Abstract
Objectives
To determine the association of Candida phenotypes, virulence factors, antifungal sensitivity and clinical response to Fluconazole in Oral leukoplakia (OL).
Methods
Sterile swabs were obtained from oral lesions in immunocompetent subjects [30 Homogenous (HOL), 31 Non- Homogenous (NHOL] and normal buccal mucosa in 30 age and sex-matched healthy controls (C). Candida phenotypes, virulence factors (Secreted Aspartyl Proteinase (SAP), Phospholipase (PL), Biofilm formation (BF) and antifungal sensitivity were determined. Clinical features (Size, Erythema, thickness, oral burning sensation (VAS scores) before and after Fluconazole therapy in OL were recorded by two calibrated observers.
Results
Candida was associated with OL (p < 0.01). Candida albicans was the most common phenotype sensitive to Fluconazole. SAP, PL and BF activity was significantly high in NHOL. Strong positive correlation was seen between SAP, and PL activity and pre-treatment VAS scores in NHOL. There was significant reduction in VAS scores, size of lesion [HOL (p < 0.001) NHOL (p < 0.05)], erythematous areas (67.8%) in NHOL and thickness of lesions (42.6%) in both types OL after Fluconazole therapy with substantial inter-observer agreement. Non albicans candida (NAC) species had similar virulence profiles but resistant to Fluconazole and showed minimal clinical improvement.
Conclusions
Virulence activity of Candida in OL increases severity of lesions. Fluconazole is effective against virulent Candida albicans, causes clinical improvement and down-staging from high –risk NHOL to low-risk HOL which can reduce risk of malignant transformation. Detection of highly virulent NAC infection and antifungal sensitivity is recommended in OL recalcitrant to Fluconazole therapy.
Keywords: Albicans, Candida, Antifungal drugs, Aspartyl proteinase, Biofilm formation, Cancer prevention, Oral leukoplakia, Phospholipase
Highlights
-
•
Oral leukoplakia is associated with highly virulent forms of Candida that can induce inflammation.
-
•
Virulence activity of Candida is increased in Non- Homogenous lesions and increases severity and oral burning sensation.
-
•
Virulence profile of Non albicans Candida is similar to Candida albicans but resistant to Fluconazole.
-
•
Fluconazole causes down- staging from high-risk to low-risk lesions, in Candida albicans associated Oral Leukoplakia.
-
•
Identification of Non albicans Candida species and antifungal sensitivity is recommended in recalcitrant lesions.
1. Introduction
Oral Leukoplakia (OL) is a common oral potentially malignant disorder (OPMD) associated with tobacco and alcohol use. It has a high malignant transformation rate of (0.13–34%) into Oral Squamous Cell Carcinoma (OSCC).1 The two common types of OL are Homogenous Oral Leukoplakia (HOL) which has a uniform, flat, thin, smooth/wrinkled/corrugated surface throughout the lesion whereas Non-homogenous Oral leukoplakia (NHOL) has a mixture of red and white lesions with a irregularly speckled/nodular/verrucous surface.2
The etiological role of Candida in OSCC and OL has been a matter of debate over the years but candidal invasion has been associated with significant risk factor for malignant transformation in OL.3,4The association has not been proven to be causal as yet but believed to be incidental due to favourable oral environmental factors and surface provided by OL for adherence of Candida, a common commensal of the oral cavity. Candida super-infection has been found in 6.8–100% of OL and is higher in non-homogenous leukoplakia. OL with Candida is also associated with epithelial hyperplasia, atypia (14.3–56.3%), mild to severe dysplasias (20–100%) and malignant transformation ((2.5–28.7%)).4,5 The carcinogenic potential of Candida in OL has been found to be due to the nitrosation potential of Candida, which was found to be higher from organisms isolated from non-homogenous leukoplakia than homogenous forms and their ability to deliver these nitrosamines into the epithelium via hyphal invasion.4,6 Candida can also produce acetaldehyde, a carcinogen with mutagenic capacity, from precursors in the oral cavity like ethanol and glucose, especially in patients with smoking and alcohol habits.7
The ability of Candida albicans to colonize penetrate and damage the host depends on its virulence factors like phenotypic switching, dimorphism, adhesive properties, extracellular enzyme production, and biofilm formation.8,9 Candida albicans produces extracellular enzymes like Secreted Aspartyl Proteinases (SAPs) which help in initial penetration and production of nitrogen during colonization while Phospholipases (PLs) causes lysis and cell death by disturbing cellular membranes. SAPs and PLs have been found to have a distinct role in the induction of epithelial pro-inflammatory cytokine response that may play a role in pathogenesis of oral cancer.9, 10, 11 The most important virulence factor of Candida albicans is the ability to form biofilm (BF), altering the microbiota of the oral cavity, disrupting the host immune response and promoting resistance to common antifungals.12,13 Non albicans Candida (NAC) species are now commonly seen due to development of invasive medical therapies, HIV infection, widespread use of broad –spectrum antibiotics, low dose antifungal prophylaxis and are found to be more virulent than Candida albicans.14
The presence of Candida albicans and NAC infection in OL and their relevant virulence factors like SAP, PL and BF activity can play a role in chronic inflammation –related oral carcinogenesis. Their correlation with clinicopathological characteristics response to antifungal therapy and sensitivity to common antifungal drugs has not been explored before but can have important therapeutic and prognostic significance in the management of OL.
