Abstract
Viruses are among the most infectious pathogens, responsible for the highest death toll around the world. Lack of effective clinical drug for most of the viruses emphasizes the rapid and accurate diagnosis at early stages of infection to prevent rapid spread of the pathogens. Nanotechnology is an emerging field with applications in various domains, where nano‐biomedical science has many significant contributions such as effective delivery of drugs/therapeutic molecules to specific organs, imaging, sensitive detection of virus, and their accurate tracking in host cells. The nanomaterials reported for virus detection and tracking mainly include magnetic and gold NPs, ZnO/Pt‐Pd, graphene, and quantum dots (QDs). In addition, the single virus tracking technology (SVT) allowed to track the life cycle stages of an individual virus for better understanding of their dynamics within the living cells. Inorganic as well as non‐metallic fluorescent materials share the advantages of high photochemical stability, a wide range of light absorption curves and polychromatic emission. Hence, are considered as potential fluorescent nano‐probes for SVT. However, there are still some challenges: (i) clinical false positive rate of some detection methods is still high; (ii) in the virus tracking process, less adaptability of QDs owing to larger size, flicker, and possible interference with virus function; and (iii) in vivo tracking of a single virus, in real time needs further refinement. In the future, smaller, non‐toxic, and chemically stable nanomaterials are needed to improve the efficiency and accuracy of detection, and monitoring of virus infections to curb the mortalities.
This article is categorized under:
Therapeutic Approaches and Drug Discovery > Nanomedicine for Infectious Disease
Biology‐Inspired Nanomaterials > Protein and Virus‐Based Structures
Keywords: detection, nanoparticles, single virus tracking, virus
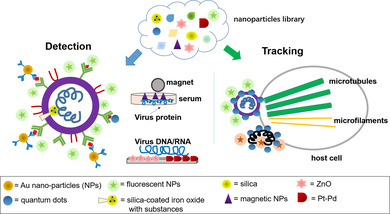
Nano‐particles and their applications in virus detection and tracking to comprehend infection mechanisms.
Abbreviations
- AuNPs
gold NPs
- CMC
carboxymethylcellulose
- EDTA•2Na
ethylenediamine tetraacetic acid disodium salt
- GFETs
graphene field‐effect transistors
- HAT
human α‐thrombin
- HBsAg
hepatitis B surface antigen
- NPs
nano‐particles
- OSiNDs
organo silica nanodots
- PEDV
porcine epidemic diarrhea virus
- QDs
quantum dots
- RSV
respiratory syncytial virus
- SVT
single virus tracking technology
1. INTRODUCTION
Viruses are one of the most infectious pathogens causative of deadliest diseases in humans. Although the pathogenic mechanism of viruses is diverse, all existing viruses need a host to maintain their existence (Liu et al., 2016; Pallas & García, 2011). Viral infections result in millions of deaths and huge economic losses annually (Baldwin & Weder di Mauro, 2020). SARS is one of such examples, where the first outbreak in 2002 caused by SARS associated coronavirus emerged in China crossed the borders and caused significant mortality and devastated the economy (LeDuc & Barry, 2004). Recently in late 2019, the new outbreak of coronavirus caused by SARS‐CoV‐2 has resulted in over 1 million deaths globally by October 4, 2020 (World Health Organization, 2020). Initial non‐availability of effective clinical drugs for such viruses highlighted the need of rapid and accurate diagnosis in the early stage of infection to limit pathogen outburst. Furthermore, precise tracking of pathogens within the host cell is need of the hour to determine the pathogenic mechanism and to pave the way for drug and vaccine development (Nikaeen et al., 2020; L.‐J. Zhang et al., 2020).
Nanotechnology, with the improvement and application of the nano‐particles (NPs)/nanocarriers with dimension in the nanoscale range (1 ~ 100 nm) (Gong et al., 2018; Piao et al., 2019; B. Zhang, Gao, et al., 2018), has been widely utilized in a variety of fields, including medicine (Emerich & Thanos, 2003), pharmacy (Li et al., 2020), biological detection (Chen et al., 2020; J. Cui et al., 2020; Peng, Huang, et al., 2020), optics (Ochmann et al., 2017; Peng, Qin, et al., 2020), and agriculture (Curtis & Wilkinson, 2001; Prasad et al., 2017). The extremely small size of NPs allows efficient entrance into living system such as animal and human body. Furthermore, the strong encapsulation effect of NPs protects the materials until delivered to the target sites (L. Wang et al., 2018; S. Wang et al., 2016; W. Yang et al., 2015). Recently, nano‐biomedical science has been the object of a substantial amount of attention (Peng et al., 2018; Zhao, Piao, Peng, Wang, Zhang, et al., 2018), including effective delivery of drugs, genes and therapeutic molecules to specific organs or cells, imaging, sensitive detection of virus at early stages, and accurate tracking in host cells (Chen et al., 2019; Gong et al., 2016; Kumar et al., 2013; Patra et al., 2018; Peng et al., 2019; Riley & Vermerris, 2017; S. Wang et al., 2016; W. Yang et al., 2015; Zhou et al., 2019). A variety of nanomaterials for virus detection and tracking have been invented, contributing to the elucidation of virus infection mechanisms, such as magnetic and gold (Au) NPs, ZnO/Pt‐Pd, graphene and quantum dots (QDs) (Chan & Nie, 1998; Wen et al., 2014; L.‐J. Zhang et al., 2020).
2. APPLICATION OF NANOTECHNOLOGY IN VIRUS DETECTION
Early detection of pathogen is key to curb the outbreak. The combination of nanotechnology and gene detection methods is considered to be an effective design to improve the sensitivity and specificity in virus nucleic acid sequence detection (J. Zhang et al., 2017). The advantages of nanomaterials significantly shorten analysis time, improve sensitivity, and provide pivotal references for early diagnosis. Gene sequence specific detection has been widely accepted and used for diagnosis of viruses. Advancement in diagnostics tools and 2002 SARS‐CoV outcomes consequently helped to shorten the detection time from 5 months in 2002 to 3 weeks during COVID 19 pandemic (Z. Wu & McGoogan, 2020). The rapid detection of the nucleic acid sequence of SARS‐CoV‐2 from suspected patients combined with nanotechnology contributed to the early confirmation of viral infection which helped to limit the mortalities (Udugama et al., 2020).
2.1. Application of magnetic NPs in virus detection
Magnetic NPs have been reported to be used for detecting SARS‐Cov‐2 RNA and host antibody response (Valdiglesias & Laffon, 2020). It was shown that silica‐coated iron oxide NPs have strong affinity for SARS‐CoV‐2 RNA, as the cracked open the virus. The magnet was used to separate the RNA coated NPs from the sample solution. This method is simple and economical, helping to extract RNA from patient samples efficiently (Islam & Ahsan, 2020).
