral antioxidants supplementation is a major shortcoming that makes comparisons between different studies difficult. The evaluation of the length of treatment in the analyzed studies also does not help in this regard, as it is variable, with 15 (15.5%) studies reporting the treatment for an unclear amount of time or less than 3 months (Table 2). This might result in difficulties to observe any significant influence on human spermatogenesis.
More recently, a systematic review and meta-analysis with data from seven RCTs using L-carnitine (LC) and L-acetyl carnitine (LAC) as treatment (LC 2 g/day+LAC 1 g/day in six studies and LC 150 mg/day+LAC 50 mg/day in one study during 12 or 24 weeks) enrolling a total of 693 patients, concluded that a combined therapy of LC and LAC is effective in men with idiopathic oligoasthenoteratozoospermia [122]. This conclusion was supported by a significant increase in forward sperm motility and total motile sperm count. All the other sperm characteristics analyzed including semen volume, sperm concentration and percentage of abnormal spermatozoa showed no change. Since most of the selected studies lacked consistent and detailed information, pregnancy of the female partner as an endpoint was not considered in the analysis. Nevertheless, the authors found that the combined therapy of LC+LAC could lead to higher pregnancy rates. Although this meta-analysis provides evidence for a positive effect of dietary supplementation with LC+LAC for 3–6 months in men with idiopathic oligoasthenoteratozoospermia seeking fertility treatment, there are several limitations that hamper a robust conclusion. First, the clinical diagnosis of idiopathic oligoasthenoteratozoospermia is open to interpretation. Thus, each study may have considered and recruited distinct types of patients. In addition, since the studies had a significant variance regarding the number of selected patients (21 patients in two studies and up to 175 patients in one study), this impedes the robustness of the conclusions. The most striking limitation, common to most studies, is the fact that the bioavailability of the compounds is unknown. Moreover, the mechanisms by which they target testicular function and exert their action is not well established and these studies do not provide evidence for a synergistic action versus a single compound action, nor do they clearly show how the compounds act.
CRITICAL EVALUATION OF THE NECESSITY OF ADDITIONAL DOUBLE-BLIND, RANDOMIZED, PLACEBO-CONTROLLED TRIALS
An important issue that must be pointed out in this context is that out of 90 studies that reported the effects of antioxidant treatment on semen parameters, 70 were low-quality studies, whereas only 20 were ranked as high-quality. The overall statistical analysis of both low- and high-quality studies indicates that the antioxidant treatment has a significant positive effect on semen parameters. A similar result could still be obtained for the effect of the antioxidant treatment on seminal oxidative stress and SDF. Only 60 of these studies evaluated oxidative stress markers. For the reproductive outcome, however, only a total of 19 studies, 5 of which were of high-quality, have reported this outcome parameter. Out of these 5 studies, 3 reported a positive effect, while in 2 studies either no effect or a negative effect was observed. These data clearly indicates that the number of high-quality studies is too low to obtain significant results. Some of the reasons are that fertilization is a multifactorial process. In this case the man is treated for oxidative stress, which reflects in improvements of semen parameters and seminal oxidative stress, even in high-quality studies. However, when looking at fertilization, pregnancy and live birth rates oocyte quality has to be considered as a confounding variable. These factors are also the reason why the number of studies necessary (n=202) to obtain a significant result for the high-quality studies is so high and is therefore unrealistic. Secondly, the general cost for high-quality double-blind, randomized, and placebo-controlled studies with a sufficiently high number of participants is also very high.
Smits et al (2019) [11] performed an extensive meta-analysis to evaluate if dietary supplementation with oral antioxidants was effective and safe. The authors analysed 61 studies involving 6,264 subfertile men seeking fertility treatment. The authors found that 18 different oral antioxidants were used in these studies. The most relevant conclusion was that oral antioxidant supplementation can improve the reproductive capacity of subfertile men and even enhance live birth rates. However, the evidence collected was considered of low or very low-quality due to serious study limitations, making the comparison and/or aggregation to perform robust statistical analysis difficult. The studies also showed a significant variation in the antioxidant supplementation regimens (type, dose, or even combined intake). Some studies used a placebo group to compare with, while others chose to compare with no treatment or treatment with another antioxidant.
The endpoint for couples attending a fertility treatment has to be clinical pregnancy or live birth, but most studies fail to present these data. Only 12 of the 44 studies that were included in the previously cited meta-analysis reported on clinical pregnancy and live birth, which is considered a major limitation [11]. The latter evidence has also been highlighted in our systematic analysis of the literature, where only 22 (22.7%) and 4 (4.1%) out of 97 studies reported pregnancy and live birth rates as a clinical outcome, respectively. Pregnancy and live birth are highly influenced by a wide variety of embryological and female factors. This highlights one of the major problems in literature as the studies on the use and effectiveness of antioxidants are mostly focused on the males and not on the couple. Studies need standardization not only regarding the selection of the males, but also the female partners to evaluate clinical pregnancy, achievement of live birth, and the influence of the treatment. Many confounding factors may interfere with such outcomes including female age, ovarian reserve, anatomic, inflammatory and endocrinal disorders, and as such, the impact of any fertility treatment can only be assessed after adjusting for such confounding factors. All these factors determine the type of ART treatment that is employed, namely in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), and eventually also have a significant impact on the reproductive outcome. Hence, the outcomes, positive or negative, can be skewed due to numerous confounding factors. Consequently, the focus of the effectiveness of an antioxidant treatment of men should be whether or not the treatment improves seminal parameters and sperm function, rather than reproductive outcomes which are influenced by numerous other variables that are, i) not/insufficiently considered in recent studies, and ii) can have a significant negative effect on blastulation, embryo development, onset/continuation of pregnancy, and live birth. Furthermore, in order to be able to judge the effectiveness of treatment, it is necessary to specify the andrological condition for which patients are being treated. Without this, one might include patients with a condition in a study which are not or only poorly responding to the treatment. In turn, this would negatively affect the outcome of the study.
