Summary
The current pandemic caused by SARS‐CoV‐2 virus infection is known as Covid‐19 (coronavirus disease 2019). This disease can be asymptomatic or can affect multiple organ systems. Damage induced by the virus is related to dysfunctional activity of the immune system, but the activity of molecules such as C‐reactive protein (CRP) as a factor capable of inducing an inflammatory status that may be involved in the severe evolution of the disease, has not been extensively evaluated. A systematic review was performed using the NCBI‐PubMed database to find articles related to Covid‐19 immunity, inflammatory response, and CRP published from December 2019 to December 2020. High levels of CRP were found in patients with severe evolution of Covid‐19 in which several organ systems were affected and in patients who died. CRP activates complement, induces the production of pro‐inflammatory cytokines and induces apoptosis which, together with the inflammatory status during the disease, can lead to a severe outcome. Several drugs can decrease the level or block the effect of CRP and might be useful in the treatment of Covid‐19. From this review it is reasonable to conclude that CRP is a factor that can contribute to severe evolution of Covid‐19 and that the use of drugs able to lower CRP levels or block its activity should be evaluated in randomized controlled clinical trials.
Keywords: C‐reactive protein, COVID‐19, SARS‐CoV‐2, severe evolution
Abbreviations
- Covid‐19
coronavirus 2019 disease
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- CRP
C‐reactive protein
- RAS
Renin‐Angiotensin System
- ACE2
angiotensin II converting enzyme
- ACE
angiotensin I converting enzyme
- nCRP
native C reactive protein
- mCRP
monomeric C reactive protein
- mCRPm
mixed C reactive protein
- IL‐6
interleukin‐6
- IL‐1
interleukin‐1
- PaO2/FiO2
partial pressure of arterial oxygen to fraction of inspired oxygen ratio
- RNA
ribonucleic acid
- TMPRSS2
transmembrane serine protease 2
- Ang II
angiotensin II
- Ang 1‐7
angiotensin 1‐7
- ADAM17
disintegrator and metalloproteinase 17
- AT1
angiotensin II receptor 1
- AT2
angiotensin II receptor 2
- NF‐kB
nuclear factor‐kappa B
- TNF
tumour necrosis factor
- GADD153
growth arrest and DNA damage 153
- JAK
janus kinase
1. INTRODUCTION
SARS‐CoV‐2 starts its pathogenetic process through renin‐angiotensin system (RAS) activation, binding to the angiotensin II converting enzyme (ACE2) and originating a series of pro‐inflammatory events that can induce a cytokine storm. 1 , 2 The C‐reactive protein (CRP) is a molecule produced by the interaction of SARS‐CoV‐2 with ACE2, 3 , 4 , 5 which is not only an indicator of acute phase of inflammation but also has been related to prognosis and severity of Covid‐19. 5 , 6 , 7 Therefore, CRP can be an important factor in the cellular damage during Covid‐19. This review aims to describe the different mechanisms by which SARS‐CoV‐2 can induce cell damage during the infectious process by increasing CRP and the options that could be considered to counteract CRP in this disease.
2. METHODS
A literature search of Covid‐19 and immunity, inflammatory response and C reactive protein using the NCBI‐PubMed database to find the articles published from December 2019 to December 2020 was performed. All terms were searched as general terms to obtain the maximum search results.
