Arising from Pokharel et al. Nature Communications 10.1038/ncomms6883 (2014)
In the previous publication, some of us reported the conversion of a copper(I) complex to a copper(II) oxalate complex, and claimed that this conversion involved a reduction of CO2 to oxalate (C2O42−). Herein, we show that the oxalate is produced not by reduction of CO2, but by reaction of ascorbate with oxygen. We also present new results that explain in a more comprehensive way the behaviour of these copper compounds under O2 and CO2.
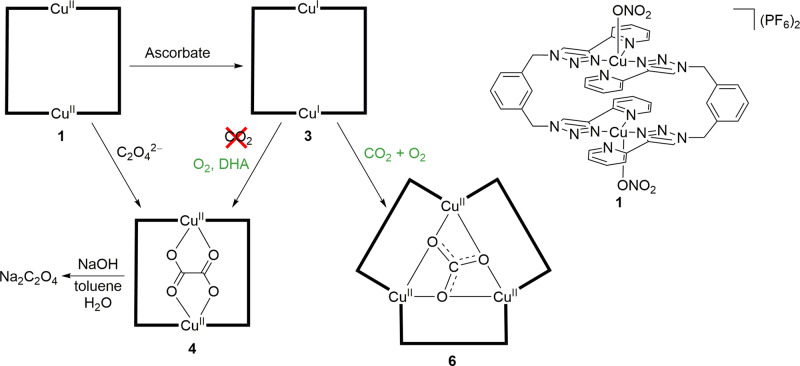
Selective reduction of carbon dioxide to C≥2 compounds using homogeneous metal complexes is a challenging transformation. Only a limited number of examples have been reported over the past decades1–12. In contrast, there has been a vast increase in reported catalysts for selective CO2 reduction to C1 compounds13–15. Among the examples reported for the reductive coupling of CO2 to oxalate is a dinuclear Cu complex introduced by some of us in 2014 (ref. 16). The in situ generated Cu(I) complex [Cu2(m-xpt)2](PF6)2 (3) formed by reduction of the Cu(II) precursor (1) with sodium ascorbate generated an oxalate-bridged dinuclear complex (4), proposed to occur via reductive coupling of atmospheric CO2 (Fig. 1). Release of the oxalate by addition of mineral acids was described, potentially enabling stepwise conversion of CO2 into oxalic acid using sodium ascorbate as a comparatively mild reductant.
Fig. 1. Reactions of Cu(II) complex 1 and the Cu(I) complex 3 obtained by reduction of 1 with ascorbate.
For the formation of oxalate complex 4 from 3, CO2 was previously reported to be required. We show here that the reaction requires ascorbate or dehydroascorbic acid (DHA), and oxygen. If ascorbate and DHA are absent, oxidation of 3 in air produces 6. All reactions were conducted in DMF, except for the removal of oxalate from 4 (Note: 1, 3, and 4 represent the same compounds as in ref. 16).
Interestingly, oxidation of ascorbic acid by transition metal compounds, especially those of copper, has been well-known for more than a century17,18. Since then, the reaction mechanisms for such oxidations have been intensely studied18–22. More specifically, oxidative degradation of ascorbic acid by (a) inorganic oxidants (sodium periodate23, sodium hypoiodite24); (b) oxygen25,26; and (c) O2 in the presence of Gd27,28, Co27, Pd29, Pt29, Cd30, Fe31, or Cu32 compounds is reported to yield oxalate as a degradation product (see Supplementary Fig. 21 for a typical reaction sequence).
We now report that the true origin of the oxalate in the communication published in 2014 is not CO2, as it was described, but oxidative degradation of sodium ascorbate.
A first hint towards the oxidative degradation pathway as the origin of oxalate was obtained when treatment of the in situ generated Cu(I) complex [Cu2(m-xpt)2](PF6)2 (3), formed via reaction of the Cu(II) precursor 1 with sodium ascorbate in DMF, with CO2 over 6 days did not result in the previously described colour change from yellow to green (Supplementary Fig. 1) and no Cu(II) species was detected by UV/Vis spectroscopy (Supplementary Fig. 2). However, after introduction of air, oxidation of the Cu(I) complex 3 was observed and followed by UV/Vis spectroscopy over 189 h, resembling the UV/Vis spectra reported in the previous publication.
The product obtained from this reaction after slow evaporation of the solvent was identical to the reported oxalate complex 4, as evident from the IR spectrum (Supplementary Fig. 3).