We have identified 21 studies investigating the effects of antioxidant treatment on semen quality that were published from January 2019 to July 2020. The majority of these studies showed an improvement in semen parameters and sperm function. With a more detailed assessment of quality reported, 13 of the 21 were ranked as low-quality. Steiner et al [99] and Micic et al [94] strictly excluded couples with female factors, while most of the studies inadequately documented such criteria in their methodologies. From the molecular point of view, this integrative focus on the couple is essential. The sperm-oocyte interaction is sensitive to the redox balance, and excessive ROS in spermatozoa can lead to impaired oocyte function [123]. Hence, the effects of sperm oxidative damage go far beyond fertilization since they can have a negative impact on embryo development or even pregnancy loss [124,125]. On the other hand, the oocyte has the capability to repair damages in spermatozoa [126], and this is another unknown variable that needs to be considered when thinking of reproductive outcomes as a therapeutic effect of treating men with antioxidants. A number of critical points can further be pointed out from individual studies. Schisterman et al [46] lost 31% of their subjects during follow-up and a substantial number of couples received non-specified treatment off-site, which could alter the study results. The Steiner et al's [99] study was terminated early as they failed to show a >10% difference in pregnancy/live birth rates. Moreover, the authors failed to confirm adherence with antioxidant treatment, and ovarian stimulation was used in couples who did not conceive after 3 months of therapy, which could affect the outcomes. Terai et al [98] included men between 20 and 60 years of age, which is atypical of the reproductive age group. Abstinence was defined as 4 or more days without an upper limit [98]. Advanced male age and delayed abstinence are associated with increased oxidative stress measures and with alteration in semen parameters [127,128]. Furthermore, Terai et al [98] did not include a placebo group, with an experimental group investigating a Chinese herbal formula. Hadi et al [45], Alahmar et al [97], Hamidian et al [121], Salehi et al [42], Hasoon [43], Gambera et al [93], Arafa et al [25], Nazari et al [101], and Jannatifar et al [92] all reported improved semen parameters, but these studies are uncontrolled open label trials and are relatively underpowered. Furthermore, Alkumait et al [100] and Terai et al [98] were unblinded trials with relatively small sample sizes (n=51 and 31, respectively). On the other hand, the reviewed studies also have several positive virtues that can be pointed out. For example, the study by Agarwal et al [129] investigated changes in protein expression following antioxidant therapy, thereby enhancing our understanding of the physiologic alterations at the molecular level. Several studies assessed the effect of antioxidant therapy on SDF levels (Table 2). SDF is increasingly being utilized in the evaluation of male factor infertility and is believed to be an important determinant of fertility potential [130]. Furthermore, SDF is considered as an indirect measure of oxidative stress and can validate the benefits of antioxidant therapy in restoring the body's redox potential.
Therefore, for the above discussed reasons, having more double-blind RCT studies with a large enough sample size are neither feasible nor could they provide the expected clear result in terms of improved live birth rates after the antioxidant treatment.
STRENGTH WEAKNESS OPPORTUNITY THREAT (SWOT) ANALYSIS
1. Strengths
Antioxidant supplementation for the treatment of male infertility has been increasingly investigated in the past decade. Reports suggest that different antioxidant formulations were used to improve sperm quality and function in infertile men with various clinical circumstances (Table 2). These improvements were reflected on reproductive outcomes such as pregnancy rate (Table 2). An increasing number of studies have investigated the effect of antioxidant therapy on measures of oxidative stress, perhaps indicating it is a feasible treatment approach/option for patients with alteration in seminal redox potential.
2. Weaknesses
The contradictory results in reproductive outcomes seen in a number of studies can be considered as the main factor limiting the routine use of antioxidants for the treatment of male infertility, while female and embryological confounding factors were not taken into account (Table 2). Yet, in these studies, the male was treated with the false expectation that this treatment would automatically increase the success of the reproductive outcome. In addition, the low level of evidence was extrapolated from the clinical trials reporting benefit due to non-homogenous study designs, or inconsistencies in the treatment regimens (individual or combined) used (Table 2). Furthermore, the majority of these studies failed to adjust for confounding factors (e.g., female factors) that are essential for conception or establishment of pregnancy (Table 2).
3. Opportunities
Selection of suitable candidates for antioxidant supplementation by oxidative stress measurement seems a logical approach [25]. Indeed, the concept of MOSI has been recently proposed. The classification may guide the identification of a specific group of idiopathic infertile men who will be more likely to benefit from the treatment [2]. Secondly, evaluation of the sperm proteome of idiopathic infertile men before and after oral oxidant supplementation offers a window to better understanding the molecular mechanisms associated with sperm function (Fig. 3) [129]. Oral antioxidants may be an alternative cost-effective treatment option for infertile couples who desire to avoid assisted reproduction.
Fig. 3. Strength Weakness Opportunity Threat (SWOT) analysis. SWOT has been conducted to describe the impact of antioxidant supplementation in the treatment of male infertility. ART: assisted reproductive technology.
4. Threats
Despite the conclusion from Cochrane collaborations that oral antioxidant therapy may improve semen parameters and the likelihood of pregnancy, the lack of sufficient high-quality evidence still hinders a consensus among clinicians [11]. In addition, the wide variation in the treatment regimen raises concerns about overzealous use of antioxidants. The detrimental effects of reductive stress may be as pathological as that of oxidative stress [23]. Moreover, the often unpredictable outcome after antioxidant supplementation in the context of multiple confounding factors in reproduction may delay the definitive treatment, particularly for couples of advanced age.
CLINICAL GUIDELINES
While antioxidant supplementation is frequently utilized for the treatment of male factor infertility, no clear recommendations exist endorsing their use for specific clinical indications. Therefore, we aimed to develop clinical practice guidelines based on the available evidence to help in identifying the clinical circumstances in which antioxidant supplementation appears to be most beneficial. This systematic review included 97 articles, which investigated antioxidant treatment for various etiologies of male infertility. Very few studies explored the effect of antioxidants on semen quality of men with genitourinary inflammation (n=3), a hyperinsulinemic state (n=1) and recurrent pregnancy loss in female partners (n=1) (Table 2), and are hence insufficient for evidence-based recommendations for their use. On the other hand, there are some perceived high-quality studies available but, as highlighted above, due to the difficulties recruiting a sufficient number of patients and the small number of these studies, a statistical analysis will be underpowered and therefore does not provide the required answer. As we have shown in our analysis, the number of such studies conducted under the given circumstances and with a pre-selected set of criteria would have to be unreasonably high. Yet, looking at the diverse groups of conditions where oxidative stress is significantly involved and an antioxidant treatment would make sense, it is clear that in absence of prior testing for oxidative stress and without proper identification of suitable patient groups, the treatment will fail. Hence, appropriate patient identification is essential for the success of the treatment. We were able to formulate recommendations for antioxidant treatment for men with abnormal semen quality, IMI, UMI, and clinical varicocele based on studies reviewed (Table 2). However, it should be noted that our recommendations are based on the previously published studies, which, in our analysis, cannot be considered as high-quality because a number of variables (such as female factor, inclusion criteria, sample size esteem, etc.) were not properly reported. Therefore, our recommendation is that antioxidant treatment is possible and can result in improved male seminal parameters if the condition is caused by oxidative stress. It is self-evident that the therapy has to be monitored to not only avoid over-dosage of antioxidants, but also to see if it is successful or if alternative treatments are to be considered.