3. C‐REACTIVE PROTEIN OVERVIEW
C‐reactive protein is an inflammatory protein of the pentraxin family and is produced in response to the acute inflammatory phase. It was first discovered in 1930 by Tillet and Francis 8 in response to pneumococcal infection. Transcriptional induction of the CRP gene primarily occurs in hepatocytes in response to increased levels of inflammatory cytokines, especially interleukin‐6 (IL‐6) with IL‐1 enhancing the effect. 9 , 10 , 11 , 12 The human CRP gene is found at 1q23.2 on the long arm of chromosome 1, and to date, there have been no allelic variations or genetic deficiencies discovered for this gene, although some polymorphisms have been identified. 13 C‐reactive protein shows high expression during inflammatory conditions such as rheumatoid arthritis, some cardiovascular diseases and infection. 14 There are many factors that can alter CRP levels, including age, sex, smoking status, weight, lipid levels and blood pressure. 13
The increase of CRP in infections occurs mainly in bacterial infections; however, it cannot identify the type of bacterial infection. 15 , 16 The main role of CRP in bacterial inflammation tends to centre around the activation of the complement molecule C1q leading to opsonisation of pathogens. In the presence of calcium, CRP binds to polysaccharides such as phosphocholine on the microorganisms and triggers complement activation by the classical pathway activating C1q. 17 In addition, CRP binds to Fc receptors on the cell surface leading to the release of pro‐inflammatory cytokines. 18 Thus, CRP is not only a marker of inflammation, but also contributes to the inflammatory response. Regarding to increased levels of CRP in SARS‐CoV‐2 infection, high levels of CRP have been associated with mortality from this infection. 6 CRP has been identified as a molecule capable of causing damage during SARS‐CoV‐2 infection. 19 , 20
4. LEVELS OF C REACTIVE PROTEIN AND COVID‐19 EVOLUTION
C‐reactive protein has been used for a long time as an indicator of acute phase inflammation, 9 , 12 ; however, in the current Covid‐19 pandemic it is related to tissue damage and poor prognosis of the disease. In this regard, high levels of CRP in the early stage of Covid‐19 have been associated with lung damage and the severity of the disease. 3 , 4 , 5 Analysis of lung alterations assessed by computerized tomography shows that high levels of CRP are present before the appearance of lung lesions, giving to CRP predictive values of severity. 21 The progression to pneumonia has been associated and correlated to the increased circulating CRP levels. 22 , 23 Studies involving CRP levels and respiratory function showed inverse correlation between elevated CRP levels with decreased partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2), suggesting that CRP is a predictor factor of lung failure. 24 Other studies show the association of CRP with other parameters in the evolution of Covid‐19. In this context, high levels of CRP with low levels of albumin have been associated with increased mortality. 3 , 6 Elevated CRP/lymphocyte ratio has been used as important predictor factor for the use of intensive care unit. 19 Analysis of CRP and other cytokines show predictive value for the severity of Covid‐19. High levels of CRP and IL‐6 (a hepatic inducer of CRP) and IL‐10 have been used as predictive factors for Covid‐19. 25 , 26 High correlation has been reported between CRP levels and IL‐10. 26 Increased levels of CRP and IL‐6 were predictive for the severity of COVID‐19 in hypertensive patients. 27 Meta‐analysis studies show that high levels of CRP with leukocytosis are predictive of poor prognosis in patients with Covid‐19. 28 The persistence of high levels of CRP in individuals who have died from Covid‐19, suggests that CRP is a predictor for SARS‐CoV‐2 induced lethality. 29 Although high levels of CRP have been associated with poor prognosis and mortality from Covid‐19, the type of CRP isoforms should be studied since some isoforms have pro‐inflammatory properties and others are anti‐inflammatory molecules. 7 Considering these findings, the presence of elevated CRP levels in association with the deleterious evolution of Covid‐19, suggest that CRP is involved in the cellular damage that leads to the failure of different organ systems in this infection.
