Abstract
Early airway responses to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection are of interest since they could decide whether coronavirus disease‐19 (COVID‐19) will proceed to life‐threatening pulmonary disease stages. Here I discuss endothelial‐epithelial co‐operative in vivo responses producing first‐line, humoral innate defence opportunities in human airways. The pseudostratified epithelium of human nasal and tracheobronchial airways are prime sites of exposure and infection by SARS‐CoV‐2. Just beneath the epithelium runs a profuse systemic microcirculation. Its post‐capillary venules respond conspicuously to mucosal challenges with autacoids, allergens and microbes, and to mere loss of epithelium. By active venular endothelial gap formation, followed by transient yielding of epithelial junctions, non‐sieved plasma macromolecules move from the microcirculation to the mucosal surface. Hence, plasma‐derived protein cascade systems and antimicrobial peptides would have opportunity to operate jointly on an unperturbed mucosal lining. Similarly, a plasma‐derived, dynamic gel protects sites of epithelial sloughing‐regeneration. Precision for this indiscriminate humoral molecular response lies in restricted location and well‐regulated duration of plasma exudation. Importantly, the endothelial responsiveness of the airway microcirculation differs distinctly from the relatively non‐responsive, low‐pressure pulmonary microcirculation that non‐specifically, almost irreversibly, leaks plasma in life‐threatening COVID‐19. Observations in humans of infections with rhinovirus, coronavirus 229E, and influenza A and B support a general but individually variable early occurrence of plasma exudation in human infected nasal and tracheobronchial airways. Investigations are warranted to elucidate roles of host‐ and drug‐induced airway plasma exudation in restriction of viral infection and, specifically, whether it contributes to variable disease responses following exposure to SARS‐CoV‐2.
Keywords: human airways, humoral innate defence, microvascular permeability, plasma exudation, pseudostratified epithelium, viral infection
1. INTRODUCTION
Unprecedented, joint research efforts currently concentrate on coronavirus disease‐19 (COVID‐19). Naturally, immune mechanisms of advanced COVID‐19, involving severe pulmonary circulation and alveolar pathophysiology, attract major attention. 1 Early host responses to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection are also of special interest since they could decide whether or not mild disease stages will proceed to life‐threatening COVID‐19. At initial, mild stages the apparently capricious COVID‐19 disease seems confined to nasal and tracheobronchial airways. Reflecting acknowledged concepts in immunology, cellular innate immunity takes centre stage in current discussions of early respiratory tract defence. 1 , 2 Focus is on cell‐produced interferons. An intriguing duality of IFN beta has been demonstrated in mice, with initial protection and, at later stages, worsening of pulmonary infection. 3 Thus, acute respiratory distress syndrome (ARDS)–like conditions with involvement of a leaky pulmonary circulation may be addressed in mouse studies. By contrast, this is not so with the airway plasma exudation response (discussed below). Further aspects of obvious interest include molecular mechanisms by which interferon defence may be hampered by immune evasion of SARS‐CoV‐2. 1 , 2 Notwithstanding their complex immune actions, interferons are forwarded as tentative treatments in COVID‐19. 2
Currently, any consideration of endothelial‐epithelial permeability and ‘plasma leak’ in relation to COVID‐19 concerns pathogenic roles in severe disease stages involving ARDS characterized by a leaking pulmonary circulation and alveolar injury. 1 Occurrence and potential contributions of physiological plasma exudation responses in early airways defence, and in regeneration of pseudostratified epithelium, are not discussed. Hence, for this review it seems important to emphasize differences between responses of the superficial subepithelial microcirculation, which runs all along the conducting airways, and the pulmonary microcirculation whose vital functions must always be protected.
Here a range of observations are collated indicating that plasma exudation is an early host response to viral infections of human nasal and tracheobronchial airways. This aspect warrants a brief update on mechanisms of airway plasma exudation and what defence opportunities this response may bring. The present focus is on findings in human airways in vivo with additional key observations in guinea‐pig airways that exhibit human airway‐like features regarding mucosal anatomy and plasma exudation responses. The present discussion is novel by its focus on respiratory viral infection.
2. PASSIVE DIFFUSION OF PLASMA PROTEINS AND PLASMA EXUDATION
An early study by Stockley et al 4 may illustrate major ways of entry of plasma proteins into airway secretions at baseline without active inflammation/infection and at active airways infection:
2.1. Passive diffusion
At baseline, there is a small degree of passive diffusion of serum proteins with sputum levels inversely correlated with the size (Stokes radii) of the proteins. Large proteins such as IgM occurring at concentrations greater than predicted by this relationship are considered to be locally produced. 4
2.2. Plasma exudation
The data obtained by Stockley et al 4 at bronchial infection showed a different picture. Their illustrated attempt at demonstrating size‐selectivity was telling; serum/sputum ratios for plasma macromolecules such as alpha2‐macroglobulin and IgM, corrected for albumin, actually did not differ much from 1. 4 Since alpha2‐macroglobulin is now a widely acknowledged marker of plasma exudation (discussed under separate heading below), these data are strong early hints at a non‐sieved transmission of plasma at inflammation/infection. However, the research that would reveal the nature of human airway plasma exudation had not arrived. Lacking a research paradigm, Stockley et al 4 thus resorted to discuss their infection data by belittling statements such as ‘when inflammation occurs, protein transudation would increase, exceeding local protein production …’.
Lack of size‐selectivity is a key defining property with the airway microvascular‐epithelial response that, in want of anything better, is called plasma exudation. This review iterates detailed features of plasma exudation as a physiological response of intact airways and at sites of epithelial regeneration (vide infra). Thus, the present discussion concerns opportunities of plasma exudation to contribute to local antiviral defence.
Proteins that appear together on the airway surface, at concentration ratios similar to their ratio in the circulation, would have moved by the plasma exudation mechanisms. Since very large plasma proteins are exuded, it seems reasonable to extrapolate, as done in Figure 1, and infer that practically all plasma molecules would be exuded together.
FIGURE 1.