1. Abnormal semen quality
Antioxidants have long been investigated as a therapeutic option to counteract the harmful effects of ROS toxicity on various body systems. The reproductive system is one good example, as seminal oxidative stress is believed to be a common pathophysiology and various oxidative stress-associated aetiologies can alter sperm quality and function. Results of our review demonstrated that the majority of low-quality studies reported a significant improvement in conventional semen parameters and measures of sperm function. However, this result was not observed by high-quality studies. The recent Cochrane review revealed a somewhat similar result [11]. While an improvement in conventional semen parameters was noted over time, the findings were not reliable as a great deal of heterogeneity was observed across the included studies. Moreover, antioxidants were found to lower SDF compared to placebo.
Recommendation: antioxidants can improve conventional semen parameters and measures of sperm function (grade C recommendation).
2. Varicocele
Varicocele is the most common correctable cause of male infertility, prevalent in about 40% of men with primary infertility and up to 80% of men with secondary infertility [131]. Several studies have confirmed the presence of higher levels of oxidative stress in infertile men with varicocele in comparison to fertile men with or without varicocele and infertile men with idiopathic infertility [132,133,134,135,136]. This finding may justify the utility of antioxidants as a medical treatment strategy for varicocele. Nonetheless, in most patients, varicocelectomy remains the gold standard modality that results in sustained improvement in semen parameters and natural conception [137].
Most of the studies exploring the effect of antioxidant supplementation on semen parameters and sperm function were of low-quality. While the majority of these studies reported an improvement in outcome, the evidence supporting antioxidant use as a sole treatment for varicocele is not sufficient. Our sample size calculation confirms the need for further research in this regard in order to obtain a statistically significant effect. However, the reported improvement may be clinically relevant supporting antioxidant use as an adjunct therapy to varicocele ligation. A recent systematic review and meta-analysis explored antioxidant efficacy on improving semen quality after varicocelectomy [138]. The authors included 6 RCT with 576 patients receiving various antioxidant regimens or placebo following varicocelectomy. Significant improvements in sperm concentration (p<0.001), total motility (p=0.03), progressive motility (p<0.001) and normal morphology (p<0.001) were reported for the treatment group. However, pregnancy rate did not improve (p=0.36). Nonetheless, this finding confirms the presence of an additive effect of the antioxidant therapy in patients undergoing varicocelectomy.
Recommendation: antioxidants in addition to varicocele ligation result in further improvement in semen parameters (grade C recommendation).
3. Unexplained male infertility and idiopathic male infertility
Antioxidants are also commonly utilized for the treatment of patients with UMI or IMI. The former is defined by failure of conception despite having normal semen parameters, while the latter is characterized by the presence of semen abnormalities due to unknown etiology. The prevalence of UMI and IMI ranges between 6%–27% and 30%–58%, respectively [3,139]. Oxidative stress is believed to play a significant role in the pathophysiology of infertility of unknown origin and has been identified in 30%–40% of patients with UMI and up to 80% of patients with IMI [140,141,142].
Our analysis revealed that antioxidant use in men with IMI and UMI resulted in significant improvement in semen parameters and sperm function, as reported by high-quality studies. While the majority of low-quality studies echoed similar improvements, a larger number of studies are required to reach statistical significance.
A systematic review of 32 studies which assessed the impact of antioxidant therapy in IMI patients revealed an improvement of semen parameters, with the biggest benefit observed in sperm motility [143]. Fewer studies have assessed antioxidant treatment in patients with UMI. A recent study included 29 UMI patients who were treated with a combination of antioxidants for 3 months. The authors reported a significant increase in progressive motility (p=0.002), and a decrease in SDF (p=0.03) and ORP levels (p=0.02) following treatment. Greco et al [117] randomized 64 patients with UMI and elevated SDF to either treatment with vitamins C and E for 2 months or placebo. While there was no significant difference in semen parameter results, significant reduction in SDF was noted in the treatment group (p<0.001).
Recommendation: antioxidants significantly increase sperm quality in men with IMI and UMI (grade B recommendation).
CURRENT STATE AND FUTURE RECOMMENDATIONS FOR ANTIOXIDANT RESEARCH
The bivalent action of ROS as essential signaling molecules in physiological functions for sperm to fertilize an oocyte [144,145,146], as well as in mediating a detrimental effect on sperm functionality [147,148,149], suggests that appropriate clinical indications for prescribing antioxidants and monitoring the treatment are critical clinical considerations. Therefore, it is crucial to continue to study the physiological and pathophysiological mechanisms of ROS in the male reproductive tract and its relevance for sperm production and conception. In this regard, the physiological needs of functional spermatozoa, e.g., for the induction of capacitation and acrosome reaction, have to be considered. It appears that three aspects of sperm physiology have to be addressed, namely i) preservation and support of metabolism, ii) improvement of sperm maturation and function, and iii) protection against ROS-related trauma. Therefore, the correct ratio and concentration of antioxidants is essential for this therapeutic effect to occur and any new studies must take this into account.
Standardization of oxidative stress measurement must also be implemented, not only because a reproducible baseline is needed, but also because many of the current studies are either lacking or using different oxidative stress measurement and are therefore difficult to compare. The assessment of oxidative stress may also facilitate the identification of the best candidates and responders to antioxidant therapy (Fig. 4) [2].
Fig. 4. Treatment options for male oxidative stress infertility (MOSI). OS: oxidative stress, ORP: oxidation-reduction potential, MAGI: male accessory gland infection.
Recently, the MiOXSYS system has be proposed as a standardized assessment of seminal oxidative stress due to its ease of use, cost effectiveness, and reproducibility, as well as proposed evidence based clinical guidelines [150,151]. The MiOXSYS system measures the overall redox balance in seminal fluid to directly evaluate oxidative or reductive stress. Monitoring the treatment with antioxidants is another important aspect as overtreatment could lead to reductive stress-related infertility because the little amount of ROS, which is essential to trigger physiological functions of spermatozoa for it to fertilize oocytes, would be scavenged if the antioxidants are overdosed.
For a safe implementation of oral antioxidants use in a clinical setting, it is important not only to discover the molecular mechanisms of action of the bioactive compounds, but also the secondary effects that may arise. Personalized or adjusted prescription of oral antioxidants can enhance efficacy, without promoting over-dosage and deleterious health effects. Methodology and couple selection must be well reported. Doses and duration of the treatment should be adjusted according to several factors including the detected levels of ROS and antioxidant enzymes in seminal fluid and spermatozoa. Bioavailability of the compounds and their mechanism of action should also be thoroughly studied. More time-points to detect the oxidative balance of spermatozoa and seminal fluid should also be considered. Finally, one should not only look at the oral antioxidant therapy as a clinical treatment of the male and then expect that this treatment will result in the live birth of a healthy baby, but rather consider reproduction as a joint responsibility of both partners where a male and a female equally contribute to the reproductive outcome. Currently, the female partner is not examined for possible oxidative stress, but there is also a lack of knowledge about the impact of ROS and the redox level (oxidative and reductive stress) in the female reproductive system on oocyte development and maturation as well as on embryo development. A study by Ufer et al (2010) [152] indicated that proper embryo development depends on finely tuned redox control. On the other hand, elevated levels of antioxidants may result in teratogenic developments [153].