5. SARS‐COV‐2 INFECTION AND C‐REACTIVE PROTEIN PRODUCTION
An important step in SARS‐CoV‐2 infection is the binding of viral S protein to its receptor ACE2, a member of the RAS. 1 This viral infection causes a disease with multi‐organ dysfunctions involving the respiratory, renal, cardiovascular, central nervous and gastrointestinal systems. 2
Structurally, SARS‐CoV‐2 is a spherical virus covered by a lipid envelope. Its genome, formed by a chain of RNA in a positive sense, is covered by a nucleocapsid. Externally, this virus presents important proteins for its pathogenesis. The S protein (spikes) that is important for the binding to its ACE2 receptor, the M protein that provides the structural support, the E protein necessary for the assembly of the virus and a haemagglutinin esterase. 30 , 31 The viral S protein binds to ACE2 after proteolytic modification of both. Before protein S binding to ACE2 occurs, protein S is proteolytically modified by several proteases, especially TMPRSS2 (transmembrane serine protease 2), L‐cathepsin and B‐cathepsin, but other proteins such as trypsin, factor X, elastase and furin may also be involved. 32 , 33 , 34 Binding of the modified S protein to ACE2 facilitates the entry of the virus into the cell and decreases the expression of ACE2 on the cell surface. 35 , 36 The ACE2‐bound virus is introduced into the cell by endocytosis. 37 Initially, ACE2 plays a protective role against the harmful effects of angiotensin II (Ang II) (inflammation, fibrosis, oxidative stress, vasoconstriction, cancer) by transforming Ang II into Ang 1‐7, which acting on its receptor Mas, generates effects contrary to Ang II. 38 The cellular internalization of the virus/ACE2 complex leaves an increased Ang II activity and represents a stimulus for the expression of ADAM17 (a disintegrin and metalloproteinase 17) on the cellular surface. ADAM17 has a proteolytic action on ACE2 leaving a decrease of this molecule on the cell surface. 39 As a result of the increased activity of Ang II on its AT‐1 receptor and through the nuclear translocation of NF‐kB, 40 Ang II induces the production of CRP, pro‐inflammatory cytokines, oxidative stress, fibrosis, vasoconstriction and increases the activity of ADAM17, 41 among other harmful effects. In addition, ADAM17 has a proteolytic action on pro‐TNF‐alpha in the cell membrane, transforming it into the active form of the molecule, which, when released into the extracellular medium, interacts with its receptor inducing the production of additional ADAM17. 42 , 43 As a result of the increased ADAM17 activity on ACE2 and the internalization of the virus/ACE2 complex, there is a drastic reduction of ACE2 on the cell surface and an increase of this molecule in the extracellular space. 39 This process leads to an exaggerated function of Ang II due to the deterioration of the conversion of Ang II into Ang 1‐7, which leads to inflammatory effects and a drastic increase in the production of cytokines with the consequent deleterious effects mediated by the RAS. 39 , 44 Under this mechanism SARS‐CoV‐2 can induce the production of CRP mediated by increased Ang II activity (Figure 1). Therefore, the hyperactivity of Ang II is involved in the severity of Covid‐19. Decreasing RAS activity by blocking Ang II receptors (AT1) and by using ACE inhibitors improve Covid‐19 evolution and decrease the production of proinflammatory cytokines, especially IL‐6 a high inducer of hepatic CRP. 45 In the same context, the blockade of Ang II production reduces the content of CRP in the circulation. 46 Other studies show that ACE inhibition decreased the presence of pneumonia in Covid‐19 accompanied by decreased circulating levels of CRP. 47 These data indicate the role of Ang II as an inducer of CRP production during Covid‐19.
FIGURE 1.
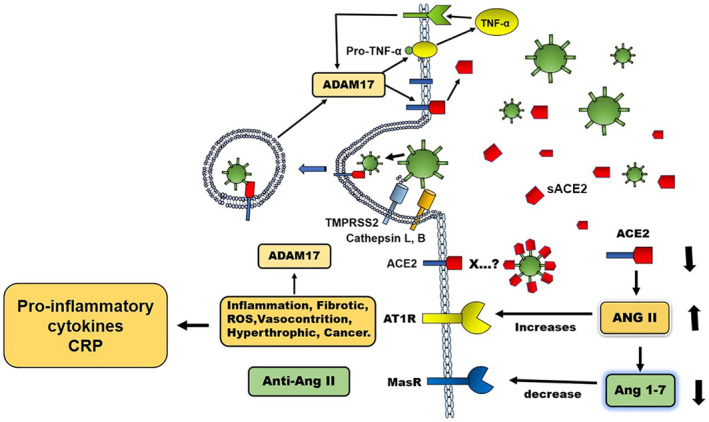
Renin angiotensin system (RAS) in the pathogenesis of SARS‐CoV‐2 infection. The viral S protein binds to Angiotensin I converting enzyme‐2 (ACE2) after proteolytic modification by TMPRSS2 (serine transmembrane protease 2) and cathepsin L. The binding of the ACE2‐modified S protein facilitates the entry of the virus into the cell and decreases the expression of ACE2 on cell surface. The cellular internalization of the virus/ACE2 complex decreases ACE2 and increases the activity of Angiotensin II (Ang II) and the expression of ADAM17 (disintegrin and metalloproteinase 17) on the cell surface, which, when acting on ACE2, decreases the expression of this molecule on the cell surface and increases it in the extracellular environment (sACE2). The increased activity of Ang II on AT1 receptor induces the production of pro‐inflammatory cytokines, oxidative stress (ROS), fibrosis, vasoconstriction, C‐reactive protein (CRP) and increases the activity of ADAM17. ADAM17 also acts on pro‐TNF‐alpha in the membrane producing the active molecule that interacts with its receptor and induces the production of additional ADAM17. The activity of ADAM17 on ACE2 and the internalization of the virus/ACE2 complex reduces ACE2 on the cell surface and increases this molecule in the extracellular space. This process induces an increase of Ang II activity by means of a deteriorated conversion of Ang II into Ang 1‐7 that leads to a drastic increase of cytokine production with the consequent deleterious effects