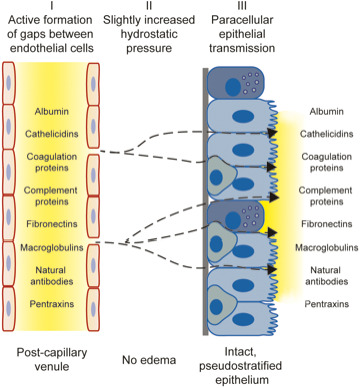
Plasma exudation in uninjured airways: In response to viral/bacterial infection, allergic inflammation and topical challenges with autacoids, plasma macromolecules move to the surface of the pseudostratified epithelial barrier of human nasal and tracheobronchial airways. As reflected by a well‐maintained ratio between small (albumin) and large (alpha2‐macroglobulin) plasma proteins during their brief journey from the intravascular compartment to the surface of an intact mucosa, the exudation of bulk plasma is defined as a largely a non‐sieved process. Hence, as illustrated and discussed in this review, practically all circulating plasma molecules could be expected to be components of the airways surface exudate. This opportunity for a first‐line airways defence is precisely localized to mucosal sites affected by exposure to environmental challenges. Yellow colour = plasma proteins‐peptides. Modified from references 12, 13 and 51
3. PERSISTENCE OF OLD CONCEPTS REGARDING INCREASED APPEARANCE OF PLASMA PROTEINS IN AIRWAY SURFACE LIQUIDS
When increased levels of plasma macromolecules appear in airway surface liquids, if at all explained, this has for a long time been considered a pathogenic feature and a sign of epithelial injury. Even in the 1980s, I thought so too. 5 Importantly, this old concept was still established at a time when gross physiological in vivo events increasingly became considered a mature research field; future medical revelations would be entirely based on advances based on added reductive biology approaches. 6 This circumstance has retarded dissemination and discussion of novel key physiological data and associated novel understanding of features and mechanisms of plasma exudation. This is not a singular example that I have encountered. Similar fates thus concern several other central airway events where original in vivo discoveries and the new research paradigms they underpin have taken unreasonably long times before being acknowledged. 7
The accepted modus operandi amongst researchers in leading positions has clearly been to simply ignore occurrence and thus disregard potential roles of plasma exudation in airways defence. Evidence for this attitude may be best represented by current state of the art‐reviews with high conceptual impact, whether it concerns airways anti‐infective defence in general 8 or more specific discussions related to COVID‐19. 1 Yet, work from different laboratories 25‐30y ago had demonstrated that human nasal infections in vivo with rhinovirus as well as coronavirus 229E associated with an early local plasma exudation response. 9 , 10 Albumin and larger plasma proteins such as fibrinogen were exuded into nasal surface liquids at active viral infections. Already then, the possibility was considered that the exuded plasma was not only a sign of and player in the inflammatory process, but could also have a role in antiviral defence contributing to resolution of the infection. 10 This idea was strengthened by emerging in vivo data demonstrating non‐injurious and swift exudation of bulk plasma in human airways without increasing inward mucosal perviousness. This and other aspects of airway plasma exudation mechanisms have previously been updated in separate reviews that, based on in vivo data, challenge several paradigms in airway research. 11 , 12 , 13 In this review, key original observations are listed under appropriate headings. The general idea is that basic physiological co‐operation between airway microcirculation and the juxtapositional pseudostratified epithelial lining (Figure 1) are designed to provide potent opportunities for early innate immune responses to environmental challenges.
4. SUPERFICIAL AIRWAYS MICROCIRCULATION DISTINCT FROM PULMONARY CIRCULATION
A profuse microcirculation network, carrying systemic, oxygenated blood, runs just beneath the pseudostratified epithelial lining cells of human nasal and tracheobronchial airways. Its ubiquitous presence is important. It caters for local precision of plasma extravasation responses. Its well‐controlled duration at the endothelial level, the extravasation being self‐abortive unless challenges increase, is also important. 11 , 13 Following mucosal challenge‐induced extravasation of plasma in nasal and tracheobronchial airways, the plasma‐derived macromolecules are swiftly transmitted across the intact, pseudostratified epithelial lining in humans and guinea pigs. Crucially, the epithelial passage occurs without altering the normal barrier function of the mucosa. 12 , 13
Airway mucosal challenges with microbes, autacoids and allergens cause localized and actively controlled extravasation of bulk plasma. This occurs between transiently separated endothelial cells in post‐capillary venules belonging to the airway subepithelial microcirculation. 12 Such conspicuous endothelial responsiveness is well reflected by effects of histamine applied topically on the mucosa of human upper airways or inhaled into the bronchi. 14 , 15 , 16 Importantly, histamine‐type autacoids may not increase permeability of the pulmonary circulation. 17
Hence, the systemic mucosal microcirculation of conducting airways differs distinctly from the relatively non‐responsive, low‐pressure pulmonary microcirculation that non‐specifically and almost irreversibly leaks plasma in ARDS, apparently reflecting dying pulmonary cells. 1 , 3 In current literature on COVID‐19, plasma leak is discussed/depicted exclusively in relation to the non‐specific ARDS‐like lung injury. 1 , 3 Airway microcirculation seems entirely undiscussed.
It is not known what mechanisms may disallow a mild COVID‐19 to proceed from conducting airways towards involvement of alveolar sites and pulmonary microcirculation. The present idea that variable airways innate humoral immune responses could be a contributing factor in the early phase of COVID‐19 is forwarded as a complement to current views of cellular early innate immunity. 1 , 2 , 8
5. VIRAL INFECTION‐INDUCED HUMAN AIRWAY PLASMA EXUDATION
Observations on human respiratory viral infections suggest their consistent association with airway plasma exudation. Proud et al 9 demonstrated that natural rhinovirus infections exhibited significant nasal mucosal exudation of albumin, kininogens and bradykinin associated with common cold symptoms. In accord with occurrence of exudative hyperresponsiveness in allergic airways disease, 12 Pizzichini et al 18 demonstrated several hundred‐fold increases in sputum fibrinogen levels in asthmatic patients naturally infected by influenza A and B. In a cohort of healthy subjects, it was noted that those who resisted infection at nasal rhinovirus inoculation exhibited exudative hyperresponsiveness compared to those who became infected by the same procedure. 19 Nasal challenge with influenza A (H3N2) produced illness associated with increased nasal lavage fluid levels of complement (C3a, C5a)—maximal levels were detected during the recovery phase. 20 It is not known whether local cell‐produced complement has contributed.
Åkerlund et al 10 further demonstrated association between symptoms and exudation of fibrinogen at coronavirus 229E inoculation‐induced common cold. Their finding was corroborated and expanded by Winther et al, 21 who demonstrated that rhinovirus infection‐induced exudation of albumin was associated with fibrinogen molecules (fibrinogen, fibrinogen degradation products, cross‐linked fibrin) in nasal lavage liquids. The time course of occurrence of the fibrinogen molecules on the human airway surface correlated with albumin and coincided with cold symptoms from 2 to 7 days following inoculation. 21 Åkerlund et al 10 also discussed fibrinogen exudation as a mode of host defence in coronavirus 229E‐induced cold.