2. Materials and Methods
A prospective case-control open-label observational pilot study was designed to determine the association of Candida phenotypes, virulence factors (SAP, PL, Biofilm formation) and antifungal sensitivity with clinicopatholologic characteristics in OL and therapeutic response to Fluconazole. Ethical clearance from Institute Ethics Committee and written informed consent from study subjects was obtained before recruitment. The study included 61 adult immunocompetent subjects with clinical diagnosis of OL [30 Homogenous OL, 31 Non- Homogenous OL (Speckled/Verrucous)] who had not been under treatment for the same or any antifungal therapy for past 6 months. The control group (C) included 30 approximately age and sex matched healthy subjects reporting to the OPD for other routine dental problems, who did not have any tobacco, arecanut or alcohol habits and did not have any OPMD. Only subjects with fairly good oral hygiene (simplified oral hygiene index score (OHI) 0–3, periodontal screening and recording (PSR) code 0–2) were included in the study. Subjects with any history of significant and serious uncontrolled systemic disease, allergy to Fluconazole, malignancy and pregnant women were excluded. Subjects with history of recent major/minor surgery and predisposing factors for oral candidiasis like diabetes/endocrine disorders, xerostomia, very poor oral hygiene, removable prosthesis, prolonged corticosteroid/antibiotic/immunosuppressant/antibacterial mouthwash radiotherapy, radiation/chemotherapy, auto immune disorders and primary/secondary immune deficiencies, nutritional deficiencies and hospitalized debilitated patients were excluded. Known conditions with genetic susceptibility to oral candidiasis like Chronic mucocutaneous candidiasis, Autoimmune polyendocrinopathy, candidiasis, and ectodermal dystrophy (APECED, Hyper IgE Syndrome (HIES), Familial Candidiasis were also excluded from the study.
2.1. Clinicopathological characteristics
The history of tobacco/arecanut/alcohol habits (frequency, duration, current status), OHI and PSR index was recorded. The clinical characteristics (Size, Erythema, Thickness, Oral burning sensation [Visual Analogue Scale (VAS) Score 0–10] were recorded with photographic records of oral lesions before and after Fluconazole therapy. Any lesion suspicious of malignancy with induration/ulceration was not included in this study. The clinical size was determined in the widest dimension of the lesion in the horizontal and vertical plane without stretching the buccal mucosa/tongue using dividers and scale and recorded as per size (L) classification of OLEP system in OL. The study subjects with any harmful oral habits (Tobacco/Areca nut/Alcohol) underwent behavioural intervention for habit cessation. Tablet Paracetamol 500 mg (maximum daily dose 2gm) was advised as a rescue medicine for symptomatic pain relief after 4 mm punch biopsy. The H & E histopathological features with PAS staining was recorded as per standard WHO criteria and staging done as per OLEP system in OL.15
2.2. Collection, transport and processing of oral samples
Two sterile swabs from each study subject (pre-wet with sterile normal saline) was taken from the oral lesion and buccal mucosa from the control subjects and coded before further processing in Microbiology. The collected swabs were examined by direct microscopy with 10% KOH, cultured on Sabouraud dextrose agar (SDA) plates at 37 °C followed by further speciation as per standard mycological procedures.