6. MECHANISMS OF CELL DAMAGE MEDIATED BY C‐REACTIVE PROTEIN
The pathogenesis of CRP is mediated by its isoform types. CRP has three different isoforms, native CRP (nCRP), monomeric (mCPR) and mixed isoform (mCRPm). In this respect, the nCRP is the native protein that is formed by five monomers (penta‐monomeric). 48 This molecule presents two ligands at opposite sides of the molecule, one of which binds calcium and the other interacts with the C1q of the complement and with Fc receptors. 14 This isoform is synthesized mainly in the liver but is also synthesized by other cells such as endothelial cells, macrophages, lymphocytes, muscle cells and adipocytes. 49 , 50 , 51 , 52 This form is stored in the endoplasmic reticulum and is slowly released into the circulation, except in states of inflammation, where it is rapidly eliminated to the circulation by the action of pro‐inflammatory cytokines. 53 The nCRP dissociates and gives rise to monomers (mCRP). 54 These two isoforms have different biological properties during the inflammatory process, 55 a phenomenon related to the points where the ligands of each molecule join. 56 There is a third isoform, mCRPm, which originates when nCRP partially dissociates and leaves an isoform that retains part of nCRP. This occurs when nCRP is bound to the cell membrane, leaving mCRPm with a high capacity to activate complement. 57
Complement activation is a crucial stage in CRP biology. 58 This molecule activates complement through the classical pathway activating C1q. 59 In this regard, the activation of C1q induces the chain activation of C4, C2 and C3. Activated C3 can induce the following effects: opsonisation, through the production of C3b and C4b; cellular lysis, through the activation of C5‐C9 leading to the membrane attack complex (MAC); and inflammation, through the production of C3a and C5a. 60 C‐reactive protein is most effective activating the early stages of complement inducing inflammatory and opsonisation effects. 48 C‐reactive protein is known to rise in infectious and inflammatory processes, but the role of each isoform is little known. mCRP can bind to the classical complement activation inhibitor C4bp, obtaining high degree of control over this complement activation. 61 There is evidence that mCRP has greater proinflammatory capacity than nCRP, by activating more complement and producing more MAC and inducing chemotaxis of monocytes and attraction of leukocytes to sites of inflammation through activation of the Fcy‐RI and Fcy‐RIIa pathways. 58 C‐reactive protein is deposited locally at the sites of inflammation and tissue damage and binds to the damaged cell membrane activating complement and contributing to the inflammatory process. 62 , 63 However, other studies report that CRP is preferably found in the fluid phase rather than on the inflamed tissue. 64 Although the main action of CRP is complement activation, this molecule when binding to Fc receptors induces the production of proinflammatory cytokines. 9 In addition, this molecule has the capacity to recognize both its own and foreign antigens. 59
CRP induces apoptosis by several mechanisms: (1) induction of pro‐apoptotic cytokines such as TNF‐α and IL‐1‐β and induction of reactive oxygen species through activation of Fc‐γ receptors. 65 , 66 (2) Induction of p53 up‐regulation altering the cell cycle through activation of Fc‐γRII. 67 (3) Activation of genes related to the expression of adhesion molecules and chemotactic cytokines. 68 (4) Induction of GADD153 gene expression related to cell cycle arrest and DNA damage. 69 (5) Activation of caspase‐3 70 which additionally promotes the opsonisation of apoptotic cells. 71 , 72
Other studies have shown that nCRP inhibits endothelial nitric oxide synthase leading to deleterious effects by decreasing nitric oxide production, increasing adhesion molecule expression and inducing vasoconstriction and inflammation. 73 , 74 , 75 , 76 This effect is mediated by the activation of the NADPH oxidase and the p38 mitogen‐activated protein kinase (MAP kinase) pathways. 48
Due to the multiple deleterious effects on the body mediated by CRP, the relationship of increased CRP levels to the poor prognosis and severity of Covid‐19 is plausible (Figure 2).