Magnetic NPs are also used to reach the target as carriers. By immobilizing hepatitis B surface antigen (HBsAg) to carboxylated magnetic NPs, HBsAg could successfully select DNA aptamers against HBsAg after 13 selection rounds. Then the chemiluminescence aptasensor, according to the magnetic separation and immunoassay, was prepared to detect HBsAg in serum or purified protein. The detection limit of 0.1 ng/ml was only one‐fifth compared with that of ELISA, indicating that this aptasensor is a better choice to detect hepatitis B virus infection (Figure 1) (Xi et al., 2015).
FIGURE 1.
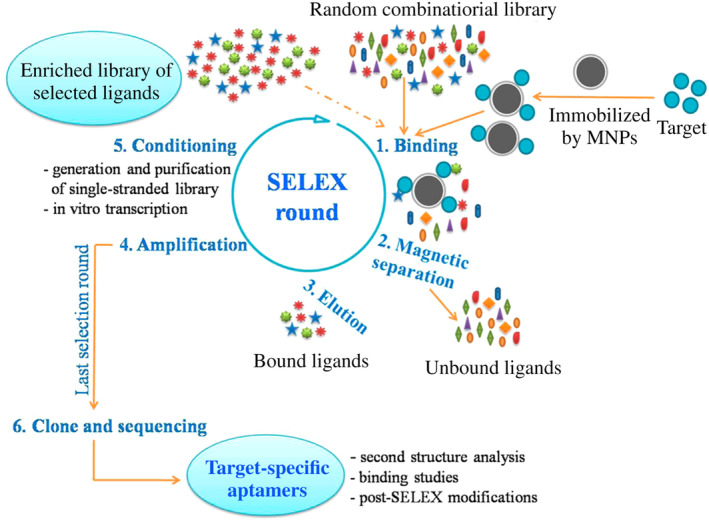
In vitro selection of target‐specific aptamers based on magnetic separation by SELEX (Xi et al., 2015). The SELEX process consists five main steps: binding, magnetic separation, elution, amplification, and conditioning. The last SELEX round is terminated after the amplification step, followed by cloning and sequencing of the PCR products
2.2. Application of AuNPs in virus detection
The AuNPs have been successfully utilized in target specific detection (Zhao et al., 2016). Sona Nanotech (Halifax, Canada) reported a rapid lateral‐flow immunochromatographic assay, for qualitative screening of patients with COVID‐19 infection within 5–15 min. The antibody tests utilize AuNPs to examine the COVID‐19 biomarkers, such as IgG and IgM, by integrating nanorod technology into a disposable lateral flow test platform (Loeffelholz & Tang, 2020).
Wu et al. designed an enhanced fluorescence enzyme‐linked immunosorbent assay based on the fluorescence “turn‐on” signal triggered by human α‐thrombin (HAT). The detection antibody Ab2 and HAT were marked with AuNPs to become a detection probe. The fluorescence quenched bisamide derivative of Rhodamine 110 was the substrate of HAT. Following the sandwich immune reaction, HAT was able to catalyze the cleavage of the fluorescence quenched substrate and produce the intensive fluorescent signal. Hence, enabled to detect the ultra‐low levels of alpha fetoprotein and HBsAg as was 10−4 times of the conventional fluorescence assay and 10−6 times of the conventional ELISA (Figure 2) (Y. Wu, Guo, et al., 2017).
FIGURE 2.
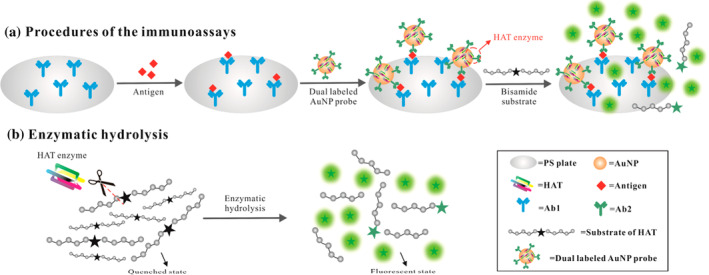
The enhanced fluorescence enzyme‐linked immunosorbent assay for the detection of alpha fetoprotein and HBsAga (Y. Wu, Guo, et al., 2017). (a) Procedures of the proposed immunoassays with dual labeled AuNP probes (multi‐HAT‐AuNP‐Ab2); (b) enzymatic hydrolysis of the bisamide Rhodamine 110 substrates
AuNPs and carboxymethylcellulose (CMC) biopolymer could be used as an in situ reducing agent and surface stabilizing ligand for aqueous colloid production. With modification of gp41 glycoprotein receptor or human immunodeficiency virus (HIV) monoclonal antibodies, the AuNPs‐CMC nanocolloids could be used in the laser light scattering immunoassay (Caires et al., 2019).
Wang et al. established a nano nested PCR method for detection of porcine epidemic diarrhea virus (PEDV) variants and classical strains based on AuNPs. The sensitivity of nano nested PCR method was 100 times higher than that of ordinary PCR methods. This method could identify PEDV variants specifically, without detecting DNA or cDNA from even classical PEDV strains, indicating that it could be applied to the PEDV variants specific detection (K. Wang et al., 2017).
Zhao et al. demonstrated an enzyme‐free assay, based on the strong chelation of Au3+ ions by ethylenediamine tetraacetic acid disodium salt (EDTA•2Na, the chelator). The low‐cost EDTA•2Na, did not rely on the ambient temperature and unstable H2O2, was labeled by the targeted moieties, and can regulate the production of AuNPs plasma signal with marked tonality and outstanding reliability. The detection sensitivity of EDTA•2Na regulated bioassay was enhanced by three orders of magnitude with incorporation of silica‐NPs. (Zhao, Piao, Peng, Wang, Gao, et al., 2018).
Mozhgani et al. reported that AuNPs combined with a scanning tunneling microscopy based vertically configured electrical detection system was developed to detect viruses. HIV virus was introduced to the Au surface by antibody fragment, forming a sandwich‐like configuration. Then the tunneling current is in response to the distance from the tip to the surface, and the localized AuNPs produce a pulse current peak at the localization position. The frequency of the peak current was used to quantify the number of HIV particles according to the immune response associated with Au NPs (Mozhgani et al., 2020).
2.3. Application of QDs in virus detection
QDs have attracted great attention because of their great optical and semiconductor properties, exemplified photostability, high quantum yield, and narrow emission spectrum with adjustable size (Algar et al., 2011; Y. Cui et al., 2012; Gao et al., 2004; Wegner & Hildebrandt, 2015; Y. Wu, Peng, et al., 2017).
Ahmed et al. reported a self‐assembled nanostructure of chiral QDs and AuNPs as a chiro‐immunosensor to detect infectious bronchitis virus (IBV) from chicken blood samples. They found that the detection limit, 47.91 EID/50 ml egg infection dose (EID), was quite efficient in examination of the target virus (Ahmed et al., 2017).
Researchers also established a method for detecting IBV using zirconium quantum dots (ZrQDs) and magnetic plasma (MP) NP. IBV antibodies were bound to the ZrQDs and MPNPs, and no attraction or binding between the nanomaterials was observed. Thereafter, the addition of the virus promoted the formation of a magnetoplasmonic‐fluorescent nanohybrid structure, which was rapidly separated by the additional magnet (Ahmed et al., 2018).