Since the reaction seemed to require air for the formation of oxalate, we suggested that oxidation of the ascorbate might be the true origin of the oxalate. Therefore, the previously published results might have eventuated from oxygen contamination of the reaction mixtures utilised for the labelling studies and the UV/Vis spectroscopic study.
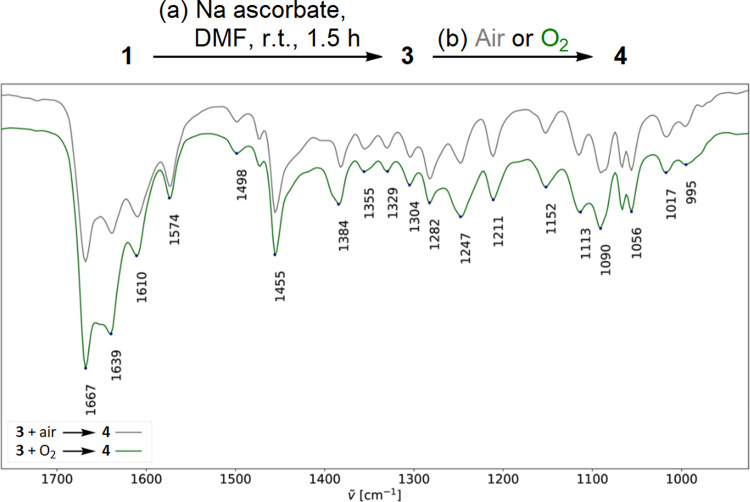
To test this hypothesis, we prepared the Cu(I) complex 3 in situ using sodium ascorbate, and exposed it to oxygen in the absence of air and CO2. Indeed, oxidation of the Cu(I) complex 3 in the presence of O2 occurred within a few minutes, as evidenced by a characteristic colour change from yellow to green (Supplementary Fig. 5) and after 5 days of reaction time, a yellow to green solid was obtained after removal of the solvent. As suspected, the solid product was identical to that obtained via the reaction of the in situ generated Cu(I) complex 3 with air, as evident from X-ray analysis and FTIR spectroscopy (see spectra in Fig. 2).
Fig. 2. Formation of the oxalate complex 4 from 3 requires O2 and does not require CO2.
FTIR spectra for the products obtained when 3 (prepared in situ from 1 and ascorbate) was exposed to air (grey) or pure O2 (green) are identical (details are given in the ESI).
Complex 4 was even obtained from a mixture of Cu(II) complex 1 and DHA in air, demonstrating that Cu(I) is not required for oxalate formation.
The Cu(I) complex [Cu2(m-xpt)2]2+ can also be prepared without ascorbate or dehydroascorbic acid, for example, by reaction of Cu(BF4)2 with Cu foil in DMF in the presence of m-xpt. This yellow solution of [Cu2(m-xpt)2](BF4)2 (3a, identical to 3 except for the counterion) (a) does not react with CO2; (b) reacts with air to produce the new trinuclear Cu(II) carbonate complex [Cu3(m-xpt)3(μ3-CO3)](BF4)4 (6); and (c) can be converted to the oxalate complex [Cu2(m-xpt)2(μ-C2O4)](BF4)2 (4b) by reaction with air or O2, but only if DHA is added. These observations are also in accordance with ascorbate being the source of oxalate. The structures of 6 and 4b were determined by X-ray analysis (see Supplementary Fig. 13).
In the previous publication, an isotope labelling experiment was conducted by treating in situ generated 3 with 13CO2. In mass spectrometry experiments performed on 4 at that time, we did not observe signals attributable to oxalate-containing product ions; however, its FTIR spectrum appeared to show a shift of ΔṽCO = −19 cm−1. Since this shift was only half the expected magnitude, we re-performed the labelling studies. In the new experiments, treatment of in situ generated 3 with 13CO2–O2 (1:1) produced only unlabelled 4, whose ESI-MS shows a monoisotopic ion at 1147.1321 amu for [Cu2(m-xpt)2(μ-C2O4)](PF6)+ (see Supplementary Fig. 9). The 13C-labelled oxalate complex 4-13C2 was obtained, for reference, by reaction of the starting complex 1 with (Bu4N)2(13C2O4); monoisotopic ion 1149.1373 amu. This analysis clearly demonstrates that oxalate does not arise from CO2 reduction. FTIR spectra of the new products show ΔṽCO = −39 cm−1, close to the expected value (see Supplementary Fig. 8). A similar value for ΔṽCO is also estimated based on DFT calculations; detailed results are given in the ESI.
