Abstract
Recipients of allogeneic hematopoietic stem cell transplantation (allo‐HSCT) are an immunocompromised group who are likely to develop severe complications and mortality because of coronavirus disease 2019 (COVID‐19). We report here a 61‐year‐old male patient of primary myelofibrosis who underwent an allo‐HSCT 6 years earlier, had chronic graft‐versus‐host disease (cGVHD) involving the liver, lung, eyes, and skin, (with recurrent episodes of pulmonary infections) who developed severe COVID‐19. The patient was treated with tocilizumab, and a combination of lopinavir/ritonavir, ribavirin, interferon‐β1b. He was discharged after 31 days with full recovery. Tocilizumab, a humanized monoclonal antibody against IL6, has been shown to benefit respiratory manifestations in severe COVID19. However, this is first report, to our knowledge, of its use and benefit in a post HSCT recipient.
Keywords: allogeneic, coronavirus disease (COVID19), GVHD, stem cell transplant, tocilizumab
1. INTRODUCTION
Although majority of cases with SARS COV‐2 infection have mild symptoms or are asymptomatic, 10% patients have a severe disease. 1 As of 10th January 2021, according to current WHO data, there are 88 million cases worldwide, with 2.1% mortality rate. 2
A study from China which analysed 1590 COVID patients across 575 hospitals, showed that cancer patients are at higher risk of developing SARS‐CoV‐2 infection, and five times more likely to develop severe events such as use of mechanical ventilator, and death, compared to non‐cancer patients. 3 Amongst cancer patients, those with hematological malignancies, who undergo HSCT and develop complications like GVHD, are even more susceptible to death from COVID‐19 because of profound immunosuppression. 4 There is paucity of literature on COVID‐19 in HSCT. We describe here the course and outcome of a patient who, after HSCT (complicated with extensive chronic GVHD including lung), developed severe COVID‐19 and recovered completely.
2. CASE
A 55‐year old male underwent a matched sibling allogeneic HSCT in January 2014 for JAK2‐negative primary myelofibrosis. His comorbidities at time of transplant included restrictive lung defect, remote hepatitis‐B infection, chronic kidney disease [estimated glomerular filtration rate (eGFR):38 mL/min] and prior splenectomy complicated by portal vein thrombosis. Conditioning used was reduced intensity [Fludarabine 40 mg/m2 × 5 days with 4 Gy total body irradiation (TBI)]. Cyclosporine and Methotrexate were employed for GVHD prophylaxis. He received a pre‐emptive donor lymphocyte infusion (DLI) on day90 post‐HSCT, in view of decreasing donor chimerism. Six months post‐HSCT (3 months post DLI), he developed extensive chronic GVHD (cGVHD) involving skin, eyes, oral cavity, lungs, and liver. He was treated with mycophenolate mofetil, imatinib, and topical therapies. Bone marrow examination at 1‐year post HSCT showed remission without increased reticulin. He was on regular 3‐monthly follow‐up and continued to be on topical steroids, imatinib, inhaled steroids (fluticasone), azithromycin, montelukast (FAM protocol), and bronchodilators. Over the next 4 years (2016‐May 2020), he required six indoor admissions for treatment of recurrent respiratory infections.