Currently, there is significant heterogeneity in trials reporting antioxidants on male infertility. This includes the condition investigated, type of antioxidant used, duration of the study, and the outcomes measured (Table 2). Furthermore, it is essential to select previously studied antioxidant candidates as well as new potential compounds, and also determine if they act better alone or in synergy with other antioxidants as has been shown in some studies [154]. Due to several factors, including the high costs and the high number of participants needed to reach a statistically robust study, the individuals recruited in any new study must be well selected based on strict inclusion/exclusion criteria. This means the patients should have the same infertility diagnosis and similar semen analysis: for example, mixing patients with azoospermia, mild oligozoospermia or asthenozoospermia should be avoided. Meeting these criteria will likely be difficult at a single institution and does not even include recruiting patients for an adequate control group. Use of placebo in the control group is also mandatory to avoid bias. Notably, confounding factors, including dietary habits, are often overlooked in most RCTs, but when patients are being enrolled in a study with antioxidant supplementation, baseline antioxidant intake and diet should be evaluated in both partners, male and female. Furthermore, an appropriate primary outcome, such as decrease in oxidative stress or SDF, should also be evaluated. Finally, it is critical to set a correct time period for the study. A short to medium time period of 3 to 6 months is considered ideal to study the antioxidant effects on semen parameters.
UNRESOLVED QUESTIONS
What makes the situation even more complicated is that there are no dose-response studies in humans to pinpoint the optimum antioxidant dosages needed to produce improvement in semen quality. We also still do not know the normal physiological range of the redox levels in both males and females. For spermatozoa, a recent study by Panner Selvam et al (2020) [24] indicated normal redox values between −9.76 and 1.48 mV/106 sperm/mL. Although these values already indicate oxidative or reductive stress conditions, the normal physiological range will be much narrowed in between these values. In the female, one might have to consider variations depending on the menstrual cycle and/or the onset of pregnancy. Besides, these values might change with age and/or health status. Furthermore, the effective bioavailability of antioxidants in the testes, the epididymis and the semen is still unknown. Since many antioxidants (e.g., co-enzyme Q10, vitamin E, and carnitines) can easily cross the blood-testis barrier, a proper balance for the redox level has to be achieved because an over-dosage might lead to reductive stress, which has been shown to be as harmful as oxidative stress [22].
CONCLUSIONS
This systematic review identified a significant number of well-designed studies that unequivocally show the beneficial effects of oral antioxidants in improving semen parameters and pregnancy outcome. However, despite the safety and efficacy of the antioxidant therapy, five main factors have hindered its wide acceptance and implementation in the treatment of male infertility: i) lack of randomized placebo-controlled studies that show the safety and efficacy of antioxidants in improving pregnancy rates in infertile couples; ii) type of antioxidant to be used; iii) dose; iv) duration of treatment; and v) costs.
Although randomized placebo-controlled studies are regarded as the gold standard in the validation of the safety and efficacy of therapies, given the fact that the occurrence of a pregnancy is a multifactorial process mainly determined by the genomic quality of the egg, to show the impact of antioxidant therapy on pregnancy outcome in randomized placebo-controlled studies would be unrealistic and extremely difficult to perform. Given the difficulty in carrying out these randomized placebo-controlled studies and the existence of significant clinical evidence supporting the safety and efficacy of antioxidants in improving pregnancy outcome in infertile couples, the use of antioxidant therapy should be recommended.
To answer the question of what type of antioxidant should be used, we believe that antioxidants that readily cross the blood-testis and blood-epididymis barriers should be recommended. The formulation should be well-balanced as lipid-soluble and water-soluble antioxidants together with other factors are closely interacting, thereby regenerating lipid-located antioxidants. If this balance is not given, it may not only result in suboptimal antioxidant effects, but also in paradoxical prooxidant effects due to interference in redox reactions.
Concerning the dose to be used, the dose should be high enough to restore the normal physiological cellular functions by reducing oxidative stress without compromising the physiological role of ROS in sperm maturation and fertilization reactions. An overdosage which may lead to reductive stress should be avoided.
The duration of an antioxidant therapy would have to be adjusted according to the place where the damage occurs. If it is in the epididymis, a treatment course of at least two weeks should be sufficient to counteract ROS-induced damage. In addition, since oxidative stress in the epididymis is a constitutive process and antioxidants have no side-effects, antioxidant therapy should be recommended until pregnancy is achieved. This would apply to couples undergoing timed intercourse as well as to couples undergoing in vitro fertilization. On the other hand, if the oxidative damage is occurring in the testes such as in the case of clinical varicocele, the duration of antioxidant treatment should be of at least three months.
Finally, the cost argument has to be seen from the perspective of the sponsors of high quality randomized, double-blind placebo-controlled clinical trials. Natural antioxidant formulations have very low cost, and their use would be amply justified based on efficacy and safety as well as for cost-saving aspects for patients and health systems. The problem for the possible funding by the pharmaceutical industry of the studies is to recover the high costs of a trial on a cheap antioxidant, which can be available over the counter, and for which not even intellectual property rights are available.
In conclusion, the use of antioxidants that readily cross the blood-testis and blood-epididymis barriers should be recommended. Their efficacy, lack of side-effects and low costs should encourage their wider acceptance and implementation among infertility specialists for their use in the treatment of male infertility and infertile couples. Despite these favorable aspects of antioxidant treatments for idiopathic and UMI, more high-quality research is needed to understand the effects of ROS and antioxidants on the human fertilizing potential in both male and female. However, the practicality of conducting such studies remains questionable.
ACKNOWLEDGEMENTS
Authors are thankful to the artists from the Cleveland Clinic's Center for Medical Art & Photography for their help with the illustrations. The study was supported by the American Center for Reproductive Medicine (Andrology Research Fund #500000105879).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: AA.
- Writing — original draft: all the authors.
- Writing — review & editing: all the authors.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200196.
