Abstract
COVID-19 is a disease first identified in Wuhan City, Hubei Province, China, in December 2019, causes by a SARS-COV-2 virus infection. By 27 October 2020, 43,921,473 confirmed cases were reported worldwide, with 1,166,389 COVID-19 deaths. Conjunctivitis has been reported in adults and pediatric patients with COVID-19.
Objective:
The aim of this meta-analysis is to estimate the odd Ratio (ORs) of conjunctivitis in patients with COVID-19.
Methods:
A systematic review and meta-analysis have been performed using the PubMed and Google Scholar literature search. The ORs of conjunctivitis in adults and pediatric patients is the outcome of this meta-analysis.
Results:
There have been 1041 articles published since the outbreak in December 2019, according to the latest literature. For the meta-analysis, 20 studies with a total of 3383 participants were included. The odds ratio (ORs) of conjunctivitis was 0.01 (95% confidence interval [CI]: 0.00–0.02). No bias has been reported.
Conclusion:
Conjunctivitis is the most common ocular manifestations reported in adults. This comprehensive meta-analysis quantifies the existing evidence linking conjunctivitis with COVID-19 and highlights the high percentage of heterogeneity that is shown in the current studies. Finally, it offers a single review article which includes all the current articles available for COVID-19 and conjunctivitis in adults and children.
Keywords: COVID-19, meta-analysis, SARS-CoV-2, viral conjunctivitis
Introduction
COVID-19 is a disease first identified in Wuhan City, Hubei Province, China, in December 2019, caused by a SARS-COV-2 virus infection.1 In February, COVID-19 was formally announced by the World Health Organization (WHO) as COVID-19, formerly recognized as 2019 Novel Coronavirus (2019-nCOV) respiratory disease. The ambiguity of COVID-19 as it infects patients can vary from asymptomatic infection to serious illness and mortality. The case report of COVID-19 patients on a cruise’s ship (Diamond Princess ship) was one of the very early cases of a patient diagnosed with COVID-19 with viral conjunctivties.2
SARS-COV and SAR-COV-2 have been explained to have identical findings linked to the same coronavirus family. In addition, it was emphasized that the infection is spread by the tears.3 Although the likelihood of coronavirus spreading through tears appears small, it may survive in the conjunctiva, even in the absence of signs of conjunctivitis, suggesting the importance of using eye protection to avoid infection from external droplets and aerosols.4 The key components considered to understand the “Ocular Pathway” are angiotensin-converting-enzyme-2 (ACE2) receptors and TMPRSS2 protein.4 By 27 October 2020, 43,921,473 confirmed cases were reported worldwide, with 1,166,389 COVID-19 deaths. In adults and pediatric patients with COVID-19, conjunctivitis has been identified. The aim of this meta-analysis is to update eye care physicians of the ORs of conjunctivitis in COVID-19 patients as a review of the current evidence.
Methods
Trials identification and data consideration
The standards and guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) has been followed.5,6 PubMed search and other search engines “Google Scholar” have been used from December 2019 to 27 October 2020. The key words used were “COVID-19,” “Conjunctivitis” used individually or in combination. We selected randomized trials, observational studies, case series or case reports, and letters of research, letters to editors for confirmed cases of COVID-19 for the literature review, but only retrospective studies and observational studies for the meta-analysis. According to current scientific literature since the outbreak in December 2019 there have been 1041 papers written. The selected studies applied no language or other restrictions.
For bias detection, in each eligible study, a 7-point predefined quality control was used. The corresponding risk of bias was categorized as low (L), high (H), or unknown (U) to each quality item according to Higgins and colleagues5,7 Unknown is used to judge insufficient information. The Complete outcome data were judged as “Low risk” or “high risk” or “unknown.” The “low risk” is used when follow up percentage of participants lost was lower than 5% and “high risk” when follow up loss percentage was more than 20%.8,9 For other potential sources of bias, including the bias source, including the funding source reported in each protocol, the term “other bias” was used.5,10
The overall treatment effect was calculated and the study weight for each study was calculated. Due to the larger sample size of some of the studies, the study weight was calculated and the “true effect” for each study is shown. The larger sample size provides more information than a small sample size.