2.4. Application of other nanomaterials in virus detection
In recent years, various types of metal nanomaterials and non‐metal particles have been used in the virus detection. Kim et al. (2019) showed that the coplanar‐gate graphene field‐effect transistors (GFETs) built on flexible polyethylene terephthalate substrates could help in virus detection. The antibody was set on the surface of graphene as a probe molecule using 1‐pyrenebutanoic acid succinimidyl ester, which was pre‐immobilized on the graphene surface. They proposed that the method can be used for diagnosis under saline conditions, as the GFETs performance was not significantly affected by Na+ and Cl− (Kim et al., 2019).
Furthermore, the electrochemical DNA sensing techniques along with NPs for signal amplification have higher sensitivity and specificity for the target DNA. It has been demonstrated that using methylene blue as intercalation agent, the electrochemical gene sensor based on fluorine doped zinc oxide glass plate modified by ZnO/Pt‐Pd turned out highly specific in detecting DNA sequence of Dengue virus (Singhal et al., 2017).
Ochmann et al. (2017) proposed a purely physical fluorescence enhancement method for amplification of target signals from a single fluorescent dye by several orders of magnitude. For amplification, DNA origami‐based optical antennas were used to create a hot spot using metal NPs (Figure 3). By equipping hot spots with molecular beacon structures, in addition to the plasmonic signal enhancement their model also ensured high specificity against target nucleic acid. This method was applied to detect the specific artificial viral DNA/RNA in buffer as well as in heat inactivated blood serum, with ability to discriminate closely related pathogens having small nucleotide variations (Ochmann et al., 2017).
FIGURE 3.
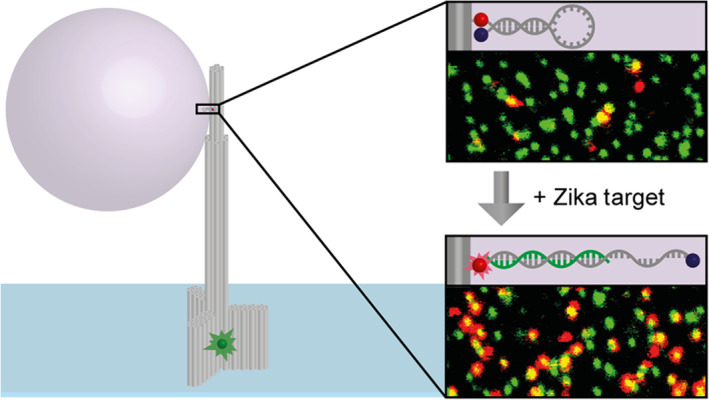
A model to detect the virus specific artificial DNA/RNA with high sensitivity against small nucleotide variations (Ochmann et al., 2017). A DNA origami optical antenna with a height of about 125 nm was used to create a hot spot using metal nanoparticles, where the plasma effect enhanced the fluorescence signal
3. APPLICATIONS OF NANOTECHNOLOGY IN VIRUS TRACKING AND VIRUS INFECTION MECHANISM RESEARCH
3.1. The processes of virus entry
Successful binding of the virus to the receptor site on the cell surface is the point where virus enters the host cell (Marsh & Helenius, 2006; Mercer et al., 2010; Mercer & Helenius, 2009; Smith & Helenius, 2004). Viruses have various mode of genome delivery to initiate replication in host cells. Endocytosis or micropinocytosis is adapted by majority of the viruses, where they finally inject their genome in endosomes. Some enveloped viruses can fuse on the plasma membrane, such as coronavirus or HIV, whereas other viruses, such as influenza, fuse within endosomes. The location and mechanism of fusion could be affected by variety of environmental factors, including lipid composition, ions, pH, and protease activity.
3.2. Single virus tracking technology
Nanotechnology plays a pivotal role in the mechanism of tracking virus entry. The single virus tracking technology (SVT) makes it possible to track the different stages of a single virus in its life cycle, thus providing dynamic insights into the basic process of virus occurrence in living cells (Liu et al., 2020). Based on fluorescence imaging, SVT functions by revealing unreported infection mechanisms with differential selection of fluorescent labeling. SVT requires special fluorescent labels with smaller size, higher brightness, and enhanced photostability. In order to fit this standard and improve the imaging ability of SVT, a variety of biocompatible fluorescent NPs have been developed to help single virus labeling. The fluorescent NPs share unique chemical and optical advantages, including greater brightness and photostability compared with organic dyes (Brandenburg & Zhuang, 2007; Huang & Xie, 2014; Liu et al., 2016; Parveen et al., 2018). QDs and metal NPs are the commonly used NPs in single virus tracking research (Liu et al., 2020).
3.3. QDs used for single‐virus tracking
Utilizing the high‐photoluminescence QDs for SVT to label viruses provides an intuitive tool to help understand the process of virus infection. Due to their excellent brightness and photostability, QDs can be tracked for a longer time under low laser intensity which makes them suitable for obtaining time‐course images or Z‐stacks for 3D reconstruction. Liang et al. (2019) labeled the porcine reproductive and respiratory syndrome virus (PRRSV) with QDs and observed the process during viral infection in live cells. QD technology clearly captured that PRRSV vibrated on the plasma membrane and entered cells through the endoplasmic mediated cell entry pathway. After entering the cells, PRRSV moved along microtubules, microfilaments, and vimentin cytoskeleton elements. During this transport, the virus particles also contacted non‐muscle myosin heavy chain II‐A, which are the small spheres in cytoplasm.
Wen et al. established a method of labeling the internal nucleocapsid of virus using QDs. In the process of virus replication in host cells, the baculovirus nucleocapsid is self‐biotinylated and then marked with streptavidin‐conjugated QDs. This kind of labeling technique has proved to be efficient, reliable, specific, and permit maintenance of virus infectivity (Wen et al., 2014).
Lipid‐biotin conjugates and streptavidin‐QD were used to mark viral lipid membranes and to light them up, respectively. This lipid‐specific QD based labeling was rapid and highly efficient while preserving the morphology and infectivity of viruses. Hence, a handy and sturdy tool to facilitate the SVT by following the virus infection path for better understanding of the complex infection mechanisms (L.‐J. Zhang et al., 2020).
QDs encapsulated fluorescent virus particles and SVT helped to unravel that the individual HIV viruses could regulate subtle rearrangement in the cortical actin barrier, which opens a door and promotes virus entry (Yin et al., 2020). Actin binding protein, α‐actinin, performs an essential role in the dynamics of actin when HIV enters living cells. The α‐actinin‐derived peptide, actin binding site 1 peptide (abs1p), was then used to block HIV infection (Yin et al., 2020).