In the previous publication, the IR absorption at ca. 1670 cm−1 in 4 was assigned to the oxalate C–O stretching vibration. However, as demonstrated in Supplementary Fig. 14, this absorption is caused by co-crystallized DMF in 4 (ṽCO for the bound oxalate is 1639 cm−1). In the previous experiment with 13CO2, 4 appeared to show an absorption at 1650 cm−1; we now know that this sample did not contain 13C2O42−. The spectra in Supplementary Fig. 7 suggest that different samples of 4 may show varying absorption in the 1670–1640 cm−1 region. This variability may have led to the incorrect assignment of an apparent 13C shift in the previous work.
Due to this complexity of the IR spectra, we searched for additional experimental evidence for the formation of oxalate from the reaction under O2 atmosphere. We repeated the previously described oxalate removal by treatment with aqueous HNO3 (ref. 16), but we could not detect the expected H2C2O4 by 13C NMR spectroscopy. Therefore, we adapted a procedure which was utilised for the isolation of Na2C2O4 from similar Cu oxalate complexes33. We used this procedure to isolate Na2C2O4 (verified by 13C NMR spectroscopy), from samples of 4 obtained by reaction of in situ generated 3 with (a) air (i.e. O2 + CO2; Supplementary Fig. 18), and (b) pure O2 (i.e. without CO2; Supplementary Fig. 16). In the latter case, the isolation of Na2C2O4 from 4 was conducted under argon, so the isolated oxalate could not be formed by any reaction requiring CO2.
In summary, we have demonstrated that the Cu complex reported in the previous communication does not form oxalate via CO2 reduction. Instead, oxalate forms by oxidative degradation of ascorbate. This was finally evidenced by the reaction conducted under an atmosphere of O2, giving rise to the same oxalate complex described earlier (ref.16) from which sodium oxalate was removed and identified by NMR spectroscopy. In addition, the same product was obtained from reactions of the Cu(I) complex [Cu2(m-xpt)2]2+ with O2 or air in the presence of DHA. In experiments with [Cu2(m-xpt)2]2+ under 13CO2 + O2, 13C was not incorporated into the oxalate product. In contrast, a new trinuclear Cu(II) carbonate complex, [Cu3(m-xpt)3(μ-CO3)]4+, has been isolated, when [Cu2(m-xpt)2]2+ was treated with CO2 and O2 in the absence of sodium ascorbate or DHA. Since reproducibility is not always given for challenging transformations, such as the reductive coupling of CO234, this report clearly highlights the importance of further mechanistic investigations on previously published systems.
Supplementary information
Acknowledgements
M.M. is grateful to the Fonds der Chemischen Industrie for a Kekulé scholarship. Research at Louisiana State University was supported by the West Professorship.
Author contributions
F.K., M.M., D.B.C., and U.R.P. performed the synthetic work, and conducted the spectroscopic analyses. F.R.F. performed X-ray analysis. F.K., M.M., M.B., and A.W.M. contributed to writing.
Data availability
Accession codes: The X-ray crystallographic data for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1976241 (4b) and 1976240 (6). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Other data are available from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fatemeh Khamespanah, Maximilian Marx.
Contributor Information
Andrew W. Maverick, Email: maverick@lsu.edu
Matthias Beller, Email: Matthias.Beller@catalysis.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-21817-w.