Lacking specific information, reviewers have based discussions on the presumption that SARS‐CoV‐2 data would recapitulate data obtained with other coronaviruses. Using this reasoning, coronavirus 229E, a common cold virus, has attracted attention with regard to antibodies following infection. 1 For the present discussion, however, observations at the start of infection are of interest. The following points summarize features of airways plasma protein exudation in human subjects developing common cold after nasal inoculation with coronavirus 229E:
Reflected by a mean 100‐fold increase in nasal lavage fluid levels of fibrinogen, the viral infection‐induced common cold associated with individually variable, significant exudation of bulk plasma. 10
Considering its duration and association with both symptom development and decline, it was suggested that the exuded plasma, along with a somewhat delayed increase in interferon production, contributed to resolving common cold. 10 , 22
Since nasal airway absorption permeability was not increased, the human nasal response to coronavirus 229E likely reflected plasma exudation without epithelial injury/loss. 23
There was marked 24‐hour variation, nasal mucosal surface levels of fibrinogen peaking in early morning hours. 24
As evidenced by a leftward shift in dose response to topical histamine, the archetypal exudative autacoid, the nasal mucosa developed exudative hyperresponsiveness (=increased innate defence alert?) following resolution of the cold. 23
It emerges that plasma exudation is a consistent feature in virally infected human airways. However, this possibility needs to be assessed in a wider context than hitherto, and involving COVID‐19. In addition, we need to learn about pathways linking airway cell recognition of microbial challenges with release and operative roles of vascular permeability‐inducing autacoids in the airway mucosa. Vasoactive mediators involved in viral infection‐induced plasma exudation thus remain to be defined. Bradykinin, which is potent inducer of airways plasma exudation, would emerge as a result rather than a cause of viral infection‐evoked plasma exudation. As reviewed elsewhere, 13 neurogenic plasma exudation may not be involved because it occurs in rodent airways but not in human airways. Although histamine is a common exudative autacoid in the airways, it seems to have no role in viral infection‐induced plasma exudation. 25
Interestingly, rhinovirus‐ and coronavirus 229E‐induced common cold has caused prompt increase in nasal lavage fluid levels of plasma exudation indices, 9 , 10 whereas a delayed increase in interferon levels 22 and epithelial interferon gene expression 26 have been reported. Of further note, early neutralization of viral infection in the absence of interferons has been demonstrated in picornavirus conjunctivitis 27 ; it cannot be excluded that IgM 28 and other plasma proteins have contributed.
6. LARGE PROTEINS ARE PREFERABLE MARKERS OF PLASMA EXUDATION
Small plasma proteins such as albumin slowly seep through airways microvascular‐epithelial barriers under baseline conditions and may thus accumulate especially in bronchial airway surface liquids. This may confuse interpretation of sputum and BAL fluid data, where relatively high baseline levels of albumin mean that fold increases of this protein at exudation of bulk plasma may be less than with large plasma proteins. Illustrative examples include viral infection‐ as well as allergen challenge‐induced plasma exudation as reflected by albumin and fibrinogen levels in sputum 18 and bronchial lavage fluid, 29 respectively. However, any‐size protein can be a marker of acute plasma exudation responses in the human nose where repeated, efficient irrigation 9 , 14 , 30 has produced near zero baseline levels of mucosal surface proteins. In the case of plasma exudation induced by a variety of challenges, data indicate that the human nose represents the tracheobronchial airways but not the most peripheral lung supplied by the pulmonary circulation. 31
Considering its non‐sieved nature, 12 plasma exudation in human airways would be best reflected by large proteins such as fibrinogen and alpha2‐macroglobulin. 12 , 13 Exudation of alpha2‐macroglobulin suggests an opportunity for the entire plasma cascade systems to defend on challenged, yet intact, mucosal surfaces 12 (Figure 1).
Plasma exudation has also been the sole source of antimicrobial peptides such as cathelicidin, 13 , 32 which is at variance with current views that it is exclusively produced by local cells. Reflecting current focus on mechanistic research, local airway cells seem to be a favoured source of several human nasal and bronchial surface proteins, including complement, even in cases when plasma exudation is the likely source (reviewed in 12).
7. PLASMA EXUDATION FEATURES IN HUMAN AIRWAYS IN VIVO
The last three decades have witnessed an enormous advance in our views of the complexity of early respiratory antimicrobial defence mechanisms involving local cells and their interactions, and with mouse studies on the centre stage. 1 , 2 , 8 This impressive development regarding cell mechanisms may have contributed to eclipse the possibility that also humoral mechanisms could be involved in early innate immune responses. Nevertheless, during recent decades different aspects of plasma exudation in human airways have emerged, which are compatible with roles in first‐line respiratory defence (Figure 1). Key observations in vivo in human airways are listed:
Topical mucosal exposure to histamine caused endothelial‐epithelial exudation of plasma in nasal airways that was prompt, dose‐dependent, reversible, repeatable, and not reduced by corticosteroids. 33
A nasal decongestant, oxymetazoline, did not reduce histamine‐induced plasma exudation also suggesting that the rich superficial blood flow of human airways (not to be confused with sinusoid vasculature responsible for nasal blockade) can be reduced without affecting the exudation. 34
Plasma exudation, not local cells, was in vivo source of bronchial mucosal surface levels of cathelicidin, a major antimicrobial peptide. 13 , 32
Similar ratios between alpha2‐macroglobulin or fibrinogen and albumin in circulation and nasal plasma exudate indicated that the endothelial‐epithelial passage of plasma approaches a non‐sieved process. 13 , 35 Lack of size‐selectivity would mean associated passage of all potent plasma‐derived defence systems (complement, coagulation, etc, Figure 1).
Despite the bulk nature of epithelial transmission of macromolecules, plasma‐exuding airways maintained a normal mucosal absorption barrier. This unidirectional feature was equally valid for acute challenge‐induced plasma exudation 36 and for the sustained baseline airways exudation of bulk plasma occurring in allergic rhinitis and asthma. As reviewed elsewhere, 12 , 13 the novel concept of fully maintained epithelial barrier function in exudative diseases such as asthma and rhinitis is based on numerous well‐controlled human in vivo studies.