Although photoluminescence‐QDs opened the door for SVT to comprehend the virus infection mechanisms, yet labeling of viral internal components was still a challenge. To meet this challenge, Yang et al. devised a strategy for QDs labeling of virus nucleic acid using the clustered regularly interspaced short palindromic repeats imaging system. QDs were conjugated to viral nucleic acid through Cas9/gRNA complex, which is inactivated by nuclease in living cell nucleus. The QDs were then packaged into virus when virion assembled. Real time monitoring of virus‐QDs in Vero and HeLa cells proved the effectiveness of this system in viral infection studies (Y.‐B. Yang et al., 2020).
Sun et al. elaborated another application of QDs. They reported that during the virus internalization process, using QD based SVT and polychromatic imaging, dynamin was involved in the clathrin mediated pathway during influenza virus uptake as well as during the maturation and membrane division of clathrin‐coated pits (Sun et al., 2017).
Concerning virus transportation research, L.‐J. Zhang, Xia, et al. (2018) reported that QDs based SVT and polychromatic imaging are efficient in resolving the puzzle of viruses transport from actin roadway to microtubule highway (Figure 4).
FIGURE 4.
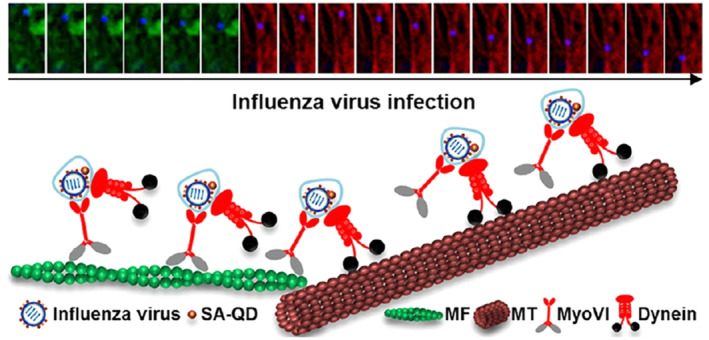
A “driver switchover” mechanism of influenza virus transport from microfilaments to microtubules (L.‐J. Zhang, Xia, et al., 2018). The switch between microfilament (MF)‐ and microtubule (MT)‐based retrograde motor proteins, myosin VI (myoVI) and dynein, took over the seamless transport of viruses from MFs to MTs during their infection. After entering the virus through endocytosis, both types of motor proteins are attached to the virus‐carrying vesicles. MyoVI used dynein to drive the virus on the MF on the virus‐carrying vesicle hitchhiking. After the role exchanges at the actin‐microtubule intersections, dynein moves the virus along the MTs toward the perinuclear region
3.4. Metal NPs used for single‐virus tracking
Because of their unusual physical, chemical, and excellent optical properties, much attention has been paid to the fluorescent metal NPs in biological and biomedical applications (Jin et al., 2016; Shang et al., 2011; L. Zhang & Wang, 2014). AuNPs have unique optical properties, with no photobleaching and less biodegradation. The scattering light signal of AuNPs required for dark field microscope or optical microscope gives an ideal image contrast and excellent temporal resolution. SVT based on AuNPs has been used to study the dynamic organization and heterogeneity of cell membrane. Wan et al. reported that, with AuNPs as light scattering labels, the respiratory syncytial virus (RSV) maintained its virulence and could be successfully tracked for a longer time with dark‐field microscopic imaging techniques (Wan et al., 2014).
However, currently accurate detection of multiple species by scattered light is difficult, hence restricted their application in SVT. Marjomäki et al. (2014) developed a site‐specific protocol for enterovirus capsid and monodispersed AuNPs. Covalent conjugation of functionalized monodispersed gold clusters with 1.5‐nm metal cores was induced on viral surfaces. Then water‐soluble Au(paramercaptobenzoic acid) clusters were synthesized and functionalized with maleimide linkers to target cysteines of the viral capsid proteins and conjugated to enteroviruses echovirus 1 and coxsackievirus B3. Transmission electron microscopy images and quantitative analysis of known virus structures showed that the bound gold clusters had high affinity and mutual ordering on the surface of the virus, and there was an obvious difference between the clusters and the target cysteine sites close to the virus surface. Infectivity of the viruses was not compromised by loading of gold clusters on the virus (Figure 5).
FIGURE 5.
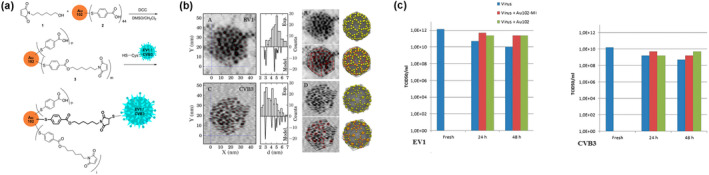
A site‐specific protocol for enterovirus capsid and monodisperse AuNPs (Marjomäki et al., 2014). (a) Synthesis of the maleimide functionalized Au102(pMBA)44 clusters and site‐specific conjugation to enteroviruses. (b) The quantified and positional analysis of the Au102‐MI clusters in the TEM images of cluster−virus conjugates. (c) The quantitative analysis of EV1 and CVB3 virus
3.5. Potential inorganic nonmetallic fluorescent nanoprobe for single‐virus tracking
The toxicity of QD precursors restricts the biological applications both in vitro and in vivo. In order to overcome this problem, fluorescent inorganic nonmetallic materials, with low toxicity, simple synthesis processes, and easy biomolecule coupling, provide an alternative fluorescent reporter (Liu et al., 2020). Inorganic fluorescent non‐metallic materials consist of oxides, phosphates or borates, carbides, aluminates, nitrides, halogen compounds, silicates, and borides. They share the advantages of a wider range of light absorption curves, higher photochemical stability, and polychromatic emission over the previous nanomaterials. Shen & Xia (2014) developed a quick and easily operated strategy named “synthesis modification integration” to prepare fluorescent nitrogen points (N‐dots) with 2‐azidoimidazole and aqueous ammonia using a microwave‐assisted method (Figure 6). Combined with AuNPs as nanoprobes, N‐dots fluorescence was used to detect and image cysteine in complex biological samples. In addition, green‐emitting organo silica nanodots (OSiNDs) were synthesized by a one‐step hydrothermal reaction. OSiNDs were proved to be lysosomal tracers. Whether they could be used in the virus tracking research needs further investigation (Shen & Xia, 2014).
FIGURE 6.