In May 2020, at the age of 61 years, he presented to the emergency room with a 3‐day history of high‐grade fever, sore throat, and anorexia. On admission, he was febrile (103F), hemodynamically stable with normal oxygen saturation and clear lungs. Broad spectrum antibiotics and intravenous hydration were initiated. Baseline investigations revealed a normal hemogram with lymphopenia [Absolute lymphocyte count(ALC)‐1.12 × 109/L (10% of differential), Neutrophil/lymphocyte ratio‐6.4], normal liver function tests, elevated CRP (11 mg%) [normal reference interval (RI): 0‐0.33 mg%], procalcitonin (11.3 ng/mL) (normal: <0.5 ng/mL) and creatinine (1.9 mg/dL, eGFR: 42 mL/min). Coagulation parameters (PT/PTT), including D‐Dimer (<200 ng/mL) were normal with elevated fibrinogen (1100 mg/dL) (normal RI‐150‐400 mg/dL). His SARS‐CoV2 RT‐PCR was positive and at the time of hospitalization, he had mild COVID19 (score 5), as per ordinal scale. 5 In view of persistent fever, he was started on triple combination of lopinavir‐ritonavir(LPV/r) (400 mg/100 mg BD), ribavirin (RBV) (200 and 400 mg alternate‐day as eGFR‐40 mL/min), Interferon β‐1b (IFNβ‐1b) [8MU] (as per protocol published by Hung et al) 6 along with prophylactic low‐molecular weight heparin (LMWH) (1 mg/kg subcutaneously OD). Over the next 48 hours (day 3 admission), he deteriorated with persistent fever (Tmax: 104°F), tachypnoea, and hypoxemia (SpO2: 92% on room air). X‐ray chest revealed bilateral pulmonary infiltrates with possible left lower zone consolidation (Figure 1A). Within 72 hours of admission, he progressed to a severe COVID19 (score 3) disease, according to the ordinal scale. 5 His serum IL6 levels were increased (21 pg/mL) (normal RI: <12.7 pg/mL). Since there was evidence of elevated IL6 levels, 7 and use of anti‐IL6 therapy to blunt the inflammatory response in severe COVID‐19, 8 tocilizumab (TCZ) (400 mg) was administered (on day 3). High flow oxygen (HFO) was also supplemented through a heated humidifier and supplied through binasal cannula with air flow rate of 60 L/min and FIO2 of 70%. After TCZ, he achieved defervescence and transient improvement in respiratory distress, but continued to be hypoxic. On day 5, on account of worsening tachypnoea (RR: 42/min) and increased oxygen requirement (FiO2: 90%), second dose of TCZ (400 mg) was given. He responded dramatically within 12 hours of second TCZ (Note the substantial drop in CRP after TCZ in Figure 2). However, on day 7, he developed possibly drug‐induced psychosis. This was managed with physical restraining and sedation. His antivirals/IFN were discontinued, since the most likely culprit was RBV. 9 After cessation of his agitation, LPV/r was restarted (day 12) and continued for total 3 weeks. He was gradually weaned off his HFO by day 14. His swab for SARS‐CoV2 was monitored weekly, and he remained persistently positive until day 28. The patient was discharged on day 31 after two consecutive negative swabs (48 hours apart).
FIGURE 1.

(A) Serial chest X‐rays for comparison from baseline till day28 of COVID infection. Note the worsening of bilateral infiltrates on day3 compared to baseline. Note the dramatic improvement in x‐ray of day5 after one dose of TCZ. (B) Present non‐contrast CT scan of chest (day51 of COVID infection) [right panel] showing peripheral ground‐glassing (pointed by arrows), without any fibrosis, in comparison to his normal prior CT chest 2years back [left panel]
FIGURE 2.
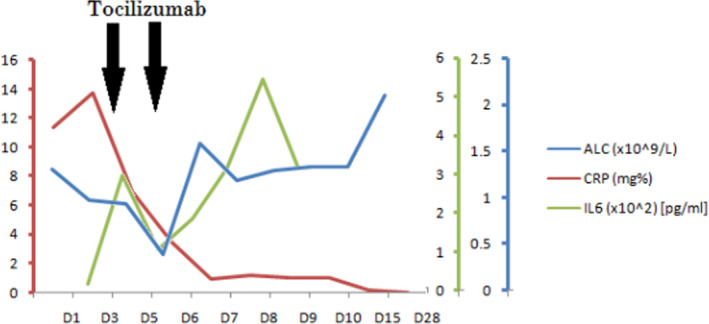
Graph showing kinetics of ALC, CRP and IL6 levels during the course of illness
On analysing the immune‐cell kinetics in our patient, we observed a significant drop in NK‐cells co‐relating with severe COVID19 in our patient (Table 1). On follow‐up (day 51), he developed IgG antibodies to SARS‐Cov‐2. Computed Tomogram of chest on day 51 (day 2196 post HSCT) revealed diffuse peripheral ground‐glassing, possibly post‐infective squeal without any air‐trapping or lung fibrosis (Figure 1B). On last follow‐up at day 229 (day 2374 post‐HSCT), he is stable without any worsening of his cGVHD.
TABLE 1.
Immune cell kinetics at various time points (low ALC, CD8, NK cells initially, at progression to severe COVID and their improvement with clinical recovery)

Abbreviations: ALC, Absolute lymphocyte count; NK, Natural Killer cells; T reg, Regulatory T‐cells.