8. PLASMA EXUDATION MECHANISMS REVEALED IN GUINEA‐PIG TRACHEOBRONCHIAL AIRWAYS
The research front in early innate immunology has moved rapidly thanks to experimental versatility provided by molecular biology of cells in culture and in vivo as well as by engineered mouse in vivo approaches. 1 , 2 , 8 Since studies of plasma exudation largely requires in vivo approaches, it is unfortunate that challenges, which are typical inducers of this response in human airways, have not produced plasma exudation in mouse airways: Extravasated plasma has been detected but there has been no sign of epithelial transmission. 37 By contrast, human‐like airways exudation of plasma is well represented in the extra‐lobar tracheobronchial airways of guinea pigs. Distinct from mice, 8 the guinea‐pig extra‐lobar airways are equipped with human‐like, pseudostratified epithelium residing just above a profuse microcirculation carrying systemic blood. 11 Hence, whilst finding a relevant mouse model for airways exudation of plasma remains a challenge, basic features of plasma exudation in humans have been well reproduced in guinea pigs. 11 , 12 , 13
The points below summarize key original guinea‐pig data that tally with, explain, and go beyond the human airway data on airway plasma exudation. Observations are made in tracheobronchial airways with an intact pseudostratified epithelial lining and with patchy sites of epithelial cell loss, respectively.
8.1. Plasma exudation in airways with an intact epithelial lining
A maintained airway absorption barrier at non‐sieved exudation is originally demonstrated both functionally and ultra‐structurally. 38 , 39 , 40
The pathways of the exudate moving paracellularly across the pseudostratified epithelium are mapped. 41
It is observed for the first time that mucosal challenge‐induced plasma exudation does not produce subepithelial oedema, 38 , 42 which has been confirmed, 43 nor is the lymph transport of proteins increased from the area of interest. 44 Hence, swift epithelial transmission emerges as an efficient elimination route for the bulk of extravasated plasma.
Crucial experiments in intact guinea‐pig tracheal tube preparations originally reveal that minimally increased hydrostatic pressures impacting on the basolateral aspect of in situ epithelial lining cells cause plasma exudation‐like, non‐injurious, unidirectional, reversible, and repeatable movement of macromolecules into the airway lumen. 45 , 46 Hence, we have a mechanism that entirely mimics and hence explains the consistent appearance on the mucosal surface of extravasated plasma in challenged, intact airways: through a simple hydrostatic pressure mechanism, plasma leaving the circulation apparently creates its own transmission between pseudostratified epithelial cells, which yield transiently without reducing normal barrier functions.
When the epithelium is injured, extravasated plasma moves particularly promptly to the surface without the slight delay observed in airways with an intact epithelial lining. 45 , 46 , 47 A few minutes of ongoing plasma extravasation thus seem needed to raise the basolateral epithelial pressure to a critical point at which epithelial junctions will yield allowing passage of bulk plasma.
The plasticity of cell junctions, allowing tight seal transmission when approached from beneath, apparently operates well for both cells and macromolecules. 13 This property of pseudostratified airway epithelium thus provides both humoral and cellular defence opportunities on the surface of the intact mucosa. Further, the ubiquitous outward para‐epithelial pathways are also silent cell elimination routes. This escape seems especially important for disease‐driving mucosal tissue granulocytes that, failing to undergo apoptosis, are eventually cleared by migration into the airway lumen at inflammation resolution. 12 By contrast, the swift movement of plasma to the mucosal surface, which counteracts mucosal oedema formation, operates acutely following extravasation.
As reviewed elsewhere, 13 shedding of epithelium in human airways in vivo involves non‐sanguineous sloughing from an intact basement membrane and seems to be exceedingly patchy (as does viral infection of epithelial cells). 13 Minimally invasive guinea‐pig approaches have met the experimental challenge of producing well‐defined shedding of pseudostratified epithelium in vivo without causing any bleeding and without injuring the basement membrane. 48 , 49 The in vivo findings listed below would belong to the picture (Figure 2) in airways exhibiting patchy sites of epithelial loss‐regeneration independent of the cause of desquamation, whether it is due to viral infection or not.
FIGURE 2.
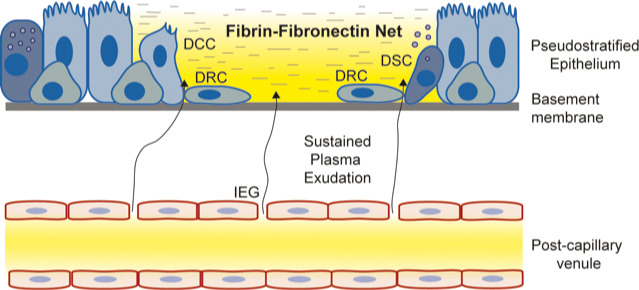
Plasma exudation at sites of epithelial loss‐regeneration: In vivo in guinea‐pig airways, with human airway‐like pseudostratified epithelium and rich subepithelial microcirculation, it has been demonstrated that the mere loss of epithelial cells, without bleeding and without injury to the basement membrane, causes prompt exudation of bulk plasma. The exudation creates and maintains a biologically active, physical fibrin‐fibronectin barrier gel restricted to the area of denudation—the plasma‐derived molecular milieu also promotes speedy epithelial regeneration in which all types of pseudostratified epithelial cells participate as stem cells. The epithelial regeneration milieu is continuously supplied with exuded plasma until the basement membrane has received a new cellular cover of poorly differentiated epithelial regeneration cells derived from ciliated, secretory and basal cells bordering the desquamated site. Hence, whether viral infection is the cause of sloughing of pseudostratified epithelium or not, vulnerable sites of epithelial regeneration would be well protected physically and biologically by exuded plasma. Yellow colour represents plasma proteins. Abbreviations: DCC = Dedifferentiating ciliated cell internalizing or shedding its cilia, flattening, and migrating; DRC = Dedifferentiated mesenchymal‐like regeneration cells migrating speedily; DSC = Dedifferentiating secretory cell releasing its secretory granules, flattening, and migrating; IEG = Venular inter‐endothelial gaps through which non‐sieved plasma proteins extravasate. Modified from references 11, 12 and 13
8.2. Plasma exudation at patchy sites of epithelial cell loss
Promptly following epithelial loss, local plasma exudation is induced selectively covering the acellular area of the epithelial basement membrane. 49 , 50
Restricted to the epithelial regeneration site, exuded plasma creates an active fibrin‐fibronectin net and barrier gel attracting and activating neutrophils. (This gel is lost by common preparations for studies of airway histology.) The site is continuously supplied with new plasma‐derived molecules creating and maintaining a fibronectin‐rich milieu suited for both defence and repair whilst protecting the denuded basement membrane. 50
During the initial regeneration phase, both ciliated and secretory cells bordering the denudation area promptly dedifferentiate into mesenchymal‐like cells. Together with basal cells they migrate at high speed and in well‐tethered fashion across the basement membrane. 49
Once a new cellular cover has been established, consisting of poorly differentiated barrier epithelium exhibiting interdigitating cellular junctions, plasma exudation ceases and the provisional fibrin‐fibronectin gel is shed. 50
As reflected by their incorporation in a major lung textbook, fundamental relevance and originality of the observations above on epithelial regeneration in airways with a pseudostratified epithelium were acknowledged at the time. 51 Curiously enough, this occurred despite the fact that we did not provide information on involved definitive molecular mechanisms. Now molecular approaches are rediscovering, apparently for the first time 52 some of the original observations including the participation of different type airway epithelial cells in repair. 49 Perhaps advanced reductive technology employed in medical‐biological experiments may allow researchers to be history‐less in some cases. 7 On this note, a milieu of plasma‐ rather than local cell‐derived proteins in airways epithelial regeneration 50 may be waiting to be rediscovered.