2.3. Determination of Phospholipase (PL) activity
Phospholipase activity was measured by growing cells on egg yolk agar medium and measuring the size of the zone of precipitation. A cell suspension of 106 yeast cells/ml in saline was prepared and 5 μl was placed on the surface of the egg yolk medium. The culture was incubated at 37 °C for 7–8 days, after which the diameter of the precipitation zone around the colony was determined. Phospholipase activity (Pz) was measured by dividing colony diameter by the diameter of the precipitation zone (Pz) around the colony formed on the plate. A Pz of 1.0 was evaluated as negative (−), 0.99–0.9 as weak (+), 0.89–0.8 as poor (++), 0.79–0.7 as moderate (+++) and 0.69 or above (++++) as strong positive. The C. albicans SC 5314 strain was used as positive control.
2.4. Determination of secreted aspartyl proteinase (SAP) activity
All isolates was tested for their ability to grow and produce a clear zone of hydrolysis in bovine serum albumin (BSA) agar. A 5 μl of 1 × 106 cells/ml was placed on solid medium and was incubated at 37 °C for 3–4 days. Subsequently, clearing of the opacity by hydrolysis of precipitated albumin was recorded and result was evaluated as that of phospholipase. The C. albicans SC 5314 strain was used as positive control.
2.5. Determination of biofilm formation (BF)
A 100 μl volume of 1 × 106 cells/ml suspension was placed on presterilized, polystyrene, flat-bottom 96-well microtitre plates and was incubated for 48 h at 37 °C for adherence and biofilm. After 48 h wells were washed with PBS and a semi-qualitative measure of biofilm was detected by XTT [2,3-bis (2-methoxy-4nitro-5-sulfo-phenyl) - 2H - tetra-zolium-5-carboxanilide] -reduction assay A colorimetric change in the XTT-reduction assay, a direct correlation of the metabolic activity of the biofilm, was then measured in a microtiter plate reader at 490 nm after 2 h of incubation. RPMI 1640 medium free of biofilm formation was included as a negative control. The absorbance values for the controls were subtracted from the values for the test wells to minimize background interference. SC5314, a standard Candida albicans strain was used as a positive control strain that allowed consistency of the result.
2.6. Antifungal sensitivity
The in-vitro antifungal susceptibility testing of all the isolates were tested using CLSI document M44-A for disk diffusion method. Briefly, sterile 90 mm petri plates containing Mueller-Hinton agar medium (Mueller-Hinton agar + 2% glucose and 0.5 μg/mL methylene blue dye) were inoculated by evenly streaking the 1 × 106 Candida cells/ml in RPMI 1640 using sterile swab over the entire agar surface. After the inoculum dried, one disk each of Fluconazole (25 μg), Voriconazole (1 μg) and Clotrimazole (50 μg) were placed onto each inoculated plate. The plates were inverted and incubated in ambient air at 35 °C for 48hrs. for Fluconazole, Voriconazole and Clotrimazole.
2.7. Antifungal therapy
The study groups (Homogenous OL and Non- Homogenous OL) were treated with antifungal therapy [Tab Fluconazole 100 mg as a mouthwash (tablet dissolved in 10 mL of drinking water and used as a rinse for 2 min) and swallowed once a day for 14 days for both topical and systemic effect]. The procedure was demonstrated to each study subject before starting the treatment. The study subjects were recalled after 14 days for review of oral burning symptoms, habit status and clinical features of oral lesions after antifungal treatment. The size, erythema and thickness was noted and recorded with post treatment photographs of oral lesions by two calibrated Oral Medicine specialists who were blinded to the results of the microbiological investigations. The reduction in erythema and thickness of the oral lesions after Fluconazole therapy were scored as [ 0- no change, 1- minimal change (Reduction in erythema/thickness < 1 cm of oral lesion, and 2- significant change( Reduction in erythema/thickness ≥ 1 cm of oral lesion).
2.8. Statistical analysis
The distribution of Candida phenotypes, virulence attributes and antifungal sensitivity in the study and control groups and clinicopathological characteristics were analysed using descriptive statistics. A comparison of the oral burning symptoms, size, erythema and thickness of oral lesions before and after antifungal therapy was done in the study group. Kohen’s kappa statistic was used to determine inter observer agreement in the reduction of erythema and thickness of the oral lesions. The results were tested for statistical significance using Chi-square, Fisher’s exact test and ANOVA where applicable.
3. Results
The distribution of demographics, oral habits, oral hygiene and periodontal status between the study and control groups is shown in Table 1. There was no significant difference in the tobacco habits, frequency and duration between the two groups. There was significant difference in the OHI and PSR index between the groups (p < 0.01) with subjects with NHOL tending to have poorer status (Score 2).
Table 1.