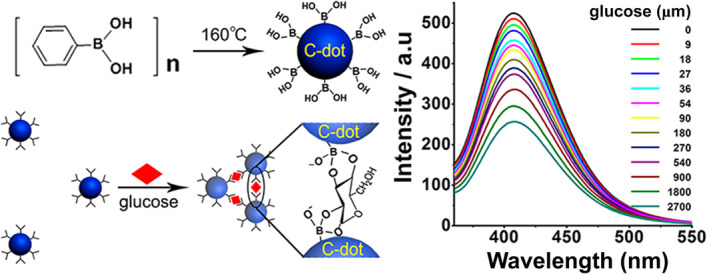
One‐step “synthesis‐modification integration” strategy for fabrication of boronic acid functionalized C‐dots (Shen & Xia, 2014)
4. CHALLENGES AND PERSPECTIVE FOR APPLICATION OF NANOTECHNOLOGY IN VIRUS
In recent years, significant research has been carried out regarding application of nanomaterials in the detection and diagnosis of viruses. However, due to the close similarity of nucleic acid sequences of some viruses, the clinical false positive rate of some detection methods is still high, resulting in misdiagnosis and waste of resources. A recent review reported the false‐negative rates from 2 to 33% in repeat sample testing from COVID‐19 suspects (Arevalo‐Rodriguez et al., 2020). Therefore, improving the specificity of clinical diagnosis using nanotechnology is need of the hour. Although considerable efforts have been made in the development of therapeutic schemes for different viruses, yet there are few drugs that effectively inhibit viruses, with the exception of vaccines.
In the virus tracking process, although QDs have shown excellent optical brightness and light stability, they still have limitations such as their larger size, flicker and potential interference with the function of viruses. In addition, the extent to which QD markers might affect the true behavior of viruses in living cells is not clear. Thus, SVT will certainly benefit from the development of new types of smaller and non‐flickering QDs to avoid these restrictions.
During viral infection, a variety of interactions occur between host cells and viruses. Super‐resolution imaging makes it possible to depict exactly this kind of cellular process on a nanoscale. The super‐resolution methods, which have optical resolution from 20 to 100 nm, can unveil the mechanism of virus infection in subcellular environment satisfactorily, and make possible revelation of the potential mechanism of virus infection by decomposing the subvirus. New imaging technology is still needed to combine SVT and super‐resolution microscopy to capture and quantitatively understand the basic processes involved in virus infection with nano spatial resolution.
SVT is limited to the in vitro study of virus infection mechanisms. By tracking individual viruses in living tissues and animals, it is possible to analyze the process of virus transmission between cells and understand how viruses break through the host's defense barrier. So far, several research groups have reported non‐invasive visualization of mice viruses (H. Pan et al., 2014; W. Pan et al., 2013). Due to the limitations of current bioimaging techniques, tracking a single virus in vivo in real time is still a challenge. Recently, near‐infrared QDs with emission ranges of 800–1600 nm were synthesized and are of particular interest in the production of ~2 nm size ultra‐small near‐infrared QDs via quasi biosynthesis (Hong et al., 2012; Jiang et al., 2012; J. J. Zhang et al., 2019; M. Zhang, Yue, et al., 2018).
With collaboration between scientists all over the world development of smaller, non‐toxic, and chemically stable nanomaterials could be achieved in near future. These novel and robust NPs would contribute to unravel the virus infection mechanisms with higher precision and accuracy, both in vivo and in vitro, supporting the development of antiviral drugs and vaccines, consequently to curb the mortalities.
AUTHOR CONTRIBUTIONS
Jun Kang: Conceptualization; writing‐original draft; writing‐review and editing. Ayesha Tahir: Writing‐review and editing. Hanjie Wang: Resources; visualization. Jin Chang: Funding acquisition; supervision.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
ACKNOWLEDGMENTS
We want to express our sincere gratitude to the laboratory partners who have patiently accompanied the entire writing and organizing process. We also would like to thank Mr. Ruoyun Zhang for providing linguistic assistance during the preparation of this manuscript.
Kang J, Tahir A, Wang H, Chang J. Applications of nanotechnology in virus detection, tracking, and infection mechanisms. WIREs Nanomed Nanobiotechnol. 2021;13:e1700. 10.1002/wnan.1700
Edited by: Andrew Wang, Associate Editor, and Nils Walter, Co‐Editor‐in‐Chief
Funding information Key project of Tianjin Foundational Research (JingJinJi) Program, China, Grant/Award Number: 19JCZDJC64100; National Key Research and Development Program of China, Grant/Award Number: 2017YFA0205104; National Natural Science Foundation of China, Grant/Award Number: 51873150
REFERENCES
- Ahmed, S. R. , Kang, S. W. , Oh, S. , Lee, J. , & Neethirajan, S. (2018). Chiral zirconium quantum dots: A new class of nanocrystals for optical detection of coronavirus. Heliyon, 4(8), e00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S. R. , Nagy, É. , & Neethirajan, S. (2017). Self‐assembled star‐shaped chiroplasmonic gold nanoparticles for an ultrasensitive chiro‐immunosensor for viruses. RSC Advances, 7(65), 40849–40857. [Google Scholar]
- Algar, W. R. , Susumu, K. , Delehanty, J. B. , & Medintz, I. L. (2011). Semiconductor quantum dots in bioanalysis: Crossing the valley of death. Analytical Chemistry, 83, 8826–8837. [DOI] [PubMed] [Google Scholar]
- Arevalo‐Rodriguez, I. , Buitrago‐Garcia, D. , Simancas‐Racines, D. , Zambrano‐Achig, P. , del Campo, R. , Ciapponi, A. , Sued O, Martinez‐Garcia L, Rutjes A, Low N, Bossuyt P M, Perez‐Molina J A, Zamora J (2020). False‐negative results of initial RT‐PCR assays for COVID‐19: A systematic review. medRxiv . [DOI] [PMC free article] [PubMed]
- Baldwin, R. , & Weder di Mauro, B. (2020). Economics in the time of COVID‐19: A new eBook. VOX CEPR Policy Portal .