Influenza viruses may cause nasal epithelial cell injury, which is not observed with common cold viruses. 53 However, since viral infection seems exceedingly patchy in the airways 13 and epithelial regeneration a prompt and speedy process, 51 induced minor epithelial injury‐loss may escape detection. RSV infection in guinea pigs has been demonstrated to cause bronchitis involving epithelial shedding 54 but few details are known about humoral responses to RSV or other respiratory viral infections in this species. Severe stages of COVID‐19 exhibiting lung injury are associated with damage to the alveolar epithelial lining in both humans and mouse models. 1 To the extent that mouse data on COVID‐19 can be translated to humans, epithelial injury would occur in both nasal and bronchiolar airways. 55 However, it is not yet known if SARS‐CoV‐2 infection causes epithelial injury/loss in human conducting airways.
9. COMPLEMENT SYSTEM IN EARLY DEFENCE
This is not a review of biological effects of plasma‐derived molecules with antiviral defence capability. However, complement proteins have received special attention in discussion of early anti‐COVID‐19 defence. Although frequently thought to be produced by local airway cells, it seems clear that plasma exudation will bring the complement system to the challenged airway mucosa in vivo in humans. 12 , 56 Similar to interferons, 1 , 2 , 3 roles of complement proteins as both antiviral and pathogenic factors may depend on the phase of COVID‐19. Java et al thus consider the complement system an important antiviral asset during the first week of COVID‐19 and warns against complement inhibition at this stage when complement is a ‘friend’. 57 Plasma exudation as a source of complement proteins is not mentioned. Instead, similar to discussions of COVID‐19 evading interferon defences, 1 , 8 there is interest in molecular pathways for immune evasion of complement‐dependent defence. 57
10. PLASMA EXUDATION DELIVERS NATURAL ANTIBODIES IN AIRWAYS ANTIVIRAL DEFENCE
Circulating natural antibodies can be expected to arrive at nasal and tracheobronchial sites of viral infection through the mechanisms of airway plasma exudation. Observations by Balfour‐Lynn et al 58 in infants with acute upper airway viral infections are supportive: nasal lavage fluid levels of secretory IgA remained unchanged, whereas total IgA was significantly elevated. 58 With reference to plasma exudation as a first‐line airways defence event, the authors inferred that plasma exudation had produced the increase in mucosal surface non‐secretory IgA. 58 In addition, Balfour‐Lynn et al noted the potential importance of plasma exudation in directing antibodies, given as iv treatment or circulating as a result of early conversion, to airway mucosal sites of infection. 58
Naturally occurring IgM, through interaction with complement, is suggested to assist in clearance of SARS‐CoV‐2 prior to development of pneumonia. 57 Intriguingly, this possibility is also forwarded as a factor causatively involved in the protection against COVID‐19 that has been observed in patients with blood group 0, harbouring both anti‐A and anti‐B IgM. 57 Further, reduced diversity of IgM has been noted in old people potentially contributing to the increased vulnerability in the elderly population. 57 In actual support of a non‐sieved nature 13 , 35 of plasma exudation, Stockley et al 4 demonstrated much increased IgM along with alpha2‐macroglobulin protein in sputum obtained from patients undergoing acute bronchial infection as compared to non‐infected conditions. Also in asthma, a disease characterized by increased baseline exudation of plasma, 12 an intriguing similarity between exudation of IgM and alpha2‐macroglobulin has been demonstrated, whether calculated as a ratio to albumin or as actual protein levels in bronchial surface liquids. 59 Indeed, it may be worthwhile to explore whether the plasma exudation feature of asthmatic bronchi 12 has contributed to unexpected but repeated observations of low risk for severe COVID‐19 in these patients. 60 The possibility for early delivery of both complement and IgM to infected airway sites should increase interest in exploration of occurrence and roles of airway plasma exudation in COVID‐19.
11. PANDORA'S BOX AND HYPOTHESIS TEST
Restricted by location and limited duration, plasma exudation is reflected by movement of bulk plasma macromolecules. Hence, this response is like opening Pandora's box of potential defence systems. Conceivably, non‐sieved plasma exudation response to airways viral infection would make immune evasion difficult. However, little is known as yet about joint effects of plasma protein systems such as would occur at highly localized epithelial sites of airway challenges. Previous studies on interactions between plasma protein systems support the importance of improved opportunities in defence. 61 However, rather than discussing effects of plasma cascade systems deposited on mucosal surfaces, the reports have so far concerned dangerous intravascular events 62 of possible relevance to coagulation cascades and thrombotic complications in late phases of COVID‐19. 1
It might be possible to tentatively test defence properties of plasma exudation in part by simple airway administration of an exudative autacoid. This mode might compete with current ideas of inhaling antimicrobial plasma proteins. 63 As discussed in this review, the opportunity for humoral innate immunity would be served by a brief spurt of plasma proteins delivered at the mucosal site of threatening or ongoing infection. Treatment‐induced sustained plasma exudation may neither be needed nor desirable.
Histamine would be suited as a prototype challenge/tentative‐treatment option. Histamine is a standard challenge agent 64 in a major model for studies of microvascular permeability mechanisms, the hamster cheek pouch, also reflecting inducement of plasma exudation in the oral cavity. Furthermore, histamine causes prompt and brief plasma exudation responses in human both nasal and bronchial airways. 14 , 16 Importantly, human safety is already demonstrated by the use of histamine inhalational challenges in large cohorts of asthmatics. 65 Initial experiments, addressing efficacy of histamine‐induced nasal plasma exudation, could involve volunteers being locally inoculated with rhinovirus using well‐established clinical trial methodology. If take rate and/or disease progression is suggesting an acceptable efficacy, and a practical dose regimen can be documented, this would be a basis for further trials eventually involving tentative use as a prophylactic measure also against COVID‐19. Special precautions should apply in the latter case to avoid sneezing and exhalations that risk spreading the disease.