Distribution of demographics, oral habits, oral hygiene and periodontal status between study and control groups.
| Controls (N = 30) | Homogenous Oral Leukoplakia (N = 30) | Non- Homogenous Oral Leukoplakia (N = 31) | Significance (p- value) | |
|---|---|---|---|---|
| Age (Years) | ||||
| Range | 21–70 | 25–69 | 26–65 | p = 0.001 |
| Mean | 40.7 | 41.0 | 50.7 | |
| Sex | ||||
| Males | 26 | 29 | 26 | |
| Females | 4 | 1 | 5 | |
| Oral Habits | ||||
| Smoking tobacco | 0 | 24 | 25 | |
| Smokeless tobacco | 0 | 12 | 11 | |
| Betel nut products | 0 | 15 | 18 | |
| Alcohol | 0 | 5 | 6 | |
| Frequency of habits/day | ||||
| 1–5 | 0 | 16 | 15 | |
| 6–10 | 0 | 9 | 8 | |
| 11–15 | 0 | 3 | 7 | |
| ≥16 | 0 | 2 | 1 | |
| Duration of habits (Months) | ||||
| Range | 0 | 12–480 | 3–480 | |
| Mean | 0 | 153.6 | 210.2 | |
| Oral Hygiene Index | ||||
| Score 1 | 19 | 11 | 7 | p < 0.01 |
| Score 2 | 11 | 19 | 24 | |
| Periodontal Screening and Recording Index | ||||
| Score1 | 19 | 10 | 7 | p < 0.01 |
| Score 2 | 11 | 20 | 24 | |
The distribution of the clinical and histopathological features between HOL and NHOL is shown in Table 2. Oral Submucous fibrosis was commonly seen in association with NHOL. The oral burning sensation ranged from 0 to 8 on VAS scale in both the groups but Scores ≥3 was more commonly seen in association with speckled type of NHOL. Tongue involvement was more commonly seen in verrucous type of NHOL. PAS staining revealed Candida in only one case of NHOL which also had severe dysplasia but no correlation was found between presence of dysplasia and Candida positivity in OL.
Table 2.
The distribution of the clinical and histopathological features between Homogenous and Non- Homogenous Oral Leukoplakia.
| Homogenous Oral Leukoplakia (N = 30) | Non- Homogenous Oral Leukoplakia (N = 31) | |
|---|---|---|
| Other oral lesions | ||
| Tobacco pouch keratosis | 4 | 1 |
| Smokers palate | 8 | 8 |
| Oral Submucous Fibrosis | 3 | 7 |
| Oral Burning sensation | 5 (Candida + ve = 2) | 16 (Candida + ve = 14) |
| Side | ||
| Unilateral | 1 | 9 |
| Bilateral | 29 | 22 |
| Extent | ||
| Localized | 14 | 13 |
| Diffuse | 16 | 18 |
| Site | ||
| Buccal Mucosa | 30 | 25 |
| Labial Mucosa | 4 | 3 |
| Commissures | 18 | 20 |
| Tongue | 2 | 9 |
| Palate | 13 | 13 |
| Gingiva | 2 | 2 |
| Histopathology | ||
| PAS stain for candida hyphae | 0 | 1 |
| Mild dysplasia | 0 | 8 (Candida + ve = 4) |
| Severe dysplasia | 0 | 1 (Candida + ve) |
| Staging (OLEP) | ||
| Stage I | 1 | 2 |
| Stage II | 8 | 4 |
| Stage III | 21 | 19 |
| Stage IV | 0 | 6 |
The distribution of Candida phenotype and virulence factors between Control, HOL and NHOL is shown in Table 3. There was significant differences in the Candida positivity between the three groups (p < 0.01) with 51.6% of NHOL, 23.3% of HOL and 13.3% of Controls showing presence of Candida. Candida albicans was the most common phenotype seen in 100% of Controls, 86% of HOL and 94% of NHOL. Non albicans candida (NAC) Candida tropicalis and Candida parapsilosis were seen in one case of HOL and NHOL respectively.
Table 3.