- Brandenburg, B. , & Zhuang, X. (2007). Virus trafficking–learning from single‐virus tracking. Nature Reviews Microbiology, 5(3), 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caires, A. , Mansur, H. , Mansur, A. , Carvalho, S. , Lobato, Z. , & Dos Reis, J. (2019). Gold nanoparticle‐carboxymethyl cellulose nanocolloids for detection of human immunodeficiency virus type‐1 (HIV‐1) using laser light scattering immunoassay. Colloids and Surfaces B: Biointerfaces, 177, 377–388. [DOI] [PubMed] [Google Scholar]
- Chan, W. C. , & Nie, S. (1998). Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science, 281(5385), 2016–2018. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Li, H. , Shi, Z. , Peng, W. , Qin, Y. , Luo, R. , Zhou, D. , Gong, X. , & Chang, J. (2020). High fluorescence quenching probe‐based reverse fluorescence enhancement LFTS coupling with IS‐primer amplification reaction for the rapid and sensitive Parkinson disease‐associated MicroRNA detection. Biosensors and Bioelectronics, 165, 112278. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Li, H. , Zhou, D. , Peng, W. , Lian, G. , Gao, W. , Gong, X. , & Chang, J. (2019). Reusable bioluminescent sensor for ultrasensitive microrna detection based on a target‐introducing “fuel‐loading” mechanism. ACS Applied Materials & Interfaces, 11, 38586–38594. [DOI] [PubMed] [Google Scholar]
- Cui, J. , Han, H. , Piao, J. , Shi, H. , Zhou, D. , Gong, X. , & Chang, J. (2020). Construction of a novel biosensor based on the self‐assembly of dual‐enzyme cascade amplification‐induced copper nanoparticles for ultrasensitive detection of microRNA153. ACS Applied Materials & Interfaces, 12(30), 34130–34136. [DOI] [PubMed] [Google Scholar]
- Cui, Y. , Gong, X. , Zhu, S. , Li, Y. , Su, W. , Yang, Q. , & Chang, J. (2012). An effective modified method to prepare highly luminescent, highly stable water‐soluble quantum dots and its preliminary application in immunoassay. Journal of Materials Chemistry, 22(2), 462–469. [Google Scholar]
- Curtis, A. , & Wilkinson, C. (2001). Nantotechniques and approaches in biotechnology. Trends in Biotechnology, 19(3), 97–101. [DOI] [PubMed] [Google Scholar]
- Emerich, D. F. , & Thanos, C. G. (2003). Nanotechnology and medicine. Expert Opinion on Biological Therapy, 3(4), 655–663. [DOI] [PubMed] [Google Scholar]
- Gao, X. , Cui, Y. , Levenson, R. M. , Chung, L. W. , & Nie, S. (2004). In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology, 22(8), 969–976. [DOI] [PubMed] [Google Scholar]
- Gong, X. , Yan, H. , Yang, J. , Wu, Y. , Zhang, J. , Yao, Y. , Liu, P. , Wang, H. , Hu, Z. , & Chang, J. (2016). High‐performance fluorescence‐encoded magnetic microbeads as microfluidic protein chip supports for AFP detection. Analytica Chimica Acta, 939, 84–92. [DOI] [PubMed] [Google Scholar]
- Gong, X. , Zhang, B. , Piao, J. , Zhao, Q. , Gao, W. , Peng, W. , Kang, Q. , Zhou, D. , Shu, G. , & Chang, J. (2018). High sensitive and multiple detection of acute myocardial infarction biomarkers based on a dual‐readout immunochromatography test strip. Nanomedicine: Nanotechnology, Biology and Medicine, 14(4), 1257–1266. [DOI] [PubMed] [Google Scholar]
- Hong, G. , Robinson, J. T. , Zhang, Y. , Diao, S. , Antaris, A. L. , Wang, Q. , & Dai, H. (2012). In vivo fluorescence imaging with Ag2S quantum dots in the second near‐infrared region. Angewandte Chemie International Edition, 51(39), 9818–9821. [DOI] [PubMed] [Google Scholar]
- Huang, L.‐L. , & Xie, H.‐Y. (2014). Progress on the labeling and single‐particle tracking technologies of viruses. Analyst, 139(13), 3336–3346. [DOI] [PubMed] [Google Scholar]
- Islam, M. A. , & Ahsan, M. Z. (2020). Plausible approach for rapid detection of SARS‐CoV‐2 virus by magnetic nanoparticle based biosensors. American Journal of Nanosciences, 6(2), 6–13. [Google Scholar]
- Jiang, P. , Zhu, C.‐N. , Zhang, Z.‐L. , Tian, Z.‐Q. , & Pang, D.‐W. (2012). Water‐soluble Ag2S quantum dots for near‐infrared fluorescence imaging in vivo. Biomaterials, 33(20), 5130–5135. [DOI] [PubMed] [Google Scholar]
- Jin, R. , Zeng, C. , Zhou, M. , & Chen, Y. (2016). Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chemical Reviews, 116(18), 10346–10413. [DOI] [PubMed] [Google Scholar]
- Kim, J. W. , Kim, S. , Jang, Y.‐h. , Lim, K.‐i. , & Lee, W. H. (2019). Attomolar detection of virus by liquid coplanar‐gate graphene transistor on plastic. Nanotechnology, 30(34), 345502. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Zhang, X. , & Liang, X.‐J. (2013). Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnology Advances, 31(5), 593–606. [DOI] [PubMed] [Google Scholar]
- LeDuc, J. W. , & Barry, M. A. (2004). SARS, the first pandemic of the 21st century. Emerging Infectious Diseases, 10(11), e26. [Google Scholar]
- Li, L. , Li, S. , Wang, S. , Xing, X. , Zhang, Y. , Su, L. , Wu, X. , Wang, H. , Chang, J. , & Gong, X. (2020). Antioxidant and anti‐glycated TAT‐modified platinum nanoclusters as eye drops for non‐invasive and painless relief of diabetic cataract in rats. Chemical Engineering Journal, 398(1), 125436. [Google Scholar]
- Liang, Z. , Li, P. , Wang, C. , Singh, D. , & Zhang, X. (2019). Visualizing the transport of porcine reproductive and respiratory syndrome virus in live cells by quantum dots‐based single virus tracking. Virologica Sinica, 35, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.‐L. , Wang, Z.‐G. , Xie, H.‐Y. , Liu, A.‐A. , Lamb, D. C. , & Pang, D.‐W. (2020). Single‐virus tracking: From imaging methodologies to virological applications. Chemical Reviews, 120(3), 1936–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.‐L. , Wang, Z.‐G. , Zhang, Z.‐L. , & Pang, D.‐W. (2016). Tracking single viruses infecting their host cells using quantum dots. Chemical Society Reviews, 45(5), 1211–1224. [DOI] [PubMed] [Google Scholar]
- Loeffelholz, M. J. , & Tang, Y.‐W. (2020). Laboratory diagnosis of emerging human coronavirus infections – The state of the art. Emerging Microbes & Infections, 9(1), 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjomäki, V. , Lahtinen, T. , Martikainen, M. , Koivisto, J. , Malola, S. , Salorinne, K. , Pettersson, M. , & Häkkinen, H. (2014). Site‐specific targeting of enterovirus capsid by functionalized monodisperse gold nanoclusters. Proceedings of the National Academy of Sciences of the United States of America, 111(4), 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, M. , & Helenius, A. (2006). Virus entry: Open sesame. Cell, 124(4), 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, J. , & Helenius, A. (2009). Virus entry by macropinocytosis. Nature Cell Biology, 11(5), 510–520. [DOI] [PubMed] [Google Scholar]
- Mercer, J. , Schelhaas, M. , & Helenius, A. (2010). Virus entry by endocytosis. Annual Review of Biochemistry, 79, 803–833. [DOI] [PubMed] [Google Scholar]