Apparently through neural reflexes, histamine produces some glandular secretion along with plasma exudation. 66 The mucus aspect is interesting. Mucus is readily produced in cell cultures and mouse models. Hence, molecular data and reviews flourish. The experimental opportunities may explain the extreme imbalance between mucus research and plasma exudation research (not lending itself to in vitro nor mouse in vivo approaches). Typically, airway mucus reviews entirely fail to even mention plasma exudation, 67 , 68 so even reviews focused on mucus defence in COVID‐19. 67 Mucus may have some barrier functions 68 conceivably attenuating both airway absorption and exudation of molecules. However, occurrence of mucus in human airways evidently has not prevented successful sampling of large plasma proteins by gentle lavage procedures—if the largest plasma proteins appear at even greater concentrations close to the epithelial apex in tethered airway secretions, that may give rise to speculations of added defence efficacy including opportunities for mucus‐plasma interactions. 13 However, how relevant that is for in vivo human airways is unknown.
It seems urgent to learn about natural occurrence of airways plasma exudation in COVID‐19 to explore associations with disease progress and guide possible intervention studies in selected cohorts. Since controlled human airway in vivo studies involving common cold are feasible, they should probably have priority in early tests of levels of antiviral efficacy along with potential utility of exogenous autacoid(histamine)‐induced plasma exudation. Separately, antiviral potentials of interactions between components of bulk plasma may be explored in human airway epithelial cell cultures subjected to viral infection.
Guinea‐pig nasal‐ and extra‐lobar tracheobronchial airways respond well with plasma exudation to many stimuli but little is known regarding mucosal effects of viral infections in guinea‐pig airways. Mice need to be humanized to get infected by SARS‐CoV‐2. However, mouse airways have not responded with plasma exudation to challenges that are consistent inducers of this response in human and guinea‐pig airways. 37 A ‘human lung only’‐mouse model has been developed whereby a subcutaneously implanted piece of human lung tissue is infected with SARS‐CoV‐2. 69 Prompt cytopathic effects and inducement of type I interferon resulted. 69 Speculatively, this model might be further developed to harbour the desired airway microcirculation with a juxtapositional, pseudostratified epithelium, which would be prerequisites for in vivo exploration of plasma exudation mechanisms.
12. CONCLUSION
The mucosal exudation response discussed in this review has so far received minimal attention in immunology literature. At best, plasma exudation may be mentioned in a sentence like this: ‘Moreover, the slightest irritation of human nasal or intestinal mucosa leads to bulk flow of serum proteins to the epithelial surface’, which is the exhaustive excerpt from a review by Brandtzaeg 70 in which he referred to the previous discussion of this topic in Scand J Immunol. 11 Dissemination thus seems warranted of basic features of the plasma exudation response towards defining it as a frequently induced local defence opportunity of intact airways mucosae and at sites of epithelial loss.
An obliging update is provided here by iterating, summarily, the basic physiology and occurrence of airway plasma exudation recently forwarded in two conceptual reviews. 12 , 13 Distinctly, here the additional focus is on occurrence of plasma exudation in infected airways. The present review thus collates a wide variety of human clinical studies involving nasal and bronchial viral infection. The reported data indicate that airway viral infections associate with early local exudation of potent plasma proteins apparently without size restriction. The data arguably need interpretation within the framework of combatting local infections. On this point, by amalgamating plasma exudation physiology with observations of exuded plasma proteins in patients with airways infection, the present review takes the first steps.
As discussed here, viral infection data both support and agree well with the novel understanding of basic features of airway plasma exudation. This statement also defines the main purpose of this review: an overlooked yet conspicuous opportunity for innate host defence is presented in order to give rise to important asks: To what extent is nasal and tracheobronchial plasma exudation an effective antiviral host response? Amongst all the potent protein systems, antimicrobial peptides and other molecules of circulating plasma that appear together on the infected airway mucosa, which immunological mechanism can be envisaged/demonstrated to actually provide viral relief? Can airway plasma exudation be induced by treatments with autacoids/drugs to fully exploit its antiviral potential? etc Inferentially, the presently forwarded aspects need consideration together with generally acknowledged (not reviewed in any detail here) antimicrobial defence capacities of the molecular content of plasma. As a corollary, investigations are warranted to elucidate any role of endothelial‐epithelial plasma exudation in human conducting airways exposed to SARS‐CoV‐2 with focus on potential attenuation of progress of COVID‐19 beyond infected airways.
CONFLICT OF INTEREST
No conflict of interest.
Persson C. Early humoral defence: Contributing to confining COVID‐19 to conducting airways?. Scand J Immunol. 2021;93:e13024. 10.1111/sji.13024
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. Vabret N, Britton GJ, Gruber C, et al. The Sinai Immunology Review Project, Immunology of COVID‐19: current state of the science. Immunity. 2020;2020(52):910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park A, Iwasaki A. Type I and type III interferons – induction, signaling, evasion, and application to combat Covid‐19. Cell Host Microbe. 2020;27:870‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type i interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe. 2016;19:181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stockley RA, Mistry M, Bradwell AR, Burnett D. A study of plasma proteins in the sol phase of sputum from patients with chronic bronchitis. Thorax. 1979;34:777‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Persson CG. Role of plasma exudation in asthmatic airways. Lancet. 1986;2(8516):1126‐1129. [DOI] [PubMed] [Google Scholar]
- 6. Persson C. Clinical research, or classical clinical research? Nat Med. 1999;5(7):714‐715. [DOI] [PubMed] [Google Scholar]