Data extraction
Inclusion criteria
(a) COVID-19 patients.
(b) Conjunctivitis was assessed, and the number of events was reported.
Exclusion criteria
a) If no data on ocular, manifestations neither conjunctivitis
b) Animal research
c) Case report, letter to the editorial or review
Quality of the comparative studies
Assessment of the quality characteristics used the following criteria: (1) random sequence generation (R), (2) allocation concealment (A), (3) blinding of participants and personnel (PB), 4) Blinding of outcome assessment (DB), (5) Incomplete outcome data (Attrition Bias) (AB), 6) Selective reporting (Reporting bias) (RB), and 7) Other bias (O). Each study was labeled with the right item either adequate (low risk of bias), unclear (unknown risk of bias), and inadequate (high risk of bias).
Literature screening and quality evaluation
One researcher independently screened the articles.
Statistical analyses
MedCalc 16.4.3 (Ostend, Belgium) software was used to statistically analyze the data and REV5. The ORs were calculated in each study. The study heterogeneity was assessed with the Cochran Q and I2 statistics. For the heterogeneity qualitative interpretation, I2 values of at least 50% were considered to reflect considerable heterogeneity, while values of at least 75% indicated large heterogeneity, as per the Cochrane Handbook.5 Publication bias was evaluated using both graphical funnel plot11 and Beggs statistical test. Random-effect model was used.
Results
Selection and characteristics of the study
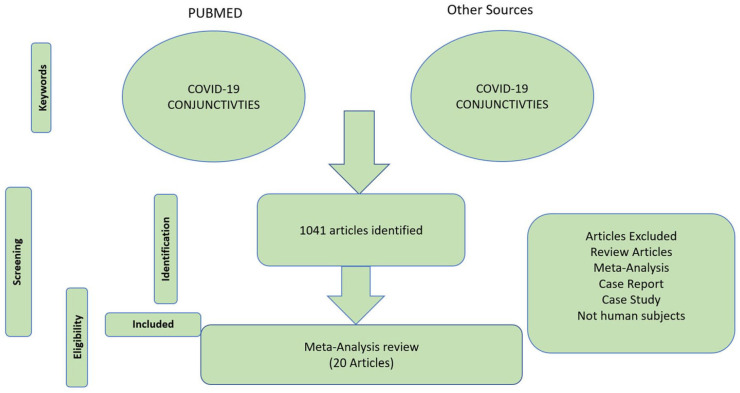
PubMed and Google Scholar Searches of database resulted in 8681 articles; 1041 results were screened after duplicates removal. For the meta-analysis reviewer, 20 articles were analyzed with a total of 3383 participants. Eighteen articles were studied in adult patients with COVID-19 and two articles were studied in pediatric patients with COVID-19.12,13 The flow chart presenting the selection of eligible studies is summarized (Figures 1 and 2). The characteristics of the included studies are summarized (Table 1). Table 1 includes first author’s name, publication year, sample size, mean or median age or range of age reported and country of the study. Finally, the number of total COVID-19 patients and number of viral conjunctivitis patients (Table 1.)
Figure 1.
Flow PRISMA chart presenting the total number of articles and the number of the included studies. Adapted from Moher and colleagues.6
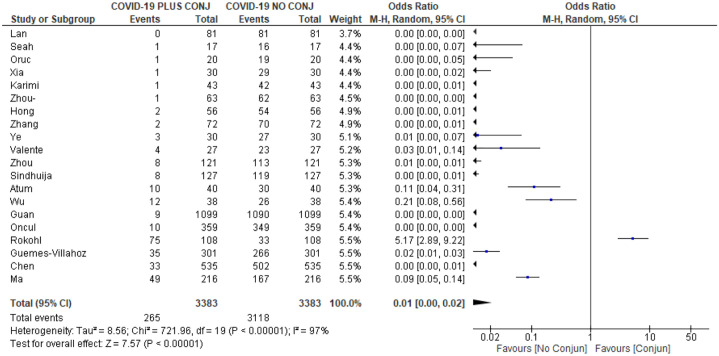
Figure 2.
Forrest plot depicting pooled analysis.
Table 1.