‘AND NOT’ Boolean operators additionally included in the keywords string to exclude animal studies
Criteria for the classification of the controlled studies into low and high quality categories
Criteria for the classification of the studies published between January 2019–July 2020 into low (total score <6) and high quality (total score ≥6)
References
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–594. doi: 10.1590/s1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 4.Leisegang K, Dutta S. Do lifestyle practices impede male fertility? Andrologia. 2020 doi: 10.1111/and.13595. [Epub] [DOI] [PubMed] [Google Scholar]
- 5.Leisegang K, Henkel R. Oxidative stress: relevance, evaluation, and management. In: Rizk B, Agarwal A, Sabanegh ES, editors. Male infertility in reproductive medicine: diagnosis and management. Boca Raton: CRC Press; 2019. pp. 119–128. [Google Scholar]
- 6.Cardoso JP, Cocuzza M, Elterman D. Optimizing male fertility: oxidative stress and the use of antioxidants. World J Urol. 2019;37:1029–1034. doi: 10.1007/s00345-019-02656-3. [DOI] [PubMed] [Google Scholar]
- 7.Kuchakulla M, Soni Y, Patel P, Parekh N, Ramasamy R. A systematic review and evidence-based analysis of ingredients in popular male fertility supplements. Urology. 2020;136:133–141. doi: 10.1016/j.urology.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011;(1):CD007411. doi: 10.1002/14651858.CD007411.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;(12):CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–124. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhling K, Schumacher A, Eulenburg CZ, Laakmann E. Influence of oral vitamin and mineral supplementation on male infertility: a meta-analysis and systematic review. Reprod Biomed Online. 2019;39:269–279. doi: 10.1016/j.rbmo.2019.03.099. [DOI] [PubMed] [Google Scholar]
- 14.McPherson NO, Shehadeh H, Fullston T, Zander-Fox DL, Lane M. Dietary micronutrient supplementation for 12 days in obese male mice restores sperm oxidative stress. Nutrients. 2019;11:2196. doi: 10.3390/nu11092196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay R, Yasmin S, Chakravarty B. Effect of continuous 6 months oral antioxidant combination with universally recommended dosage in idiopathic male infertility. IJIFM. 2016;7:1–6. [Google Scholar]
- 17.da Silva TM, Maia MCS, Arruda JT, Approbato FC, Mendonça CR, Approbato MS. Folic acid does not improve semen parametrs in subfertile men: a double-blin, randomized, placebo-controlled study. JBRA Assist Reprod. 2013;17:152–157. [Google Scholar]
- 18.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 19.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 20.Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–421. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Castagné V, Lefèvre K, Natero R, Clarke PG, Bedker DA. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience. 1999;93:313–320. doi: 10.1016/s0306-4522(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 23.Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 24.Panner Selvam MK, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic Biol Med. 2020;152:375–385. doi: 10.1016/j.freeradbiomed.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Arafa M, Agarwal A, Majzoub A, Panner Selvam MK, Baskaran S, Henkel R, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants (Basel) 2020;9:219. doi: 10.3390/antiox9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia. 2018;50:e12927. doi: 10.1111/and.12927. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: the Cambridge Quality Checklists. J Exp Criminol. 2009;5:1–23. [Google Scholar]
- 29.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 31.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roseff SJ. Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med. 2002;47:821–824. [PubMed] [Google Scholar]
- 33.Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007;47:216–221. doi: 10.1111/j.1479-828X.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 34.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–768. doi: 10.1016/s1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 35.Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7:137–144. doi: 10.1093/ecam/nem171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejarano I, Monllor F, Marchena AM, Ortiz A, Lozano G, Jiménez MI, et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J Pineal Res. 2014;57:333–339. doi: 10.1111/jpi.12172. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med. 2016;62:387–395. doi: 10.1080/19396368.2016.1246623. [DOI] [PubMed] [Google Scholar]
- 38.Hosseini J, Mardi Mamaghani A, Hosseinifar H, Sadighi Gilani MA, Dadkhah F, Sepidarkish M. The influence of ginger (Zingiber officinale) on human sperm quality and DNA fragmentation: a double-blind randomized clinical trial. Int J Reprod Biomed. 2016;14:533–540. [PMC free article] [PubMed] [Google Scholar]
- 39.Stenqvist A, Oleszczuk K, Leijonhufvud I, Giwercman A. Impact of antioxidant treatment on DNA fragmentation index: a double-blind placebo-controlled randomized trial. Andrology. 2018;6:811–816. doi: 10.1111/andr.12547. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90:627–635. doi: 10.1016/j.fertnstert.2007.07.1314. [DOI] [PubMed] [Google Scholar]
- 41.Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res. 2018;32:514–521. doi: 10.1002/ptr.5998. [DOI] [PubMed] [Google Scholar]
- 42.Salehi P, Zahra Shahrokhi S, Kamran T, Ajami A, Taghiyar S, Reza Deemeh M. Effect of antioxidant therapy on the sperm DNA integrity improvement; a longitudinal cohort study. Int J Reprod Biomed. 2019;17:99–106. doi: 10.18502/ijrm.v17i2.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasoon MA. Using of the L-arginine and co-enzyme Q10 shows improvement of the male subfertility. IJDDT. 2019;9:544–551. [Google Scholar]
- 44.Nurmawati D, Hinting A, Sudjarwo Astaxanthin improves erythrocyte sedimentation rate (ESR), Malondialdehyde (MDA), 8-hydroxydeoxyguanosine (8-OH-Dg) levels, and semen quality in human sperm. IJSTR. 2020;9:6896–6903. [Google Scholar]
- 45.Hadi AM, Abbass YI, Yadgar MA. The impact of L-carnitine supplement on semen variables and the levels of sexual hormones (serum LH, FSH, testosterone, and inhibin) in males with infertility. Medico Leg Update. 2020;20:772–776. [Google Scholar]
- 46.Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA. 2020;323:35–48. doi: 10.1001/jama.2019.18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–165. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- 48.Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 49.Oliva A, Dotta A, Multigner L. Pentoxifylline and antioxidants improve sperm quality in male patients with varicocele. Fertil Steril. 2009;91(4 Suppl):1536–1539. doi: 10.1016/j.fertnstert.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Festa R, Giacchi E, Raimondo S, Tiano L, Zuccarelli P, Silvestrini A, et al. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: an open, uncontrolled pilot study. Andrologia. 2014;46:805–807. doi: 10.1111/and.12152. [DOI] [PubMed] [Google Scholar]