Distribution of Candida phenotype, virulence factors and antifungal sensitivity between Control, Homogenous and Non- Homogenous Oral leukoplakia.
| Controls (N = 30) | Homogenous Leukoplakia (N = 30) | Non- Homogenous Leukoplakia (N = 31) | |
|---|---|---|---|
| KOH stain | 0 | 3 | 2 |
| Culture | |||
| Candida albicans | 4 | 6 | 15 |
| Candida tropicalis | 0 | 1 | 0 |
| Candida parapsilosis | 0 | 0 | 1 |
| SAP activity | |||
| Negative | 0 | 1 | 2 |
| Weak | 3 | 1 | 0 |
| Poor | 1 | 0 | 1 |
| Moderate | 0 | 1 | 5 |
| Strong | 0 | 4 | 8 |
| PL activity | |||
| Negative | 1 | 5 | 8 |
| Weak | 2 | 1 | 0 |
| Poor | 1 | 0 | 1 |
| Moderate | 0 | 0 | 7 |
| Strong | 0 | 1 | 0 |
| Biofilm formation | |||
| Negative | 1 | 2 | 2 |
| Positive | 3 | 5 | 14 |
| Antifungal sensitivity (Fluconazole) | |||
| Sensitive | 4 | 6 | 15 |
| Resistant | 0 | 1 (Candida tropicalis) | 1 (Candida parapsilosis) |
The SAP and PL activity was found to be significantly higher in NHOL as compared to HOL, C and test control (p < 0.01). Biofilm formation was seen in 71.4% of HOL and 87.5% of NHOL. The rank scale of the metabolic activity in biofilm by XTT assay was found to be Candida albicans > Candida tropicalis > Candida parapsilosis. There was no difference in the SAP/PL activity and BF ability between Candida albicans and NAC isolates in OL. All the Candida albicans isolates in the three groups were sensitive to Fluconazole and Voriconazole. Both the NAC isolates Candida parapsilosis and Candida tropicalis were found to be resistant to Fluconazole but sensitive to Voriconazole. Since there are no standard cut-off values for determining sensitivity towards Clotrimazole with disk diffusion method, definite results could not be obtained for the isolates. No adverse effects to Fluconazole was reported by any of the study subjects.
There was significant reduction in the oral burning sensation after Fluconazole therapy in both HOL and NHOL (p < 0.001). There was significant reduction in the size of the oral lesion in both HOL (p < 0.001) and NHOL (p < 0.05) after Fluconazole therapy (Fig. 1). There was also visible reduction in the erythematous areas (21/31, 67.8%) in the speckled type of NOHL (Fig. 2) and the thickness of the white patches (26/61, 42.6%) (Fig. 3) in both types OL after antifungal therapy. There was significant reduction in size and erythema in all Candida-positive NHOL. There was minimal clinical improvement in OL lesions associated with NAC after Fluconazole therapy. None of the lesions resolved completely after Fluconazole treatment. Inter-observer agreement for reduction in erythema and thickness of the oral lesions was found to be substantial with kappa statistic of 0.8 and 0.7 respectively.
Fig. 1.
a). Size of lesion in left buccal mucosa before Fluconazole therapy b). Decrease in size of lesion in left buccal mucosa after Fluconazole therapy.
Fig. 2.
a). Speckled non-homogenous oral leukoplakia in left commissure and buccal mucosa before Fluconazole therapy b). Decrease in erythematous areas in left commissure and buccal mucosa after Fluconazole therapy.
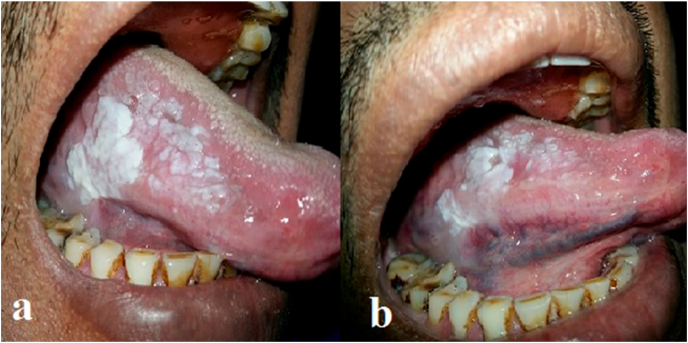
Fig. 3.
a). Thick patch in right ventro-lateral surface of tongue before Fluconazole therapy b). Decrease in thickness of white patch in right ventro-lateral surface of tongue after Fluconazole therapy.
4. Discussion
Candida is a harmless common commensal of the oral cavity which can become an opportunistic pathogen with change in local or systemic immune barriers. Normal oral carriage of Candida albicans in adult healthy population is between 2 and 69.1% depending on population studied.16 Candida albicans is the most common phenotype seen in OL but NAC species like Candida tropicalis, Candida parapsilosis, Candida glabrata, Candida krusei, Candida dubliniensis and Candida pintolopesii have also been isolated.17 We also found Candida albicans as the predominant phenotype while NAC species isolated were Candida tropicalis and Candida parapsilosis.