Characteristics of included studies.
| Study ID | Country | Conjunctivitis | Mean ± STD or median/range age (years) | Study iD | Country | Conjunctivitis | Average years |
|---|---|---|---|---|---|---|---|
| Guan and colleagues14 | China | 9/1099 | Median: 47 (range: 35.0–58.0) |
Wu and colleagues15 | China | 12/38 | 65.8 ± 16.6 |
| Xia and colleagues16 | China | 1/30 | 54.5 ± 14.2 | Zhou and colleagues17 | China | 8/121 | Median: 48 (range: 22–89) |
| Zhou and colleagues18 | China | 1/63 | Range: 18–60 | Zhang and colleagues19 | China | 2/72 | 58.7 ± 14.8 |
| Sindhuja and colleagues20 | India | 8/127 | Median 38.8 | Guemes-Villahoz and colleagues21 | Spain | 35/301 | Median: 72 |
| Chen and colleagues22,23 | China | 33/535 | Median: 44 (range: 16–68) |
Lan and colleagues24 | China | 0/81 | 41.69 ± 18.6 |
| Seah and colleagues25 | Singapore | 1/17 | Range: 18–50 | Atum and colleagues26 | Turkey | 10/40 | 41.38 ± 23.72 (range: 1–82) |
| Hong and colleagues27 | China | 2/56 | 48 ± 12.1 | Oruc and colleagues28 | Turkey | 1/20 | b |
| Valente and colleagues13 | Italy | 4/27 | 96.26 ± 76.10 (months) range (1–216 months) |
Ye and colleagues29 | China | 3/30 | 38.33 ± 23.33a |
| Ma and colleagues12 | China | 49/216 | Median: 7.25, (range: 2.6–11.6) | Öncül and colleagues30 | Turkey | 10/359 | Median: 58.5 (range:20–91) |
| Rokohl and colleagues31 | Germany | 75/108 | 37.9 ± 13.7 | Karimi and colleagues32 | Iran | 1/43 | 56 ± 13 |
The age of the 3 conjunctivitis ages was listed, rather than the average of the 30 patients.
Not listed.
The ORs ranged from 0.00 to 5.17, which reveals the high heterogeneity between individual studies. The sample size also varied between 17 and 1099 COVID-19 patients (Figure 2).
Independent studies bias risk
The risk of bias of the included studies has been reviewed and studied by the author (Supplemental Table 1). Random sequence was low in 6 studies.12,14,19,20,22,27 Allocation concealment was low in five studies.12,14,20,22,27 The blinding participants and personnel (PB) is low in 10 articles.12,20–22,26–28,30–32 The blinding of outcome assessment (DB) is low in 12 articles.12,20–22,24–28,30–32
The incomplete outcome data (Attrition Bias) (AB) is low in 19 articles.12–18,20–22,24–32
Selective reporting (Reporting bias (RB) is 16 articles.12–14,16–18,20,21,24,26–32 All other bias is low in all 20 articles12–22,24–33 (Supplemental Table 1).
Overall final analyses
The systematic review of the selected 20 articles estimated the ORs of conjunctivitis of a total of 3383 COVD-19 patients. The ORs = 0.01; 95% confidence interval (CI): [0.00, 0.02] (Figure 2). Heterogeneity was statistically significant using the I2 value of 97% and p < 0.0001. In addition, no publication bias was detected using the funnel plot inspection.
Discussion
Conjunctivitis may be the first manifestation of the COVID-19 infection.15,23,34
Most patients in the 20 articles were hospitalized with COVID-19 (Table 2). Each article had at least two groups of patients, both non-severe and severe. Other articles were grouped into three classes, moderate, severe, and critical according to the PC-NCP guidelines35 (Table 2).
Table 2.