- 7. Persson C. In vivo observations provide insight into roles of eosinophils and epithelial cells in asthma. Eur Respir J. 2019;54:1900470. 10.1183/13993003.00470-2019 [DOI] [PubMed] [Google Scholar]
- 8. Iwasaki A, Foxman EF, Molony RD. Early local immune defences in the respiratory tract. Nat Rev Immunol. 2017;17:7‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Proud D, Naclerio RM, Gwaltney JM, Hendley JO. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990;161:120‐123. [DOI] [PubMed] [Google Scholar]
- 10. Åkerlund A, Greiff L, Andersson M, Bende M, Alkner U, Persson C. Mucosal exudation of fibrinogen in coronavirus‐induced common colds. Acta Otolaryngol. 1993;113:642‐648. [DOI] [PubMed] [Google Scholar]
- 11. Persson C, Erjefält JS, Erjefält GL, et al. Contribution of plasma‐derived molecules to mucosal immune defence, disease and repair in the airways. Scand J Immunol. 1998;47:302‐313. [DOI] [PubMed] [Google Scholar]
- 12. Persson C. Airways exudation of plasma macromolecules: innate defense, epithelial regeneration, and asthma. J Allergy Clin Immunol. 2019;143:1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Persson C. Humoral first‐line mucosal innate defence in vivo. J Innate Immun. 2020;2020(12):373‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Svensson C, Baumgarten CR, Pipkorn U, Alkner U, Persson C. Reversibility and reproducibility of histamine‐induced plasma leakage in nasal airays. Thorax. 1989;44:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greiff L, Andersson M, Svensson C, Persson C. Topical azelastine has a 12‐hour duration of action as assessed by histamine challenge‐induced exudation of alpha2‐macroglobulin into human nasal airways. Clin Exp Allergy. 1997;27:438‐444. [PubMed] [Google Scholar]
- 16. Halldorsdottir H, Greiff L, Wollmer P, et al. Effects of inhaled histamine, methacholine and capsaicin on sputum levels of alpha2‐macroglobulin. Thorax. 1997;52:964‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pietra GG, Szidon JP, Leventhal MM, Fishman AP. Histamine and interstitial pulmonary edema in the dog. Circ Res. 1971;29:323‐337. [DOI] [PubMed] [Google Scholar]
- 18. Pizzichini MMM, Pizzicini E, Efthimiadis A, et al. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178‐1184. [DOI] [PubMed] [Google Scholar]
- 19. Greiff L, Andersson M, Svensson C, Linden M, Myint S, Persson C. Allergen challenge‐induced acute exudation of IL‐8, ECP and alpha2‐macroglobulin in human rhinovirus‐induced common colds. Eur Respir J. 1999;13:41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bjornson AB, Mellencamp MA, Schiff GM. Complement is activated in the upper respiratory tract during influenza virus infection. Am Rev Respir Dis. 1991;143:1062‐1066. [DOI] [PubMed] [Google Scholar]
- 21. Winther B, Gwaltney JM Jr, Humphries JE, Hendley JO. Cross‐linked fibrin in the nasal fluid of patients with common cold. Clin Infect Dis. 2002;34:708‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linden M, Greiff L, Andersson M, et al. Nasal cytokines in common cold and allergic rhinitis. Clin Exp Allergy. 1995;25:166‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greiff L, Andersson M, Åkerlund A, et al. Microvascular exudative hyperresponsiveness in human coronavirus‐induced common cold. Thorax. 1994;49:121‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greiff L, Akerlund A, Andersson M, Svensson C, Alkner U, Persson C. Day‐night differences in mucosal plasma proteins in common cold. Acta Otolaryngol. 1996;116:85‐90. [DOI] [PubMed] [Google Scholar]
- 25. Naclerio RM, Proud D, Kagey‐Sobotka A, Lichtenstein L, Hendley JO, Gwaltney JM Jr. Is histamine responsible for the symptoms of rhinovirus colds? A look at the inflammatory mediators following infection. Pediatr Infect Dis. 1988;7:218‐222. [DOI] [PubMed] [Google Scholar]
- 26. Ravi A, Chang M, van de Pol M, et al. Rhinovirus‐16 induced temporal interferon responses in nasal epithelium links with viral clearance and symptoms. Clin Exp Allergy. 2019;49:1587‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langford MP, Stanton GJ, Barber JC, Baron S. Early‐appearing antiviral activity in human tears during a case of picornavirus epidemic conjunctivitis. J Infect Dis. 1979;139:653‐658. [DOI] [PubMed] [Google Scholar]
- 28. Bours J, Reitz C, Strobel J, Breipohl W. Detection of IgM in tears during rhinoconjunctivitis. Graefes Arch Clin Exp Ophtalmol. 2005;243:456‐463. [DOI] [PubMed] [Google Scholar]
- 29. Salomonsson P, Grönneberg R, Gilljam H, et al. Bronchial exudation of bulk plasma at allergen challenge in allergic asthma. Am Rev Respir Dis. 1992;146:1535‐1542. [DOI] [PubMed] [Google Scholar]
- 30. Greiff L, Pipkorn U, Alkner U, Persson C. The nasal pool device applies controlled concentrations of solutes on human nasal airway mucosa and samples its surface exudations/secretions. Clin Exp Allergy. 1990;20:253‐259. [DOI] [PubMed] [Google Scholar]
- 31. Persson C, Svensson C, Greiff L, et al. Editorial. The use of the nose to study the inflammatory response of the respiratory tract. Thorax. 1992;47:993‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu MC, Xiao HQ, Brown AJ, Ritter CS, Schroeder J. Association of vitamin D and antimicrobial peptide production during late‐phase allergic responses in the lung. Clin Exp Allergy. 2012;42:383‐391. [DOI] [PubMed] [Google Scholar]
- 33. Greiff L, Andersson M, Svensson C, Alkner U, Persson C. Glucocorticoids may not inhibit plasma exudation by direct vascular antipermeability effects in human airways. Eur Respir J. 1994;7:1120‐1124. [PubMed] [Google Scholar]
- 34. Svensson C, Pipkorn U, Alkner U, Baumgarten CR, Persson C. Topical vasoconstrictor (oxymetazoline) does not affect histamine‐induced mucosal exudation of asthma in human nasal airways. Clin Exp Allergy. 1992;22:411‐416. [DOI] [PubMed] [Google Scholar]
- 35. Greiff L, Andersson M, Erjefalt JS, Svensson C, Persson CG. Loss of size‐ selectivity at histamine‐induced exudation of plasma proteins in atopic nasal airways. Clin Physiol Funct Imaging. 2002;22:28‐31. [DOI] [PubMed] [Google Scholar]
- 36. Greiff L, Andersson M, Akerlund A, et al. Microvascular exudative hyperresponsiveness in human coronavirus‐induced common cold. Thorax. 1994;49(2):121‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erjefält JS, Andersson P, Gustafsson B, Korsgren M, Sonmark B, Persson CGA. Allergen challenge‐induced extravasation of plasma in mouse airways. Clin Exp Allergy. 1998;28:1013‐1020. [DOI] [PubMed] [Google Scholar]