SAP protein is encoded by a family of 10 SAP genes of which SAP2 is the most abundantly secreted by Candida albicans. Sap2 (and possibly other Sap proteins) is known to be involved in all stages of infection and can degrade many human proteins, including mucin, extracellular matrix proteins, numerous immune system molecules, endothelial cell proteins, and coagulation and clotting factors, salivary lactoferrin, lactoperoxidase, cathepsin D, complement, α2 macroglobulin and cystatin A and which greatly enhances the pathogenic ability of Candida albicans. Candida albicans, Candida tropicalis and Candida parapsilosis are the most virulent of the Candida species with high proteolytic activity in symptomatic patients as compared to asymptomatic carriers.18 SAP activity has been found to be increased in OL19,20 and proteolytic activity is increased at lower salivary pH.19 Low salivary pH found in association with OPMDs, poor oral hygiene and periodontal health associated with smoking and smokeless tobacco habits may also act as contributing factors in increasing the SAP activity of Candida in OL. Phospholipases like PLA, PLB and PLC that target phospholipid substrates in host epithelial and endothelial cell membranes are the virulent factors in Candida spp that are involved in the early steps of host invasion like adherence, penetration and damage while its by - products can act as mediators and secondary messengers in signal transduction and activation of protein kinase C leading to deregulation of cell signaling.10
SAP and PL activity of Candida isolates depends on the type, the site and the stage of infection as well as the host response. No significant difference in SAP and PL activity between Candida albicans and NAC has been reported which is similar to our findings. High SAP/PL activity is seen in oral cancer as compared to other OPMDs like chronic candidiasis and atrophic lichen planus indicating an association between virulence and disease severity. A strong correlation between SAP and PL activity has been reported which indicates their synergistic nature.21 We also found a strong correlation between SAP and PL activity which increased with disease severity as they were found to be higher in Candida isolates in NHOL as compared to HOL and asymptomatic carriers.
Candida biofilms in oral mucous membranes is part of the commensal microflora of the oral cavity and play a central role in Candida pathogenesis. The biofilms formed by Candida albicans are also inherently tolerant and less susceptible to current antifungal agents due to extracellular matrix adsorption of drugs and formation of “persister” cells. The β-glucan and extracellular DNA components of the extracellular matrix promote biofilm resistance to multiple antifungals. It provides a stable environment for Candida by facilitating host immune evasion, resistance to disinfectants and antifungal agents.12 The surface of OL provides a suitable substrate for adherence, hence the ability of Candida isolates from OL to form biofilms becomes relevant. BF has been found to be significantly higher in Oral cancer and Chronic candidiasis as compared to Atrophic lichen planus and asymptomatic carriers and the ability is higher in NAC species like Candida tropicalis. We found BF ability in 82.6% of Candida isolates in OL and no significant difference in the metabolic activity between Candida albicans and NAC isolates.
Oral burning sensation was associated with Candida positivity in HOL (40%) and NOHL (87.5%) indicating that presence of burning sensation may be used as a clinical indicator for Candida infection in OL. The erythematous areas in NHOL showed significant reduction after fluconazole therapy especially in those with high SAP/PL activity. We found a strong correlation between SAP scores and pre-treatment VAS scores in OL. The burning sensation and erythema in OL could be as a result of the inflammatory response of the oral epithelial cells invoked by SAP activity. The thick white patches could be due to increased adherence of Candida to epithelial surface from PL activity which improves after antifungal therapy.22,23
Detection of Candida in OL was done using direct microscopy, culture and histopathology however culture was more sensitive as it was able to detect Candida in 92.6% of the positive samples in this study. Candidial leukoplakia/hyperplastic candidiasis also shares the same predisposing factors as OL and hence it is possible that many diffuse lesions of OL may have co-existing Candidial leukoplakia which also regress after antifungal therapy.16