Characteristics of COVID-19 patients that included in each study.
| No. of patients | Female | Male | |
|---|---|---|---|
| Guan and colleagues14 | 1099 hospitalized Only laboratory-confirmed cases |
459 | 640 |
| Wu and colleagues15 | 38 hospitalized patients with NCP | 13 | 25 |
| Xia and colleagues16 | 30 hospitalized patients with NCP | 9 | 21 |
| Karimi and colleagues32 | 43 severe COVID-19 9 patients were admitted to ICU because of respiratory failure |
14 | 29 |
| Sindhuja and colleagues20 | 127 patients | 14 | 113 |
| Zhang and colleagues19 | 72 confirmed laboratory diagnosis SARS-COV2-RT-PCR | 36 | 36 |
| Zhou and colleagues17 | 67 63 confirmed laboratories confirmed NCP 4 suspected cases of NCP |
42 | 25 |
| Zhou and colleagues18 | 121 patients | 68 | 53 |
| Guemes-Villahoz and colleagues21 | 301 patients from COVID admission unit with laboratory-confirmed SARS-COV2 infection 41 patients admitted to the intensive care unit. Age: 72 (59–82) |
121 | 180 |
| Chen and colleagues22,23 | A total of 535 COVID-19 patients (27 with conjunctival congestion) were enrolled in the study | 267 | 268 |
| Lan and colleagues24 | Hospitalized 81 patients | 48 | 33 |
| Seah and colleagues25 | 17 patients 20–75 (37) Age of patients (range, median) |
6 | 11 |
| Atum and colleagues26 | 40 patients tested positive Rt-PCR of nasopharyngeal and oropharyngeal swabs. 41.38 ± 23.72 years Range: 1–82 years |
15 | 25 |
| Hong and colleagues27 | 56 hospitalized patients who were discharged from the isolation ward and recovered well enough to return home. 48 (24–68, 12.1) Mean (range, SD), years |
25 | 31 |
| Oruc and colleagues28 | 20 patients COVID-19 patients | x | x |
| Valente and colleagues13 | 27 patients Nasopharyngeal swabs were positive for COVID-19 in all patients. Mean age: 84 months. Age range: 8 days to 210 months Children |
7 | 20 |
| Ye and colleagues29 | 30 COVID-19 patients | x | x |
| Ma and colleagues12 | 216 pediatric patients Laboratory-confirmed children with COVID-19 A median (interquartile range) age of 7.25 (2.6–11.6) years. |
82 | 134 |
| Öncül and colleagues30 | 359 COVID-19 patients Mean age of the patients was 58.5 years (20–91). 294 (81.9%) patients were treated in the inpatient clinic 65 (18.1%) patients were treated in the ICU. 11 (16.9%) of the 65 patients treated in the ICU received respiratory support with a mechanical ventilator. |
162 | 197 |
| Rokohl and colleagues31 | 108 Mean age of 37.9 ± 13.7 years (range: 18–87 years). |
57 | 51 |
ICU, intensive care unit; NCP, Novel Coronavirus Pneumonia; RT-PCR, reverse transcriptase-polymerase chain reaction; SD, standard deviation; x, no data.
The majority of patients with viral conjunctivitis were male patients (Table 3). Other ocular manifestations have been documented, such as conjunctival hyperemia25,29,31 and secretion,25,30 conjunctival discharge,29,31 eye rubbing, subconjunctival bleeding, keratitis, and vitreous hemorrhages. Oruc and colleagues, recorded that 5% of COVID-19 patients had conjunctivitis and 5% had diplopia.28 Acute conjunctivitis21 and chronic conjunctivitis22 were reported. Some studies reported conjunctivitis following the onset of COVID-19,13–20,22,24–27,29,30,32 while one study reported the diagnosis of conjunctivitis as the initial manifestation of COVID-19.12 Guemes-Villahoz and colleagues21 reported acute conjunctivitis in 13 patients prior to hospital admission, 12 patients between hospital admission and evaluation, and 10 patients at the time of evaluation. All of the included studies were hospitalized by COVID-19, except for one study included non-hospitalized patients.31 All patients were laboratory-confirmed SARS-COV2.
Table 3.