- 51.Pourmand G, Movahedin M, Dehghani S, Mehrsai A, Ahmadi A, Pourhosein M, et al. Does L-carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Urology. 2014;84:821–825. doi: 10.1016/j.urology.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Nematollahi-Mahani SN, Azizollahi GH, Baneshi MR, Safari Z, Azizollahi S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia. 2014;46:240–245. doi: 10.1111/and.12067. [DOI] [PubMed] [Google Scholar]
- 53.Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–238. doi: 10.1590/S1677-5538.IBJU.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–229. doi: 10.3109/14647273.2015.1050462. [DOI] [PubMed] [Google Scholar]
- 55.Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10:120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kızılay F, Altay B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: a randomized controlled trial. Int J Impot Res. 2019;31:424–431. doi: 10.1038/s41443-018-0109-4. [DOI] [PubMed] [Google Scholar]
- 57.Ardestani Zadeh A, Arab D, Kia NS, Heshmati S, Amirkhalili SN. The role of vitamin E - selenium - folic acid supplementation in improving sperm parameters after varicocelectomy: a randomized clinical trial. Urol J. 2019;16:495–500. doi: 10.22037/uj.v0i0.4653. [DOI] [PubMed] [Google Scholar]
- 58.Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- 59.Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–1033. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- 60.Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001;16:2338–2342. doi: 10.1093/humrep/16.11.2338. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki M, Kurabayashi T, Yamamoto Y, Fujita K, Tanaka K. Effects of antioxidant treatment in oligozoospermic and asthenozoospermic men. J Reprod Med. 2003;48:707–712. [PubMed] [Google Scholar]
- 62.Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81:93–98. doi: 10.1016/j.fertnstert.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. Sperm quality improvement after natural antioxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 2008;10:201–206. doi: 10.1111/j.1745-7262.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 64.Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235. doi: 10.1016/j.fertnstert.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad MK, Mahdi AA, Shukla KK, Islam N, Rajender S, Madhukar D, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94:989–996. doi: 10.1016/j.fertnstert.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 66.Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest. 2011;34:e224–e228. doi: 10.3275/7572. [DOI] [PubMed] [Google Scholar]
- 67.Shukla KK, Mahdi AA, Mishra V, Rajender S, Sankhwar SN, Patel D, et al. Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online. 2011;22:421–427. doi: 10.1016/j.rbmo.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic antioxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 69.Safarinejad MR. Effect of pentoxifylline on semen parameters, reproductive hormones, and seminal plasma antioxidant capacity in men with idiopathic infertility: a randomized double-blind placebo-controlled study. Int Urol Nephrol. 2011;43:315–328. doi: 10.1007/s11255-010-9826-4. [DOI] [PubMed] [Google Scholar]
- 70.Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. doi: 10.2147/IJGM.S16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Safarinejad MR, Shafiei N, Safarinejad S. A prospective double-blind randomized placebo-controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia. Phytother Res. 2011;25:508–516. doi: 10.1002/ptr.3294. [DOI] [PubMed] [Google Scholar]
- 72.Safarinejad MR. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: an open-label prospective study. Int Urol Nephrol. 2012;44:689–700. doi: 10.1007/s11255-011-0081-0. [DOI] [PubMed] [Google Scholar]
- 73.Abad C, Amengual MJ, Gosálvez J, Coward K, Hannaoui N, Benet J, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia. 2013;45:211–216. doi: 10.1111/and.12003. [DOI] [PubMed] [Google Scholar]
- 74.Ajayi R, Okhowat J, Spitzer D, Schechinger B, Zech NH. Impact of antioxidative supplementation on semen quality according to MSOME criteria. JBRA Assist Reprod. 2013;17:27–31. [Google Scholar]
- 75.Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Effect of coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014;46:177–183. doi: 10.1111/and.12062. [DOI] [PubMed] [Google Scholar]
- 76.Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, et al. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia. 2014;46:956–962. doi: 10.1111/and.12180. [DOI] [PubMed] [Google Scholar]
- 77.Kobori Y, Ota S, Sato R, Yagi H, Soh S, Arai G, et al. Antioxidant cosupplementation therapy with vitamin C, vitamin E, and coenzyme Q10 in patients with oligoasthenozoospermia. Arch Ital Urol Androl. 2014;86:1–4. doi: 10.4081/aiua.2014.1.1. [DOI] [PubMed] [Google Scholar]
- 78.Thakur AS, Littarru GP, Funahashi I, Painkara US, Dange NS, Chauhan P. Effect of ubiquinol therapy on sperm parameters and serum testosterone levels in oligoasthenozoospermic infertile men. J Clin Diagn Res. 2015;9:BC01–BC03. doi: 10.7860/JCDR/2015/13617.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobori Y, Suzuki K, Iwahata T, Shin T, Sadaoka Y, Sato R, et al. Improvement of seminal quality and sexual function of men with oligoasthenoteratozoospermia syndrome following supplementation with L-arginine and Pycnogenol®. Arch Ital Urol Androl. 2015;87:190–193. doi: 10.4081/aiua.2015.3.190. [DOI] [PubMed] [Google Scholar]
- 80.Hadwan MH, Almashhedy LA, Alsalman AR. Oral zinc supplementation restores superoxide radical scavengers to normal levels in spermatozoa of Iraqi asthenospermic patients. Int J Vitam Nutr Res. 2015;85:165–173. doi: 10.1024/0300-9831/a000235. [DOI] [PubMed] [Google Scholar]
- 81.Al-Hilli AS, Al-Mousawi NAH, Ali AS. Use of Simvastation as antioxidant drug significantly decreases lipid peroxidation by utilization of malondialdehyde (MAD) level assay as an indicator of spermatozoal oxidative stress in male infertile patient. Kufa Med J. 2009;12:488–495. [Google Scholar]
- 82.Martinez AM, Sordia-Hernández LH, Morales JA, Merino M, Vidal O, Garza MRG, et al. A randomized clinical study assessing the effects of the antioxidants, resveratrol or SG1002, a hydrogen sulfide prodrug, on idiopathic oligoasthenozoospermia. Asian Pac J Reprod. 2015;4:106–111. [Google Scholar]
- 83.Gvozdjáková A, Kucharská J, Dubravicky J, Mojto V, Singh RB. Coenzyme Q10, α-tocopherol, and oxidative stress could be important metabolic biomarkers of male infertility. Dis Markers. 2015;2015:827941. doi: 10.1155/2015/827941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.ElSheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: a prospective, randomized trial. Andrology. 2015;3:864–867. doi: 10.1111/andr.12086. [DOI] [PubMed] [Google Scholar]