Fluconazole is a broad spectrum triazole that is commonly used in oral candidiasis with antifungal activity against most of the species of Candida with excellent bioavailability, low toxicity, affordability and rare adverse effects. There have been studies showing improvement and disappearance of a significant number of cases of OL with topical antifungals like nystatin and imidazoles and systemic antifungals like fluconazole in immunocompetent patients, and more toxic drugs like amphotericin B in immuno-compromised patients.24
Candida albicans the predominant phenotype detected in both HOL and NOHL was sensitive to commonly used antifungals like clotrimazole, voriconazole and fluconazole. Fluconazole therapy was effective in reduction of oral burning sensation, size, redness and thickness of the lesions and conversion of NHOL into HOL. This is a positive outcome as NHOL is at higher risk of malignant transformation as compared to HOL. Hence Fluconazole can be used as an empirical short term therapy in OL for reduction in Candida super-infection and symptoms of oral burning sensation, down-staging from high-risk NHOL to low-risk HOL lesions. Fluconazole is very effective in managing oral Candida albicans infection but may be unable to penetrate Candida biofilms. The high SAP, PL and BF activity of NAC seen in this study could be a cause for resistance to Fluconazole and minimal response to therapy.25 OL has to be kept under long term surveillance for any malignant transformation and after initial short –term course of systemic Fluconazole, topical antifungals like clotrimazole can be used prophylactically to prevent recolonization of OL surfaces with Candida albicans as an adjunct to conventional management. There has been a resurgence of fluconazole resistance in both Candida albicans and NAC oral infections hence persistent erythematous areas after common antifungal therapy require biopsy and detection of Candida species with antifungal sensitivity.25 NAC species like Candida parapsilosis and Candida tropicalis as seen in this study did not respond favourably to Fluconazole treatment at a daily dose of 100 mg for 14 days. Antifungal susceptibility could have been done by microboth dilution method to determine the minimum inhibitory concentration so that in susceptible dose dependant isolates, higher doses of Fluconazole or optimal doses of Voriconazole can be given to obtain favourable results. The virulence factors of NAC in OL can be targeted for development of alternative antifungal drugs. Novel approaches like vaccines, anti- SAP antibodies and SAP/PL inhibitors can be used in Candida species resistant to common antifungals.10,18
The virulence attributes of Candida super-infection in OL increase the severity of the lesions and risk for malignant transformation. The predisposing modifiable risk factors for Candida super-infection in OL should be identified and eliminated. Further management should aim at reducing the risk factors for Candida super-infection in OL with oral prophylaxis, maintenance of dental and periodontal health, maintenance of oral hygiene, homeostasis and salivation, reinforcement of tobacco/areca nut/alcohol habit cessation through counselling and regular follow-up. Genetic susceptibility as a risk factor for candida infection in OL can be explored in future prospective studies in Indian population as these patients may require immunotherapy as an adjunct to antifungals.26 Antifungals like Fluconazole can play a role in reducing the infection and thereby the inflammatory response induced by virulent factors of Candida albicans as evident by improvement in clinical signs and symptoms. The down-staging from high-risk NHOL to low-risk HOL can reduce risk of malignant transformation. This is a preliminary investigation and the results need to be corroborated with large scale double-blinded randomized clinical trials in different populations with OL.
Funding sources
This study was funded by the All India Institute of Medical Sciences New Delhi Intramural Research Fund (2018–2020). Reference Number A-634.
The funding agency had no other involvement in the conduct of the research project or preparation of the article.
Declaration of competing interest
The interim results were presented at the IADR conference in Vancouver Canada in 2019 and abstract published in the Journal of Dental research.
The manuscript is not under consideration for publication elsewhere. There is no conflict of interest to declare.
Acknowledgements
The authors would like to acknowledge the contribution of Mr. Prashant, Senior Technician in Department of Microbiology AIIMS New Delhi, in conducting the various laboratory investigations.
Contributor Information
Shalini R. Gupta, Email: shalinigupta@hotmail.com.
Immaculata Xess, Email: immaxess@gmail.com.
Gagandeep Singh, Email: drgagandeep@gmail.com.
Alpana Sharma, Email: dralpanasharma@gmail.com.
Nidhi Gupta, Email: 1992nidhigupta@gmail.com.
Kalaivani Mani, Email: manikalaivani@yahoo.co.in.
Sheetal Sharma, Email: sheetalsharma527@gmail.com.