- Mozhgani, S.‐H. , Kermani, H. A. , Norouzi, M. , Arabi, M. , & Soltani, S. (2020). Nanotechnology based strategies for HIV‐1 and HTLV‐1 retroviruses gene detection. Heliyon, 6(5), e04048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaeen, G. , Abbaszadeh, S. , & Yousefinejad, S. (2020). Application of nanomaterials in treatment, anti‐infection and detection of coronaviruses. Nanomedicine, 15, 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochmann, S. E. , Vietz, C. , Trofymchuk, K. , Acuna, G. P. , Lalkens, B. , & Tinnefeld, P. (2017). Optical nanoantenna for single molecule‐based detection of zika virus nucleic acids without molecular multiplication. Analytical Chemistry, 89(23), 13000–13007. [DOI] [PubMed] [Google Scholar]
- Pallas, V. , & García, J. A. (2011). How do plant viruses induce disease? Interactions and interference with host components. Journal of General Virology, 92(12), 2691–2705. [DOI] [PubMed] [Google Scholar]
- Pan, H. , Zhang, P. , Gao, D. , Zhang, Y. , Li, P. , Liu, L. , Wang, C. , Wang, H. , Ma, Y. , & Cai, L. (2014). Noninvasive visualization of respiratory viral infection using bioorthogonal conjugated near‐infrared‐emitting quantum dots. ACS Nano, 8(6), 5468–5477. [DOI] [PubMed] [Google Scholar]
- Pan, W. , Dong, Z. , Li, F. , Meng, W. , Feng, L. , Niu, X. , Li, C. , Luo, Q. , Li, Z. , Sun, C. , & Chen, L. (2013). Visualizing influenza virus infection in living mice. Nature Communications, 4, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen, N. , Borrenberghs, D. , Rocha, S. , & Hendrix, J. (2018). Single viruses on the fluorescence microscope: Imaging molecular mobility, interactions and structure sheds new light on viral replication. Viruses, 10(5), 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra, J. K. , Das, G. , Fraceto, L. F. , Campos, E. V. R. , del Pilar Rodriguez‐Torres, M. , Acosta‐Torres, L. S. , Armando Diaz‐Torres, L. , Grillo, R. , Kumara Swamy, M. , Sharma, S. , Habtemariam, S. , & Shin, H.‐S. (2018). Nano based drug delivery systems: Recent developments and future prospects. Journal of Nanobiotechnology, 16(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, W. , Huang, Y. , Zhao, Q. , Lian, G. , Chen, M. , Piao, J. , Gong, X. , & Chang, J. (2020). A fluorescent signal “removal” sensor via duplex‐specific nuclease‐aided cleavage for miRNA detection in flow cytometry. Colloids and Surfaces B: Biointerfaces, 185, 110570. [DOI] [PubMed] [Google Scholar]
- Peng, W. , Qin, Y. , Li, W. , Chen, M. , Zhou, D. , Li, H. , Cui, J. , Chang, J. , Xie, S. , Gong, X. , & Tang, B. (2020). Nonenzyme cascaded amplification biosensor based on effective aggregation luminescence caused by disintegration of silver nanoparticles. ACS Sensors, 5(7), 1912–1920. [DOI] [PubMed] [Google Scholar]
- Peng, W. , Zhao, Q. , Chen, M. , Piao, J. , Gao, W. , Gong, X. , & Chang, J. (2019). An innovative “unlocked mechanism” by a double key avenue for one‐pot detection of microRNA‐21 and microRNA‐141. Theranostics, 9(1), 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, W. , Zhao, Q. , Piao, J. , Zhao, M. , Huang, Y. , Zhang, B. , Gao, W. , Zhou, D. , Shu, G. , Gong, X. , & Chang, J. (2018). Ultra‐sensitive detection of microRNA‐21 based on duplex‐specific nuclease‐assisted target recycling and horseradish peroxidase cascading signal amplification. Sensors and Actuators B: Chemical, 263, 289–297. [Google Scholar]
- Piao, J. , Zhao, Q. , Zhou, D. , Peng, W. , Gao, W. , Chen, M. , Shu, G. , Gong, X. , & Chang, J. (2019). Enzyme‐free colorimetric detection of microRNA‐21 using metal chelator as label for signal generation and amplification. Analytica Chimica Acta, 1052, 145–152. [DOI] [PubMed] [Google Scholar]
- Prasad, R. , Bhattacharyya, A. , & Nguyen, Q. D. (2017). Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Frontiers in Microbiology, 8, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, M. K. , & Vermerris, W. (2017). Recent advances in nanomaterials for gene delivery—A review. Nanomaterials, 7(5), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, L. , Dong, S. , & Nienhaus, G. U. (2011). Ultra‐small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today, 6(4), 401–418. [Google Scholar]
- Shen, P. , & Xia, Y. (2014). Synthesis‐modification integration: One‐step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Analytical Chemistry, 86(11), 5323–5329. [DOI] [PubMed] [Google Scholar]
- Singhal, C. , Pundir, C. , & Narang, J. (2017). A genosensor for detection of consensus DNA sequence of dengue virus using ZnO/Pt‐Pd nanocomposites. Biosensors and Bioelectronics, 97, 75–82. [DOI] [PubMed] [Google Scholar]
- Smith, A. E. , & Helenius, A. (2004). How viruses enter animal cells. Science, 304(5668), 237–242. [DOI] [PubMed] [Google Scholar]
- Sun, E.‐Z. , Liu, A.‐A. , Zhang, Z.‐L. , Liu, S.‐L. , Tian, Z.‐Q. , & Pang, D.‐W. (2017). Real‐time dissection of distinct dynamin‐dependent endocytic routes of influenza A virus by quantum dot‐based single‐virus tracking. ACS Nano, 11(5), 4395–4406. [DOI] [PubMed] [Google Scholar]
- Udugama, B. , Kadhiresan, P. , Kozlowski, H. N. , Malekjahani, A. , Osborne, M. , Li, V. Y. , Chen, H. , Mubareka, S. , Gubbay, J. B. , & Chan, W. C. (2020). Diagnosing COVID‐19: The disease and tools for detection. ACS Nano, 14(4), 3822–3835. [DOI] [PubMed] [Google Scholar]
- Valdiglesias, V. , & Laffon, B. (2020). The impact of nanotechnology in the current universal COVID‐19 crisis. Let's not forget nanosafety! Nanotoxicology, 14(8), 1013–1016. [DOI] [PubMed] [Google Scholar]
- Wan, X.‐Y. , Zheng, L.‐L. , Gao, P.‐F. , Yang, X.‐X. , Li, C.‐M. , Li, Y. F. , & Huang, C. Z. (2014). Real‐time light scattering tracking of gold nanoparticles‐bioconjugated respiratory syncytial virus infecting HEp‐2 cells. Scientific Reports, 4, 4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Zhu, J. , Dong, H. , Pei, Z. , Zhou, T. , & Hu, G. (2017). Rapid detection of variant and classical porcine epidemic diarrhea virus by nano‐nest PCR. Pakistan Veterinary Journal, 37(2), 225–229. [Google Scholar]
- Wang, L. , Zhou, Y. , Wu, M. , Wu, M. , Li, X. , Gong, X. , Chang, J. , & Zhang, X. (2018). Functional nanocarrier for drug and gene delivery via local administration in mucosal tissues. Nanomedicine, 13(1), 69–88. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Yang, W. , Du, H. , Guo, F. , Wang, H. , Chang, J. , Gong, X. , & Zhang, B. (2016). Multifunctional reduction‐responsive SPIO&DOX‐loaded PEGylated polymeric lipid vesicles for magnetic resonance imaging‐guided drug delivery. Nanotechnology, 27(16), 165101. [DOI] [PubMed] [Google Scholar]
- Wegner, K. D. , & Hildebrandt, N. (2015). Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chemical Society Reviews, 44(14), 4792–4834. [DOI] [PubMed] [Google Scholar]
- Wen, L. , Lin, Y. , Zheng, Z.‐H. , Zhang, Z.‐L. , Zhang, L.‐J. , Wang, L.‐Y. , Wang, H. Z. , & Pang, D.‐W. (2014). Labeling the nucleocapsid of enveloped baculovirus with quantum dots for single‐virus tracking. Biomaterials, 35(7), 2295–2301. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2020). Coronavirus disease (COVID‐19): Weekly epidemiological update .