3. DISCUSSION
As per CIBMTR (Centre for International Blood and Marrow Transplant Research) data (updated 10.01.2021), 1179 patients have been infected with SARS‐CoV‐2 post‐HSCT, with a mortality rate (n = 179) of 15.2%, eight times as compared to normal population. 10 However, there is no standard of care for COVID management in this population. After entrance of SARS‐CoV2 though ACE2 receptor, its replication can be halted by targeting either protease enzyme (LPV/r) or RNA dependent RNA‐polymerase (Remdesivir, RBV). 11 In moderate COVID19, 5‐day remdesivir was significantly better, vs standard care, with respect to clinical improvement and hospital discharge. 12 However, in severe COVID19, there was no added benefit of 10‐day remdesivir over a 5‐day course. 5 At the time of our patient, remdesivir was not available in India. Hence, we used the triplet combination as published by Hung et al in a phase 2 randomized study. 6 They stated that early use of antivirals was beneficial, as viral load in SARS‐CoV2, similar to influenza, peaks at time of symptom presentation. Hence, using a synergistic combination of multiple drugs, would not only make the patients negative earlier (consequently leading to less infectivity), but also prevent the emergence of a drug‐resistant virus, and prevent progression to severe COVID disease. 6 Amongst Interferons, type1 cytokines (IFN‐α, IFN‐β, IFN‐ε, IFN‐κ, IFN‐ω, IFN‐δ, IFN‐ζ, IFN‐τ) upregulate molecules which inhibit viral replication, and activate NK‐cell effector functions ie essential to induce an antiviral response. 13 Since a major number of CoVs do not elicit a type‐1 IFN response, inducing them would be a relevant therapeutic strategy. 11 On this basis, a randomized trial (ACTT‐3) combining IFN‐β with an antiviral (Remdesivir), vs remdesivir alone is ongoing. 14
Nearly 20% patients develop severe COVID19 infection with progression to acute respiratory distress syndrome and admission to intensive care unit. The latter complication has been hypothesized to be because of a dysfunctional immune response after viral invasion, leading to auto‐amplification of cytokines, thereby leading to vascular leak, called as “cytokine release syndrome” (CRS). The biopsy findings of cellular alveolar exudates and interstitial lymphocyte infiltrates, along with the pro‐inflammatory cytokine profile in peripheral blood supports the implication of CRS in severe COVID19. TCZ is a humanized IgG1 monoclonal antibody against IL6 receptor. Its utility in COVID19 CRS stems both by demonstration of elevated IL6 in severe cases and from its approval for mitigating the CRS induced by chimeric antigen receptor‐T cell therapy. 15 TCZ has been used in severe COVID19, including in a fraction of patients with chronic kidney disease. 8 Similar to our case (Figures 1 and 2), they have also shown that TCZ produces rapid improvement in inflammatory markers and radiological parameters. 8 However, it has never been utilized in post‐HSCT setting. Contrary with phase 3 trial results (unpublished), 16 our report suggests that TCZ produced a dramatic clinical benefit. Moreover, in vitro and in vivo studies have shown that IL6 inversely correlates with NK‐cell function. Hence, inhibition of IL6 axis by TCZ, might indirectly enhance NK‐cell functions. 11 It is plausible that TCZ might have played a role in improving NK‐cell count after day 10 in our patient (Table 1). Additionally, Market et al, (similar to our data) have reported that reduced and/or exhausted NK‐cells may be responsible for severe COVID19 manifestations. They have also proposed that therapeutic strategies repurposing NK‐cells, might help to flatten the COVID19 curve. 11 Amongst anti‐inflammatory drugs ameliorating the cytokine storm, apart from TCZ, dexamethasone has been used, with a 28‐day mortality benefit in the landmark RECOVERY trial. However, cancer patients were not included in this trial. Moreover, use of systemic steroids might lead to slower viral clearance, as shown with SARS, MERS (Middle East Respiratory syndrome), and influenza virus. 17 The above reasons coupled with the possibility of leading to secondary infections after steroids, in a post‐transplant immunocompromised patient, compelled us to prefer TCZ.