Characteristics of conjunctivitis patients that included in each study.
| Study iD | No. of patients | Male | Female | Age (years) mean ± STD | Severity | ICU or death | Tests and comments | B or U |
|---|---|---|---|---|---|---|---|---|
| Guan and colleagues14 | 9/1099 Adults |
5: N, 4: S | CC | |||||
| Wu and colleagues15 | 12/38 Adults |
7 2 70’s 2 60’s 1 50’s 1 40’s 1 30’s |
5 3 80’s 1 70’s 1 60’s |
4: M, 2: S 6: CR |
+NPS 11 +CS 2 |
|||
| Xia and colleagues16 | 1/30 Adults |
1 | +Sputum RT-PCR +CS |
|||||
| Karimi and colleagues32 | 1/43 Adults |
+Tear RT-PCR 3 | B | |||||
| Sindhuja and colleagues20 | 8/127 Adults |
N = 8 41.13 ± 16.64 | 2/8 CC with no systemic symptoms 1/8 CC before COVID-19 symptoms. 5/8 had only CC without any associated ocular complaints |
|||||
| Zhang and colleagues19 | 2/72 Adults |
1 | 1 | |||||
| Zhou and colleagues17 | 1/63 Adults |
1 | +NPS, –CS | |||||
| Zhou and colleagues18 | 8/121 Adults |
7 S or CR 1 MD or M |
1 + SAR-CoV-2 in CS | |||||
| Guemes-Villahoz and colleagues21 | 35/301 Adults |
Male S1 1, S2 12 S3 8 Female S1 9, S2 3 S3 2 |
Acute conjunctivitis 13 before admission, 12 in the time interval between admission and evaluation 10 at the time of evaluation. 11.6% prevalence of conjunctivitis among hospitalized patients with COVID-19 |
54.29% U | ||||
| Chen and colleagues22,23 | 33/535 Adults |
Chronic conjunctivitis 30 /508 − CC 3/27 + CC N = 27 with CC Median age (IQR) – years 44(28–53.5) Female: 12 |
||||||
| Lan and colleagues24 | 0/81 Adults |
N = 3 eye discomfort, F 65 (DES) M 63 Allergic conjunctivitis F 47 (unexplained conjunctivitis, self-resolved) All three cases negative CS |
||||||
| Seah and colleagues27 | 1/17 Adults |
Conjunctival injection and chemosis during the stay in the hospital | ||||||
| Atum and colleagues26 | 10/ 40 Adults |
7 | 3 | 43.33 ± 20.79 | +CS Only one tested positive using CS |
|||
| Hong and colleagues27 | 2/56 Adults |
1 | 1 | 49.5 ± 4.95 | 9/56 showed ocular symptoms after the onset of COVID-19 | U | ||
| Oruc and colleagues28 | 1/20 Adults |
5% conjunctivitis and 5% diplopia developed in patients diagnosed with COVID-19 | ||||||
| Valente and colleagues13 | 4/27 children |
115.75 (months) ± 51.05 | 1 +CS | |||||
| Ye and colleagues29 | 3/30 Adults |
2 | 1 | 38.33 ± 26.08 | 3 MD to M | 1 Death | Clinical symptoms: hyperemia, eye pain, foreign body sensation, stickiness, or increased watery exudation. | B |
| Ma and colleagues12 | 49/216 Children |
35 | 14 | M | 9 had ocular complaints being the initial manifestations of COVID-19. | |||
| Öncül and colleagues30 | 10/359 Adults |
6 | 4 | 51.7 ± 11.95 | 3 CR 6 M 1 S |
+NPS (10) The rate of ocular disease 4/65 in intensive care patients. Conjunctival chemosis developed in two (0.56%) patients in the ICU |
||
| Rokohl and colleagues31 | 75/108 Adults |
75 M | 115 non-hospitalized individuals with COVID-19 were called and 109 of them responded 75/108 (69.4%) had at least one ocular symptom during COVID-19 Burning sensations in 39 (36.1%), Epiphora in 37 (34.3%), and Redness in 28 (25.9%), compatible with conjunctivitis. These symptoms occurred 1.96 ± 3.17 days after the beginning of COVID-19 |
B, bilateral; CC, conjunctival congestion; CR, critical; CS, conjunctival swab; DES, dry eye syndrome; ICU, intensive care unit; M, moderate; MD, mild; N, non-severe; NPS, nasopharyngeal swab; RT-PCR, reverse transcriptase-polymerase chain reaction; S, severe; U, unilateral.