References
- 1.Warnakulasuriya S., Ariyawardana A. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2016;45(3):155–166. doi: 10.1111/jop.12339. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2018;125(6):582–590. doi: 10.1016/j.oooo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Mohd Bakri M., Mohd Hussaini H., Rachel Holmes A., David Cannon R., Mary Rich A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v2i0.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla K., Vun I., Lov I., Laparidis G., McCamley C., Ariyawardana A. Role of Candida infection in the malignant transformation of oral leukoplakia: a systematic review of observational studies. Translational Research in Oral Oncology. 2019;4 doi: 10.1177/2057178X19828229. 2057178X19828229. [DOI] [Google Scholar]
- 5.Cawson R.A. Induction of epithelial hyperplasia by Candida albicans. Br J Dermatol. 1973;89(5):497–503. doi: 10.1111/j.1365-2133.1973.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 6.Krogh P., Hald B., Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis. 1987;8(10):1543–1548. doi: 10.1093/carcin/8.10.1543. [DOI] [PubMed] [Google Scholar]
- 7.Gainza-Cirauqui M.L., Nieminen M.T., Novak Frazer L., Aguirre-Urizar J.M., Moragues M.D., Rautemaa R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J Oral Pathol Med: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2013;42(3):243–249. doi: 10.1111/j.1600-0714.2012.01203.x. [DOI] [PubMed] [Google Scholar]
- 8.Priest S.J., Lorenz M.C. Characterization of virulence-related phenotypes in Candida species of the CUG clade. Eukaryot Cell. 2015;14(9):931–940. doi: 10.1128/EC.00062-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaller M., Sander C.A., Korting H.C., Mailhammer R., Grassl G., Hube B. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118(4):652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum M.A. Potential role of Phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13(1):122–143. doi: 10.1128/cmr.13.1.122-143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel J.B., Shah F.D., Joshi G.M., Patel P.S. Clinical significance of inflammatory mediators in the pathogenesis of oral cancer. J Canc Res Therapeut. 2016;12(2):447–457. doi: 10.4103/0973-1482.147765. [DOI] [PubMed] [Google Scholar]
- 12.Tsui C., Kong E.F., Jabra-Rizk M.A. Pathogenesis of Candida albicans biofilm. Pathog Dis. 2016;74(4) doi: 10.1093/femspd/ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad Khan M.S., Alshehrei F., Al-Ghamdi S.B., Bamaga M.A., Al-Thubiani A.S., Alam M.Z. Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis. Future Sci OA. 2020;6(2):FSO440. doi: 10.2144/fsoa-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muadcheingka T., Tantivitayakul P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch Oral Biol. 2015;60(6):894–901. doi: 10.1016/j.archoralbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 15.van der Waal I., Schepman K.P., van der Meij E.H. A modified classification and staging system for oral leukoplakia. Oral Oncol. 2000;36(3):264–266. doi: 10.1016/s1368-8375(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 16.Scully C., el-Kabir M., Samaranayake L.P. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5(2):125–157. doi: 10.1177/10454411940050020101. [DOI] [PubMed] [Google Scholar]
- 17.Krogh P., Holmstrup P., Thorn J.J., Vedtofte P., Pindborg J.J. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63(1):48–54. doi: 10.1016/0030-4220(87)90339-2. [DOI] [PubMed] [Google Scholar]
- 18.Naglik J.R., Challacombe S.J., Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modrzewska B., Kurnatowski P., Khalid K. Comparison of proteolytic activity of Candida sp. strains depending on their origin. J Mycol Med. 2016;26(2):138–147. doi: 10.1016/j.mycmed.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Rehani S., Rao N.N., Rao A., Carnelio S., Ramakrishnaiah S.H., Prakash P.Y. Spectrophotometric analysis of the expression of secreted aspartyl proteinases from Candida in leukoplakia and oral squamous cell carcinoma. J Oral Sci. 2011;53(4):421–425. doi: 10.2334/josnusd.53.421. [DOI] [PubMed] [Google Scholar]
- 21.Castillo G.D.V., Blanc SL de, Sotomayor C.E., Azcurra A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch Oral Biol. 2018;91:35–41. doi: 10.1016/j.archoralbio.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Naglik J.R., Moyes D.L., Wächtler B., Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microb Infect. 2011;13(12-13):963–976. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naglik J.R., Moyes D. Epithelial cell innate response to Candida albicans. Adv Dent Res. 2011;23(1):50. doi: 10.1177/0022034511399285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vizhi K. Comparison of clinical and histopathological behavior of oral leukoplakia before and after treatment with 1% topical clotrimazole - an observational study. J Basic Clin Pharm. 2017;8(4) https://www.jbclinpharm.org/abstract/comparison-of-clinical-and-histopathological-behavior-of-oral-leukoplakia-before-and-after-treatment-with-1-topical-clot-3931.html Accessed October 13, 2020. [Google Scholar]
- 25.Berkow E.L., Lockhart S.R. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–245. doi: 10.2147/IDR.S118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smeekens S.P., van de Veerdonk F.L., Kullberg B.J., Netea M.G. Genetic susceptibility to Candida infections. EMBO Mol Med. 2013;5(6):805–813. doi: 10.1002/emmm.201201678. [DOI] [PMC free article] [PubMed] [Google Scholar]