References
- 1.Cook BJ, Di Francesco GN, Abboud KA, Murray LJ. Countercations and solvent influence CO2 reduction to oxalate by chalcogen-bridged tricopper cyclophanates. J. Am. Chem. Soc. 2018;140:5696–5700. doi: 10.1021/jacs.8b02508. [DOI] [PubMed] [Google Scholar]
- 2.Angamuthu R, Byers P, Lutz M, Spek AL, Bouwman E. Electrocatalytic CO2 conversion to oxalate by a copper complex. Science. 2010;327:313–315. doi: 10.1126/science.1177981. [DOI] [PubMed] [Google Scholar]
- 3.Farrugia, L. J., Lopinski, S., Lovatt, P. A. & Peacock, R. D. Fixing carbon dioxide with copper: crystal structure of [LCu(μ-C2O4)CuL][Ph4B]2 (L = N,Nʹ,N′′-Triallyl-1,4,7-triazacyclononane). Inorg. Chem.40, 558–559 (2001). [DOI] [PubMed]
- 4.Saouma CT, Lu CC, Day MW, Peters JC. CO2 reduction by Fe(I): solvent control of C–O cleavage versus C–C coupling. Chem. Sci. 2013;4:4042–4051. doi: 10.1039/c3sc51262b. [DOI] [Google Scholar]
- 5.Klose A, et al. The metal–carbon multiple bond in iron(I)– and iron(II)–dibenzotetramethyltetra[14]azaannulene: carbene, carbonyl, and isocyanide derivatives. J. Organomet. Chem. 1999;591:45–62. doi: 10.1016/S0022-328X(99)00354-X. [DOI] [Google Scholar]
- 6.Tanaka K. Reduction of CO2 directed toward carbon–carbon bond formation. Bull. Chem. Soc. Jpn. 1998;71:17–29. doi: 10.1246/bcsj.71.17. [DOI] [Google Scholar]
- 7.Horn B, Limberg C, Herwig C, Braun B. Nickel(I)-mediated transformations of carbon dioxide in closed synthetic cycles: reductive cleavage and coupling of CO2 generating NiICO, NiIICO3 and NiIIC2O4NiII entities. Chem. Commun. 2013;49:10923–10925. doi: 10.1039/c3cc45407j. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph M, Dautz S, Jäger E-G. Macrocyclic [N42−] coordinated nickel complexes as catalysts for the formation of oxalate by electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 2000;122:10821–10830. doi: 10.1021/ja001254n. [DOI] [Google Scholar]
- 9.Fröhlich H-O, Schreer H. Einschub und reduktive Kopplung von CO2; zur Bildung von Cp2TiIIIC2O4TiIIICp2 und Cp2TiIV(−O2C(CH2)3NRCH2CH2NR(CH2)3CO2−) (R = i-C4H9) Z. Chem. 1983;23:348–349. doi: 10.1002/zfch.19830230922. [DOI] [Google Scholar]
- 10.Evans WJ, Lorenz SE, Ziller JW. Investigating metal size effects in the Ln2(μ-η2:η2-N2) reduction system: reductive reactivity with complexes of the largest and smallest trivalent lanthanide ions, La3+ and Lu3+ Inorg. Chem. 2009;48:2001–2009. doi: 10.1021/ic801853d. [DOI] [PubMed] [Google Scholar]
- 11.Evans WJ, Seibel CA, Ziller JW. Organosamarium-mediated transformations of CO2 and COS: monoinsertion and disproportionation reactions and the reductive coupling of CO2 to [O2CCO2]2−. Inorg. Chem. 1998;37:770–776. doi: 10.1021/ic971381t. [DOI] [Google Scholar]
- 12.Arnold PL, Turner ZR. Carbon oxygenate transformations by actinide compounds and catalysts. Nat. Rev. Chem. 2017;1:0002. doi: 10.1038/s41570-016-0002. [DOI] [Google Scholar]
- 13.Francke R, Schille B, Roemelt M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts. Chem. Rev. 2018;118:4631–4701. doi: 10.1021/acs.chemrev.7b00459. [DOI] [PubMed] [Google Scholar]
- 14.Windle CD, Perutz RN. Advances in molecular photocatalytic and electrocatalytic CO2 reduction. Coord. Chem. Rev. 2012;256:2562–2570. doi: 10.1016/j.ccr.2012.03.010. [DOI] [Google Scholar]
- 15.Wang W-H, Himeda Y, Muckerman JT, Manbeck GF, Fujita E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 2015;115:12936–12973. doi: 10.1021/acs.chemrev.5b00197. [DOI] [PubMed] [Google Scholar]
- 16.Pokharel UR, Fronczek FR, Maverick AW. Reduction of carbon dioxide to oxalate by a binuclear copper complex. Nat. Commun. 2014;5:5883. doi: 10.1038/ncomms6883. [DOI] [PubMed] [Google Scholar]
- 17.Creutz C. Complexities of ascorbate as a reducing agent. Inorg. Chem. 1981;20:4449–4452. doi: 10.1021/ic50226a088. [DOI] [Google Scholar]
- 18.Davies MB. Reactions of l-ascorbic acid with transition metal complexes. Polyhedron. 1992;11:285–321. doi: 10.1016/S0277-5387(00)83175-7. [DOI] [Google Scholar]
- 19.Yano Y, Takano S, Kato Y, Tagaki W. Oxidation of ascorbic acid by a trivalent copper complex. J. Chem. Soc. Perkin Trans. 1979;2:1227–1229. doi: 10.1039/p29790001227. [DOI] [Google Scholar]
- 20.Xu J, Jordan RB. Kinetics and mechanism of the reaction of aqueous copper(II) with ascorbic acid. Inorg. Chem. 1990;29:2933–2936. doi: 10.1021/ic00341a015. [DOI] [Google Scholar]
- 21.Scarpa M, Vianello F, Signor L, Zennaro L, Rigo A. Ascorbate oxidation catalyzed by bis(histidine)copper(II) Inorg. Chem. 1996;35:5201–5206. doi: 10.1021/ic9600644. [DOI] [Google Scholar]
- 22.Nayak S, Dash AC. Mechanistic study of the reactions of l-ascorbic acid and oxalic acid with an octahedral manganese(IV) complex of 1,8-bis(2-hydroxybenzamido)-3,6-diazaoctane. Transit. Met. Chem. 2006;31:316–324. doi: 10.1007/s11243-005-6389-9. [DOI] [Google Scholar]
- 23.Harkrader RJ, Plunkett LM, Tolbert BM. Periodate degradation of labeled ascorbic acid. Anal. Biochem. 1976;72:310–314. doi: 10.1016/0003-2697(76)90535-2. [DOI] [PubMed] [Google Scholar]
- 24.Herbert, R. W., Hirst, E. L., Percival, E. G. V., Reynolds, R. J. W. & Smith, F. The constitution of ascorbic acid. J. Chem. Soc. 1270–1290 (1933).