Literature on COVID19 in HSCT recipients is limited, majority as isolated cases or series. Five of these 12 cases (42%) have succumbed, usually at end of third week. 4 , 18 , 19 , 20 , 21 Importantly, 3 of 4 patients who received steroids died. 18 , 19 , 20 Half of them received antivirals [HCQS (n = 2), LPV/r (n = 2), remdesivir (n = 1), umifenavir (n = 1)]. 4 , 18 , 19 , 20 , 21 Importantly, two patients who were on Ruxolitinib for their cGVHD, are alive. 21 Unlike our case, none of them received TCZ. Moreover, at 6‐months follow‐up, there was no worsening of his lung GVHD or evidence of pulmonary fibrosis.
4. CONCLUSION
Our report points out the fact that use of TCZ in an immunocompromised patient who has significant comorbities because of his post‐transplant complications can lead to favorable outcomes.
ACKNOWLEDGEMENT
None.
Mirgh S, Gokarn A, Punatar S, et al. Clinical course of severe COVID19 treated with tocilizumab and antivirals post‐allogeneic stem cell transplant with extensive chronic GVHD. Transpl Infect Dis. 2021;23:e13576. 10.1111/tid.13576
REFERENCES
- 1. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192‐206. 10.1111/joim.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO coronavirus disease (COVID‐19) dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last updated 2021 Jan 10) [Google Scholar]
- 3. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saraceni F, Scortechini I, Mancini G, et al. Severe COVID‐19 in a patient with chronic graft‐versus‐host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib. Transpl Infect Dis. 2020;e13401. 10.1111/tid.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe covid‐19. N Engl J Med. 2020;383(19):1827‐1837. 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hung I‐N, Lung K‐C, Tso E‐K, et al. Triple combination of interferon beta‐1b, lopinavir‐ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395:1695‐1704. 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coomes EA, Haghbayan H. Interleukin‐6 in Covid‐19: a systematic review and meta‐analysis. Rev Med Virol. 2020;e2141. 10.1002/rmv.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;92(10):2042‐2049. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zareifopoulos N, Lagadinou M, Karela A, et al. Neuropsychiatric effects of antiviral drugs. Cureus. 2020;12(8):e9536. 10.7759/cureus.9536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CIBMTR . (Centre for International Blood and Marrow Transplant Research) [Internet]. Milwaukee/Minneapolis: The Medical College of Wisconsin, Inc. and the National Marrow Donor Program; 2004. Available from: https://www.CIBMTR.org/Covid19/Pages/default.aspx (last updated 2021 Jan 10) [Google Scholar]
- 11. Market M, Angka L, Martel AB, et al. Flattening the COVID‐19 curve with natural killer cell based immunotherapies. Front Immunol. 2020;11:1512. 10.3389/fimmu.2020.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324:1048‐1057. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee AJ, Ashkar AA. The dual nature of type I and type II interferons. Front Immunol. 2018;9:2061. 10.3389/fimmu.2018.02061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Library of Medicine (U.S.) . (2020) A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID‐19 in hospitalized adults (ACTT‐3). Identifier NCT04492475. Available from https://clinicaltrials.gov/ct2/show/NCT04492475
- 15. Liu D, Zhang T, Wang Y, Xia L. Tocilizumab: the key to stop coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? Front Med. 2020;7:571597. 10.3389/fmed.2020.57159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Library of Medicine (U.S.) . (2020) A randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy and safety of tocilizumab in hospitalized patients with COVID‐19 pneumonia. Identifier NCT04372186. Available from https://clinicaltrials.gov/ct2/show/NCT04372186
- 17. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid‐19 ‐ preliminary report. N Engl J Med. 2020;NEJMoa2021436. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 18. Kanellopoulos A, Ahmed MZ, Kishore B, et al. COVID‐19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020;190:e67‐e70. 10.1111/bjh.16856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Lin H, Wu Y, et al. COVID‐19 in post‐transplant patients—report of 2 cases. Am J Transplant. 2020;20:1879‐1881. 10.1111/ajt.15896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu X, Tang LV, Wang H‐F, et al. The great challenge of managing recipients of hematopoietic stem cell transplantation combined with COVID‐19. Bone Marrow Transplant. 2020;1‐5. 10.1038/s41409-020-01035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foss FM, Rubinowitz A, Landry ML, et al. Attenuated novel SARS coronavirus 2 infection in an allogeneic hematopoietic stem cell transplant patient on ruxolitinib. Clin Lymphoma Myeloma Leuk. 2020;20(11):720‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]