- 38. Persson CG, Erjefalt I, Andersson P. Leakage of macromolecules from guinea‐pig tracheobronchial microcirculation. Effects of allergen, leukotrienes, tachykinins, and anti‐asthma drugs. Acta Physiol Scand. 1986;127(1):95‐105. [DOI] [PubMed] [Google Scholar]
- 39. Luts A, Sundler F, Erjefalt I, Persson CG. The airway epithelial lining in guinea pigs is intact promptly after the mucosal crossing of a large amount of plasma exudate. Int Arch Allergy Appl Immunol. 1990;91(4):385‐388. [DOI] [PubMed] [Google Scholar]
- 40. Erjefalt I, Persson CG. Allergen, bradykinin, and capsaicin increase outward but not inward macromolecular permeability of guinea‐pig tracheobronchial mucosa. Clin Exp Allergy. 1991;21(2):217‐224. [DOI] [PubMed] [Google Scholar]
- 41. Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Epithelial pathways for luminal entry of bulk plasma. Clin Exp Allergy. 1995;25(2):187‐195. [DOI] [PubMed] [Google Scholar]
- 42. Erjefalt I, Persson CG. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2(2):93‐102. [DOI] [PubMed] [Google Scholar]
- 43. Rogers DF, Alton EW, Aursudkij B, Boschetto P, Dewar A, Barnes PJ. Effect of platelet activating factor on formation and composition of airway fluid in the guinea‐pig trachea. J Physiol. 1990;431:643‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Erjefalt I, Luts A, Persson CG. Appearance of airway absorption and exudation tracers in guinea pig tracheobronchial lymph nodes. J Appl Physiol. (1985). 1993;74(2):817‐824. [DOI] [PubMed] [Google Scholar]
- 45. Persson CG, Erjefalt I, Gustafsson B, Luts A. Subepithelial hydrostatic pressure may regulate plasma exudation across the mucosa. Int Arch Allergy Appl Immunol. 1990;92(2):148‐153. [DOI] [PubMed] [Google Scholar]
- 46. Gustafsson BG, Persson CG. Asymmetrical effects of increases in hydrostatic pressure on macromolecular movement across the airway mucosa. A study in guinea‐pig tracheal tube preparations. Clin Exp Allergy. 1991;21(1):121‐126. [DOI] [PubMed] [Google Scholar]
- 47. Greiff L, Erjefalt I, Erjefalt JS, Wollmer P, Persson CG. Effects of hydrogen peroxide on the guinea‐pig tracheobronchial mucosa in vivo. Acta Physiol Scand. 1999;165(4):415‐420. [DOI] [PubMed] [Google Scholar]
- 48. Erjefalt IA, Wagner ZG, Strand SE, Persson CG. A method for studies of tracheobronchial microvascular permeability to macromolecules. J Pharmacol Methods. 1985;14(4):275‐283. [DOI] [PubMed] [Google Scholar]
- 49. Erjefalt JS, Erjefalt I, Sundler F, Persson CG. In vivo restitution of airway epithelium. Cell Tissue Res. 1995;281(2):305‐316. [DOI] [PubMed] [Google Scholar]
- 50. Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Microcirculation‐derived factors in airway epithelial repair in vivo. Microvasc Res. 1994;48(2):161‐178. [DOI] [PubMed] [Google Scholar]
- 51. Persson CGA, Erjefält JS. Airway epithelial restitution following shedding and denudation. In: Crystal RG, West JB, Weibel ER, Barnes PJ, eds. The Lung: Scientific Foundations, 2nd edn. New York: Raven; 1997:2611‐2627. [Google Scholar]
- 52. Tata PR, Mou H, Pardo‐Saganta A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winther B. Rhinovirus infection in the upper airway. Proc Am Thorac Soc. 2011;8:79‐89. [DOI] [PubMed] [Google Scholar]
- 54. Dakhama A, Vitalis TZ, Hegele RG. Persistence of respiratory syncytial virus (RSV) infection and development of RSV‐specific IgG1 response in a guinea‐pig model of acute bronchiolitis. Eur Respir J. 1997;10:20‐26. [DOI] [PubMed] [Google Scholar]
- 55. Leist SR, Dinnon KH, Schäfer A, Tse LV, Okuda K, Hou YJ. A mouse‐adapted SARS‐CoV‐2 induces acute lung injury and mortality in standard laboratory mice. Cell 2020, in press. 10.1016/j.cell.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andersson M, Michel L, Llull JB, Pipkorn U. Complement activation on the nasal mucosal surface – a feature of the immediate allergic reaction in the nose. Allergy. 1994;49:242‐245. [DOI] [PubMed] [Google Scholar]
- 57. Java A, Apicelli AJ, Liszewski MK, et al. The complement system in Covid‐19: friend or foe? JCI insight. 2020;5:e140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balfour‐Lynn IM, Valman B, Silverman M, Webster AD. Nasal IgA response in wheezy infants. Arch Dis Child. 1993;68:472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Vyve T, Chanez P, Bernard A, et al. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol. 1995;95:60‐68. [DOI] [PubMed] [Google Scholar]
- 60. Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and non‐hospitalized patients with COVID‐19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. 10.1016/j.jaci.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ma YJ, Lee BL, Garred P. An overview of the synergy and crosstalk between pentraxins and collectins/ficolins: their functional relevance and complement activation. Exp Mol Med. 2017;49:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ekdahl KN, Terramura K, Hamad OA, et al. Dangerous liasions: complement, coagulation, and Kallikrein/Kinin crosstalk act as linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274:245‐269. [DOI] [PubMed] [Google Scholar]
- 63. Vonarburg C, Loetscher M, Spycher MO, et al. Topical application of nebulized human IgG, IgA and IgAM in the lungs of rats and non‐human primates. Respir Res. 2019;20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Persson NH, Erlansson M, Svensjö E, et al. The hamster cheek pouch – an experimental model to study postischemic macromolecular permeability. Int J Microcirc Clin Exp. 1985;4:257‐263. [PubMed] [Google Scholar]
- 65. Chinn S, Britton JR, Burney PG, Tattersfield AE, Papacosta AO. Estimation and repeatability of the response to inhaled histamine in a community survey. Thorax. 1987;42:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mullol J, Raphael GD, Lundgren JD, et al. Comparison of human nasal mucosal secretion in vivo and in vitro. J Allergy Clin Immunol. 1992;89:584‐592. [DOI] [PubMed] [Google Scholar]
- 67. Chatterjee M, van Putten PM, Strijbis K. Defensive properties of mucin glycoproteins during respiratory infections – relevance for SARS‐CoV‐2. MBio. 2020;11:e02374‐e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Button B, Cai LH, Ehre C, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wahl A, Gralinski L, Johnson C, et al. Acute SARS‐CoV‐2 infection is highly cytopathic, elicits a robust innate immune response and is efficiently prevented by EIDD‐2801. 2020 Preprint. 10.21203/rs-80404/v1 [DOI]
- 70. Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol. 2013;5(1):20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