One study confirms that conjunctivitis can be a symptom of COVID-19 infection related with more serious type of disease, which confirms previous study.36
Furthermore, unilateral conjunctivitis in a 27-year-old COVID-19 male patient, has been reported as a first manifestation.37 Only two patients with conjunctivitis had been reported in 72 laboratory-confirmed COVID-19 cases.19 In addition, only one patient had conjunctivitis and foreign body sensation (2.3%) and tears in the 43 COVID-19 patients.32 In contrast, other reports showed no ocular conjunctivitis which could be due to the very low sample size.38
The prevalence rate of acute conjunctivitis in COVID-19 was reported ranging from 1.1 to 15.9.15,39 Other studies reported acute conjunctivitis in 31.6% of patients.23,39,40
One meta-analysis study showed that the probability of conjunctivitis in patients with non-severe COVID-19 was 4 out of 173, and severe COVID-19 was 5 of 926.40 Wu et al.15 showed that only one patient had the first symptoms of conjunctivitis.
There is certainly a difference in the percentage of conjunctivitis in COVID-19 patients that contributes to the aim of this meta-analysis. In our study, we found that 1% of COVID-19 patients are likely to have conjunctivitis with a total of 3383 COVID-patients.12–22,24–32
A related point to consider is that these findings have been reviewed retrospectively, and second, clinical symptoms have not been confirmed by clinical tests, but only have been subjectively reported by patients. In addition, because conjunctival mucosa can be a point of entry of COVID-19 infection due to overexpression of ACE2 receptors in epithelium from congested conjunctiva,41 careful attention should also be given to protective measures such as the face shields and goggles.
This meta-analysis is consistent with the meta-analysis that showed that the incidence of ocular manifestations in COVID-19 patients ranged from 2% to 32%.42 This meta-analysis review studied ORs of conjunctivitis and showed that the ORs ranged from 0.00 to 0.02.
The limitation of this study is (1) pediatric COVID-patients studies are needed, only 2 of the 20 studies in which the pediatric study was conducted, (2) only one reviewer performed the meta-analysis which may lead to bias, and (3) a greater sample size is needed.
Conclusion
Viral conjunctivitis is the most common ocular manifestations reported in adults. This comprehensive meta-analysis quantifies the existing evidence linking conjunctivitis with COVID-19 and highlights the high percentage of heterogeneity that is shown in the current studies. Viral conjunctivitis has been reported in males more than females. Finally, it offers a single review article which includes all the current articles available for COVID-19 and conjunctivitis.
Supplemental Material
Supplemental material, sj-pdf-1-oed-10.1177_25158414211003368 for COVID-19 and conjunctivitis: a meta-analysis by Mashael Al-Namaeh in Therapeutic Advances in Ophthalmology
Footnotes
Author contributions: Conceived and designed the literature review: M.A. performed the article assessment, analyzed the data and wrote the paper.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was a review, and no ethical approval is required.
ORCID iD: Mashael Al-Namaeh  https://orcid.org/0000-0002-5253-1175
https://orcid.org/0000-0002-5253-1175
Supplemental material: Supplemental material for this article is available online.
References
- 1. Paudel S, Dangal G, Chalise A, et al. The coronavirus pandemic: what does the evidence show? J Nepal Health Res Counc 2020; 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Salducci M, La Torre G. COVID-19 emergency in the cruise’s ship: a case report of conjunctivitis. Clin Ter 2020; 171: e189–e191. [DOI] [PubMed] [Google Scholar]
- 3. Dockery DM, Rowe SG, Murphy MA, et al. The ocular manifestations and transmission of COVID-19: recommendations for prevention. J Emerg Med 2020; 59: 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Napoli PE, Nioi M, d’Aloja E, et al. The ocular surface and the coronavirus disease 2019: does a dual “ocular route” exist? J Clin Med 2020; 9: 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sackett DL. Evidence-based medicine and treatment choices. Lancet 1997; 349: 570; author reply 572–573. [DOI] [PubMed] [Google Scholar]
- 9. Sackett DL. Evidence-based medicine. Semin Perinatol 1997; 21: 3–5. [DOI] [PubMed] [Google Scholar]
- 10. Bero LA. Why the Cochrane risk of bias tool should include funding source as a standard item. Cochrane Database Syst Rev 2013; 12: ED000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 12. Ma N, Li P, Wang X. Ocular manifestations and clinical characteristics of children with laboratory-confirmed COVID-19 in Wuhan, China. JAMA Ophthalmol 2020; 138: 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valente P, Iarossi G, Federici M, et al. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: a preliminary report. J AAPOS 2020; 24: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 2020; 138: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 2020; 92: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.02.11.20021956v1