- 85.Montanino Oliva M, Minutolo E, Lippa A, Iaconianni P, Vaiarelli A. Effect of myoinositol and antioxidants on sperm quality in men with metabolic syndrome. Int J Endocrinol. 2016;2016:1674950. doi: 10.1155/2016/1674950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, Jahan N, Radhakrishnan G, Banerjee BD. To evaluate the efficacy of combination antioxidant therapy on oxidative stress parameters in seminal plasma in the male infertility. J Clin Diagn Res. 2016;10:QC14–QC17. doi: 10.7860/JCDR/2016/15597.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alahmar AT. Effect of vitamin C, vitamin E, zinc, selenium, and coenzyme Q10 in infertile men with idiopathic oligoasthenozoospermia. IJIFM. 2017;8:45–49. [Google Scholar]
- 88.Yamamoto Y, Aizawa K, Mieno M, Karamatsu M, Hirano Y, Furui K, et al. The effects of tomato juice on male infertility. Asia Pac J Clin Nutr. 2017;26:65–71. doi: 10.6133/apjcn.102015.17. [DOI] [PubMed] [Google Scholar]
- 89.Magdi Y, Darwish E, Elbashir S, Majzoub A, Agarwal A. Effect of modifiable lifestyle factors and antioxidant treatment on semen parameters of men with severe oligoasthenoteratozoospermia. Andrologia. 2017;49:e12694. doi: 10.1111/and.12694. [DOI] [PubMed] [Google Scholar]
- 90.Alsalman ARS, Almashhedy LA, Hadwan MH. Effect of oral zinc supplementation on the thiol oxido-reductive index and thiol-related enzymes in seminal plasma and spermatozoa of Iraqi asthenospermic patients. Biol Trace Elem Res. 2018;184:340–349. doi: 10.1007/s12011-017-1215-8. [DOI] [PubMed] [Google Scholar]
- 91.Lu XL, Liu JJ, Li JT, Yang QA, Zhang JM. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: a placebo-controlled, double-blind trial. Andrologia. 2018;50:e13033. doi: 10.1111/and.13033. [DOI] [PubMed] [Google Scholar]
- 92.Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol. 2019;17:24. doi: 10.1186/s12958-019-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gambera L, Stendardi A, Ghelardi C, Fineschi B, Aini R. Effects of antioxidant treatment on seminal parameters in patients undergoing in vitro fertilization. Arch Ital Urol Androl. 2019;91:187–190. doi: 10.4081/aiua.2019.3.187. [DOI] [PubMed] [Google Scholar]
- 94.Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia. 2019;51:e13267. doi: 10.1111/and.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33:3203–3211. doi: 10.1002/ptr.6493. [DOI] [PubMed] [Google Scholar]
- 96.Busetto GM, Del Giudice F, Virmani A, Sciarra A, Maggi M, Ferro M, et al. Body mass index and age correlate with antioxidant supplementation effects on sperm quality: post hoc analyses from a double-blind placebo-controlled trial. Andrologia. 2020;52:e13523. doi: 10.1111/and.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alahmar AT, Calogero AE, Sengupta P, Dutta S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J Mens Health. 2020 doi: 10.5534/wjmh.190145. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Terai K, Horie S, Fukuhara S, Miyagawa Y, Kobayashi K, Tsujimura A. Combination therapy with antioxidants improves total motile sperm counts: a preliminary study. Reprod Med Biol. 2020;19:89–94. doi: 10.1002/rmb2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, et al. Reproductive Medicine Network. The effect of antioxidants on male factor infertility: the Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113:552–560. doi: 10.1016/j.fertnstert.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alkumait MHMS, Abdul-Aziz MM, Nima MH. The effect of glutathione versus co-enzyme Q10 on male infertility original study. Medico Leg Update. 2020;20:409–414. [Google Scholar]
- 101.Nazari L, Salehpour S, Hosseini S, Allameh F, Jahanmardi F, Azizi E, et al. Effect of antioxidant supplementation containing L-carnitine on semen parameters: a prospective interventional study. JBRA Assist Reprod. 2020 doi: 10.5935/1518-0557.20200043. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goyal A, Chopra M, Lwaleed BA, Birch B, Cooper AJ. The effects of dietary lycopene supplementation on human seminal plasma. BJU Int. 2007;99:1456–1460. doi: 10.1111/j.1464-410X.2007.06804.x. [DOI] [PubMed] [Google Scholar]
- 103.Tartibian B, Maleki BH. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycling training. J Androl. 2012;33:449–461. doi: 10.2164/jandrol.110.012815. [DOI] [PubMed] [Google Scholar]
- 104.Williams EA, Parker M, Robinson A, Pitt S, Pacey AA. A randomized placebo-controlled trial to investigate the effect of lactolycopene on semen quality in healthy males. Eur J Nutr. 2020;59:825–833. doi: 10.1007/s00394-019-02091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vicari E, La Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil Steril. 2002;78:1203–1208. doi: 10.1016/s0015-0282(02)04350-9. [DOI] [PubMed] [Google Scholar]
- 106.Yang CC, Chen JC, Chen GW, Chen YS, Chung JG. Effects of Shao-Fu-Zhu-Yu-Tang on motility of human sperm. Am J Chin Med. 2003;31:573–579. doi: 10.1142/S0192415X03001223. [DOI] [PubMed] [Google Scholar]
- 107.Chayachinda C, Thamkhantho M, Ngamskulrungroj P. Effects of coenzyme Q10 on sperm motility of infertile men with pyospermia treated with doxycycline: a randomized controlled trial. J Med Assoc Thai. 2020;103:121–127. [Google Scholar]
- 108.Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility--a preliminary report. Int Urol Nephrol. 2002;34:369–372. doi: 10.1023/a:1024483520560. [DOI] [PubMed] [Google Scholar]
- 109.Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005;84:662–671. doi: 10.1016/j.fertnstert.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 110.Heidary M, Vahhabi S, Reza Nejadi J, Delfan B, Birjandi M, Kaviani H, et al. Effect of saffron on semen parameters of infertile men. Urol J. 2008;5:255–259. [PubMed] [Google Scholar]
- 111.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74:73–76. doi: 10.1016/j.urology.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 112.Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104:318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 113.Soleimani M, Masoumi N. The effect of grape seed extract on semen oxidative stress markers in men with idiopathic infertility: a cross-sectional before-after study. Nephro-Urol Mon. 2017;9:e13837 [Google Scholar]
- 114.Negri L, Benaglia R, Monti E, Morenghi E, Pizzocaro A, Levi Setti PE. Effect of superoxide dismutase supplementation on sperm DNA fragmentation. Arch Ital Urol Androl. 2017;89:212–218. doi: 10.4081/aiua.2017.3.212. [DOI] [PubMed] [Google Scholar]
- 115.Kopets R, Kuibida I, Chernyavska I, Cherepanyn V, Mazo R, Fedevych V, et al. Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: a randomized clinical study. Andrology. 2020;8:1184–1193. doi: 10.1111/andr.12805. [DOI] [PubMed] [Google Scholar]
- 116.Greco E, Romano S, Iacobelli M, Ferrero S, Baroni E, Minasi MG, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod. 2005;20:2590–2594. doi: 10.1093/humrep/dei091. [DOI] [PubMed] [Google Scholar]