- 25.Shin DB, Feather MS. The degradation of l-ascorbic acid in neutral solutions containing oxygen. J. Carbohydr. Chem. 1990;9:461–469. doi: 10.1080/07328309008543846. [DOI] [Google Scholar]
- 26.Kurata T, Miyake N, Otsuka Y. Formation of l-threonolactone and oxalic acid in the autoxidation reaction of l-ascorbic acid. Biosci. Biotechnol. Biochem. 1996;60:1212–1214. doi: 10.1271/bbb.60.1212. [DOI] [PubMed] [Google Scholar]
- 27.Ünaleroǧlu C, Zümreoǧlu-Karan B, Zencir Y, Hökelek T. pH-independent decomposition reactions of l-ascorbic acid in aqueous metal solutions—I. Formation and structures of CoII and GdIII oxalates. Polyhedron. 1997;16:2155–2161. doi: 10.1016/S0277-5387(96)00563-3. [DOI] [Google Scholar]
- 28.Magda, D. et al. Motexafin gadolinium reacts with ascorbate to produce reactive oxygen species. Chem. Commun. 2730–2731 (2002). [DOI] [PubMed]
- 29.Arendse MJ, Anderson GK, Rath NP. Oxidative degradation of the ascorbate anion in the presence of platinum and palladium. Formation and structures of platinum and palladium oxalate complexes. Polyhedron. 2001;20:2495–2503. doi: 10.1016/S0277-5387(01)00843-9. [DOI] [Google Scholar]
- 30.Orioli P, Bruni B, Di Vaira M, Messori L, Piccioli F. Decomposition of ascorbic acid in the presence of cadmium ions leads to formation of a polymeric cadmium oxalate species with peculiar structural features. Inorg. Chem. 2002;41:4312–4314. doi: 10.1021/ic025598l. [DOI] [PubMed] [Google Scholar]
- 31.de Ruiter G, et al. The system iron(II)/mpzbpy mediates the H2O2 oxidation of cyclohexane and cyclooctene and the aerobic oxidative cleavage of ascorbic acid to oxalate. Inorg. Chem. Commun. 2008;11:787–790. doi: 10.1016/j.inoche.2008.03.032. [DOI] [Google Scholar]
- 32.Thomas, A. M., Mandal, G. C., Tiwary, S. K., Rath, R. K. & Chakravarty, A. R. Ascorbate oxidation leading to the formation of a catalytically active oxalato bridged dicopper(II) complex as a model for dopamine β-hydroxylase. J. Chem. Soc. Dalton Trans. 1395–1396 (2000).
- 33.Takisawa H, Morishima Y, Soma S, Szilagyi RK, Fujisawa K. Conversion of carbon dioxide to oxalate by α-ketocarboxylatocopper(II) complexes. Inorg. Chem. 2014;53:8191–8193. doi: 10.1021/ic5006242. [DOI] [PubMed] [Google Scholar]
- 34.Thomas AM, Lin B-L, Wasinger EC, Stack TDP. Ligand noninnocence of thiolate/disulfide in dinuclear copper complexes: solvent-dependent redox isomerization and proton-coupled electron transfer. J. Am. Chem. Soc. 2013;135:18912–18919. doi: 10.1021/ja409603m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession codes: The X-ray crystallographic data for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1976241 (4b) and 1976240 (6). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Other data are available from the authors.