- 18. Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology 2020; 127: 982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf 2020; 18: 360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sindhuja K, Lomi N, Asif MI, et al. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: a retrospective cross-sectional study. Indian J Ophthalmol 2020; 68: 1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guemes-Villahoz N, Burgos-Blasco B, Garcia-Feijoo J, et al. Conjunctivitis in COVID-19 patients: frequency and clinical presentation. Graefes Arch Clin Exp Ophthalmol 2020; 258: 2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol 2020; 98: e951–e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol 2020; 104: 748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lan QQ, Zeng SM, Liao X, et al. [A special on epidemic prevention and control:screening for novel coronavirus related conjunctivitis among the patients with coronavirus disease 2019]. Zhonghua Yan Ke Za Zhi 2020; 56: 433–437. [DOI] [PubMed] [Google Scholar]
- 25. Seah I, Anderson D, Kang A, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Am Acad Ophthalmol 2020; 127: 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atum M, Boz AAE, Cakir B, et al. Evaluation of conjunctival swab PCR results in patients with SARS-CoV-2 infection. Ocul Immunol Inflamm 2020; 28: 745–748. [DOI] [PubMed] [Google Scholar]
- 27. Hong N, Yu W, Xia J, et al. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. Epub ahead of print 26 April 2020. DOI: 10.1111/aos.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oruc Y, Aydin S, Akkoc RF, et al. Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions. Turk J Biochem 2020; 45: 443–449. [Google Scholar]
- 29. Y e Y, S ong Y, Y an M, et al. Novel coronavirus pneumonia combined with conjunctivitis: three cases report. Chin J Exp Ophthalmol 2020; 38: 242–244. [Google Scholar]
- 30. Öncül H, Öncül Y, Alakus M, et al. Ocular findings in patients with coronavirus disease 2019 (COVID-19) in an outbreak hospital. J Med Virol 2021; 93: 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rokohl A, Loreck N, Wawer Matos PA, et al. More than loss of taste and smell: burning watering eyes in coronavirus disease 2019. Clin Microbiol Infect 2020; 26: 1560.e5–1560.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karimi S, Arabi A, Shahraki T, et al. Detection of severe acute respiratory syndrome coronavirus-2 in the tears of patients with coronavirus disease 2019. Eye 2020; 34: 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aaker GD, Myung JS, Ehrlich JR, et al. Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 2010; 4: 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daruich A, Martin D, Bremond-Gignac D. Ocular manifestation as first sign of coronavirus disease 2019 (COVID-19): interest of telemedicine during the pandemic context. J Fr Ophtalmol 2020; 43: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Health Commission of the People’s Republic of China. The guideline on diagnosis and treatment of the novel coronavirus pneumonia (NCP). 5th ed. Revised version, http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml (2020, accessed 8 February 2020).
- 36. Loffredo L, Pacella F, Pacella E, et al. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol 2020; 92: 1413–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daruich A, Martin D, Bremond-Gignac D. Unilateral conjunctivitis as first presentation of coronavirus disease 2019 (COVID-19): a telemedicine diagnosis. J Fr Ophtalmol 2020; 43: e167–e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mungmungpuntipantip R, Wiwanitkit V. Ocular manifestation, eye protection, and COVID-19. Graefes Arch Clin Exp Ophthalmol 2020; 258: 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai CC, Ko WC, Lee PI, et al. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents 2020; 56: 106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu M, Dai C, Lv X, et al. Letter to the editor: are severe COVID-19 patients more susceptible to conjunctivitis? J Med Virol 2020; 92: 2394–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li S, Li D, Fang J, et al. SARS-CoV-2 receptor ACE2 is expressed in human conjunctival tissue, especially in diseased conjunctival tissue. Ocul Surf 2021; 19: 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol 2020; 258: 1351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-oed-10.1177_25158414211003368 for COVID-19 and conjunctivitis: a meta-analysis by Mashael Al-Namaeh in Therapeutic Advances in Ophthalmology