- Wu, Y. , Guo, W. , Peng, W. , Zhao, Q. , Piao, J. , Zhang, B. , Wu, X. , Wang, H. , Gong, X. , & Chang, J. (2017). Enhanced fluorescence ELISA based on HAT triggering fluorescence “turn‐on” with enzyme–antibody dual labeled AuNP probes for ultrasensitive detection of AFP and HBsAg. ACS Applied Materials & Interfaces, 9(11), 9369–9377. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Peng, W. , Zhao, Q. , Piao, J. , Zhang, B. , Wu, X. , Wang, H. , Shi, Z. , Gong, X. , & Chang, J. (2017). Immune fluorescence test strips based on quantum dots for rapid and quantitative detection of carcino‐embryonic antigen. Chinese Chemical Letters, 28(9), 1881–1884. [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323(13), 1239–1242. [DOI] [PubMed] [Google Scholar]
- Xi, Z. , Huang, R. , Li, Z. , He, N. , Wang, T. , Su, E. , & Deng, Y. (2015). Selection of HBsAg‐specific DNA aptamers based on carboxylated magnetic nanoparticles and their application in the rapid and simple detection of hepatitis B virus infection. ACS Applied Materials & Interfaces, 7(21), 11215–11223. [DOI] [PubMed] [Google Scholar]
- Yang, W. , Guo, W. , Zhang, T. , Yang, W. , Su, L. , Fang, L. , Wang, H. , Gong, X. , & Chang, J. (2015). Synthesis of aqueous AgInS/ZnS@ PEI as a self‐indicating nonviral vector for plasmid DNA self‐tracking delivery. Journal of Materials Chemistry B, 3(43), 8518–8527. [DOI] [PubMed] [Google Scholar]
- Yang, Y.‐B. , Tang, Y.‐D. , Hu, Y. , Yu, F. , Xiong, J.‐Y. , Sun, M.‐X. , Lyu, C. , Peng, J.‐M. , Tian, Z.‐J. , Cai, X.‐H. , & An, T.‐Q. (2020). Correction to single virus tracking with quantum dots packaged into enveloped viruses using CRISPR. Nano Letters, 20(4), 2931–2931. [DOI] [PubMed] [Google Scholar]
- Yin, W. , Li, W. , Li, Q. , Liu, Y. , Liu, J. , Ren, M. , Ma, Y. , Zhang, Z. , Zhang, X. , Wu, Y. , Jiang, S. , Zhang, X.‐E. , & Cu, Z. (2020). Real‐time imaging of individual virion‐triggered cortical actin dynamics for human immunodeficiency virus entry into resting CD4 T cells. Nanoscale, 12(1), 115–129. [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Gao, W. , Piao, J. , Xiao, Y. , Wang, B. , Peng, W. , Gong, X. , Wang, Z. , Yang, H. , & Chang, J. (2018). Effective bioactivity retention of low‐concentration antibodies on HFBI‐modified fluorescence ICTS for sensitive and rapid detection of PSA. ACS Applied Materials & Interfaces, 10(17), 14549–14558. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Zhao, Q. , Wu, Y. , Zhang, B. , Peng, W. , Piao, J. , Zhou, Y. , Gao, W. , Gong, X. , & Chang, J. (2017). The construction of a novel nucleic acids detection microplatform based on the NSET for one‐step detecting TK1‐DNA and microRNA‐21. Biosensors and Bioelectronics, 97, 26–33. [DOI] [PubMed] [Google Scholar]
- Zhang, J. J. , Lin, Y. , Zhou, H. , He, H. , Ma, J. J. , Luo, M. Y. , Zhang, Z. L. , & Pang, D. W. (2019). Cell membrane‐camouflaged NIR II fluorescent Ag2Te quantum dots‐based nanobioprobes for enhanced in vivo homotypic tumor imaging. Advanced Healthcare Materials, 8(14), 1900341. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , & Wang, E. (2014). Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today, 9(1), 132–157. [Google Scholar]
- Zhang, L.‐J. , Wang, S. , Xia, L. , Lv, C. , Tang, H.‐W. , Liang, Z. , Xiao, G. , & Pang, D.‐W. (2020). Lipid‐specific labeling of enveloped viruses with quantum dots for single‐virus tracking. MBio, 11(3), e00135–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.‐J. , Xia, L. , Liu, S.‐L. , Sun, E.‐Z. , Wu, Q.‐M. , Wen, L. , Zhang, Z.‐L. , & Pang, D.‐W. (2018). A “driver switchover” mechanism of influenza virus transport from microfilaments to microtubules. ACS Nano, 12(1), 474–484. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Yue, J. , Cui, R. , Ma, Z. , Wan, H. , Wang, F. , Zhu, S. , Zhou, Y. , Kuang, Y. , Zhong, Y. , Pang, D.‐W. , & Dai, H. (2018). Bright quantum dots emitting at ∼1,600 nm in the NIR‐IIb window for deep tissue fluorescence imaging. Proceedings of the National Academy of Sciences of the United States of America, 115(26), 6590–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Piao, J. , Peng, W. , Wang, J. , Gao, W. , Wu, X. , Wang, H. , Gong, X. , Chang, J. , & Zhang, B. (2018a). A metal chelator as a plasmonic signal‐generation superregulator for ultrasensitive colorimetric bioassays of disease biomarkers. Advanced Science, 5(7), 1800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Piao, J. , Peng, W. , Wang, Y. , Zhang, B. , Gong, X. , & Chang, J. (2018b). Simple and sensitive quantification of microRNAs via PS@ Au microspheres‐based DNA probes and DSN‐assisted signal amplification platform. ACS Applied Materials & Interfaces, 10(4), 3324–3332. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Yan, H. , Liu, P. , Yao, Y. , Wu, Y. , Zhang, J. , Li, H. , Gong, X. , & Chang, J. (2016). An ultra‐sensitive and colorimetric sensor for copper and iron based on glutathione‐functionalized gold nanoclusters. Analytica Chimica Acta, 948, 73–79. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Li, L. , Li, S. , Li, S. , Zhao, M. , Zhou, Q. , Gong, X. , Yang, J. , & Chang, J. (2019). Autoregenerative redox nanoparticles as an antioxidant and glycation inhibitor for palliation of diabetic cataracts. Nanoscale, 11(27), 13126–13138. [DOI] [PubMed] [Google Scholar]