- 117.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 118.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 119.Khani B, Bidgoli SR, Moattar F, Hassani H. Effect of sesame on sperm quality of infertile men. J Res Med Sci. 2013;18:184–187. [PMC free article] [PubMed] [Google Scholar]
- 120.Bosman E, Esterhuizen AD, Rodrigues FA, Becker PJ, Hoffmann WA. Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males. Andrologia. 2015;47:974–979. doi: 10.1111/and.12366. [DOI] [PubMed] [Google Scholar]
- 121.Hamidian S, Talebi AR, Fesahat F, Bayat M, Mirjalili AM, Ashrafzadeh HR, et al. The effect of vitamin C on the gene expression profile of sperm protamines in the male partners of couples with recurrent pregnancy loss: a randomized clinical trial. Clin Exp Reprod Med. 2020;47:68–76. doi: 10.5653/cerm.2019.03188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia. 2020;52:e13470. doi: 10.1111/and.13470. [DOI] [PubMed] [Google Scholar]
- 123.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 125.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–2412. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 126.Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 127.Darbandi S, Darbandi M, Khorshid HRK, Sadeghi MR, Heidari M, Cheshmi G, et al. The effect of paternal age on semen quality and fertilization outcome in men with normal sperm DNA compaction, reactive oxygen species, and total antioxidant capacity levels. Turk J Urol. 2019;45:164–170. doi: 10.5152/tud.2019.74944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–110. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 129.Agarwal A, Panner Selvam MK, Samanta L, Vij SC, Parekh N, Sabanegh E, et al. Effect of antioxidant supplementation on the sperm proteome of idiopathic infertile men. Antioxidants (Basel) 2019;8:488. doi: 10.3390/antiox8100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–950. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agarwal A, Esteves SC. Varicocele and male infertility: current concepts and future perspectives. Asian J Androl. 2016;18:161–162. doi: 10.4103/1008-682X.172819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril. 2010;94:1531–1534. doi: 10.1016/j.fertnstert.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 133.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 134.Mehraban D, Ansari M, Keyhan H, Sedighi Gilani M, Naderi G, Esfehani F. Comparison of nitric oxide concentration in seminal fluid between infertile patients with and without varicocele and normal fertile men. Urol J. 2005;2:106–110. [PubMed] [Google Scholar]
- 135.Mostafa T, Anis T, El Nashar A, Imam H, Osman I. Seminal plasma reactive oxygen species-antioxidants relationship with varicocele grade. Andrologia. 2012;44:66–69. doi: 10.1111/j.1439-0272.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 136.Mostafa T, Anis T, Imam H, El-Nashar AR, Osman IA. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41:125–129. doi: 10.1111/j.1439-0272.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 137.Sharlip ID, Jarow J, Belker AM, Damewood M, Howards SS, Lipshultz LI, et al. Report on varicocele and infertility: an AUA best practice policy and ASRM Practice Committee report [Internet] Linthicum (MD): American Urological Association; c2001. [cited 2017 May 31]. Available from: https://www.academia.edu/21886760/Report_on_Varicocele_and_Infertility. [Google Scholar]
- 138.Wang J, Wang T, Ding W, Wu J, Wu G, Wang Y, et al. Efficacy of antioxidant therapy on sperm quality measurements after varicocelectomy: a systematic review and meta-analysis. Andrologia. 2019;51:e13396. doi: 10.1111/and.13396. [DOI] [PubMed] [Google Scholar]
- 139.Moghissi KS, Wallach EE. Unexplained infertility. Fertil Steril. 1983;39:5–21. doi: 10.1016/s0015-0282(16)46750-6. [DOI] [PubMed] [Google Scholar]
- 140.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ko EY, Sabanegh ES, Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102:1518–1527. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 142.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2017;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. 2014;2014:426951. doi: 10.1155/2014/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Lamirande E, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med. 1993;14:157–166. doi: 10.1016/0891-5849(93)90006-g. [DOI] [PubMed] [Google Scholar]
- 145.O'Flaherty C. Redox regulation of mammalian sperm capacitation. Asian J Androl. 2015;17:583–590. doi: 10.4103/1008-682X.153303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baskaran S, Finelli R, Agarwal A, Henkel R. Reactive oxygen species in male reproduction: a boon or a bane? Andrologia. 2020 doi: 10.1111/and.13577. [Epub] [DOI] [PubMed] [Google Scholar]
- 147.Dias TR, Martin-Hidalgo D, Silva BM, Oliveira PF, Alves MG. Endogenous and exogenous antioxidants as a tool to ameliorate male infertility induced by reactive oxygen species. Antioxid Redox Signal. 2020 doi: 10.1089/ars.2019.7977. [Epub] [DOI] [PubMed] [Google Scholar]
- 148.Martin-Hidalgo D, Bragado MJ, Batista AR, Oliveira PF, Alves MG. Antioxidants and male fertility: from molecular studies to clinical evidence. Antioxidants (Basel) 2019;8:89. doi: 10.3390/antiox8040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17:87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agarwal A, Arafa M, Chandrakumar R, Majzoub A, AlSaid S, Elbardisi H. A multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible method. Andrology. 2017;5:939–945. doi: 10.1111/andr.12395. [DOI] [PubMed] [Google Scholar]
- 151.Agarwal A, Henkel R, Sharma R, Tadros NN, Sabanegh E. Determination of seminal oxidation-reduction potential (ORP) as an easy and cost-effective clinical marker of male infertility. Andrologia. 2018;50:e12914. doi: 10.1111/and.12914. [DOI] [PubMed] [Google Scholar]
- 152.Ufer C, Wang CC, Borchert A, Heydeck D, Kuhn H. Redox control in mammalian embryo development. Antioxid Redox Signal. 2010;13:833–875. doi: 10.1089/ars.2009.3044. [DOI] [PubMed] [Google Scholar]
- 153.Wang CC, Rogers MS. Oxidative stress and fetal hypoxia. In: Góth L, editor. Reactive oxygen species and diseases. Trivandrum: Research Signpost; 2007. pp. 257–282. [Google Scholar]
- 154.Dias TR, Alves MG, Casal S, Silva BM, Oliveira PF. The single and synergistic effects of the major tea components caffeine, epigallocatechin-3-gallate and L-theanine on rat sperm viability. Food Funct. 2016;7:1301–1305. doi: 10.1039/c5fo01611h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘AND NOT’ Boolean operators additionally included in the keywords string to exclude animal studies
Criteria for the classification of the controlled studies into low and high quality categories
Criteria for the classification of the studies published between January 2019–July 2020 into low (total score <6) and high quality (total score ≥6)