FIGURE 2.
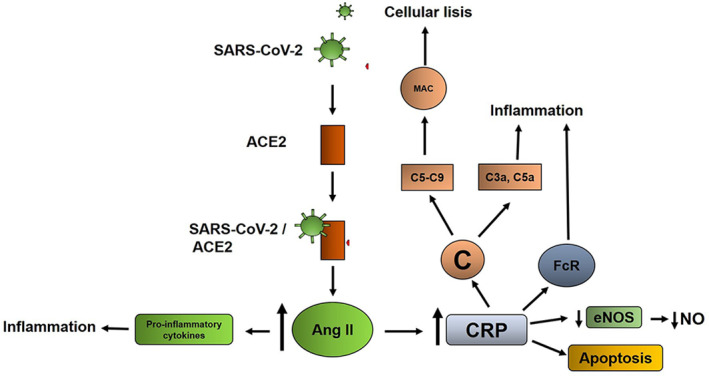
Possible mechanism of C‐reactive protein (CRP) damage during Covid‐19. After binding of SARS‐CoV‐2 to its ACE2 receptor, the complex is internalized in the cell and determines the hyperactivity of angiotensin II (Ang II), which among other effects induces the production of CRP and pro‐inflammatory cytokines. CRP induces deleterious effects in the organism mediated by activation of complement, binding to Fc receptors and induction of apoptosis. Both the production of cytokines and CRP can together be part of the cytokine storm reported in Covid‐19.
Abbreviations: C, complement; MAC, membrane attack complex; FcR, Fc receptor; eNOS, endothelial nitric oxide sintase; NO, nitric oxide.
7. DRUGS THAT BLOCK C‐REACTIVE PROTEIN WITH A POTENTIAL THERAPEUTIC EFFECT ON COVID‐19
Tetracycline is an antibiotic with antiviral properties that has been proposed to treat Covid‐19. 77 Anti‐CRP properties of tetracyclines have also been demonstrated. Doxycycline decreases the oxidizing effect of CRP in the production of 5‐α‐dihydrotestosterone by osteoblasts through its antioxidant capacity. 78 Doxycycline also decreases circulating levels of CRP and other markers of inflammation and improves glucose metabolism in db/db diabetic mice. 79 Human studies show that doxycycline reduces circulating CRP levels in patients with peripheral arterial disease, 80 with small abdominal aortic aneurysms 81 and, with periodontal disease. 82 The reduction of CRP levels may be related to the anti‐inflammatory property of tetracyclines. 77 Doxycycline can also induce the expression of Ang II AT2 receptors 83 which when stimulated can induce effects contrary to the AT1 receptor involved in the production of CRP. Minocycline diminishes the vasopressor and inflammatory effects of Ang II in rats treated with continuous infusion of Ang II 84 and in spontaneously hypertensive rats. 85 These studies suggest that tetracyclines may alter the production and/or the effect of CRP by acting on the production of Ang II‐induced CRP.
Janus kinase (JAK: JAK1/JAK2) is involved in the induction of pro‐inflammatory cytokines. 86 Inhibition of Janus kinase represses induction of CRP in human hepatocytes, 87 thus blocking drugs of this pathway represents a reasonable therapeutic strategy. Several JAK inhibitors have been reported. Baricitinib is a drug involved in blocking the passage of SARS‐CoV‐2 by endocytosis into the cell 88 and is also a potent inhibitor of the JAK1/JAK2 pathway inhibiting the proinflammatory signal of several cytokines especially IL‐6 which promotes CRP synthesis involved in the cytokine storm reported in Covid‐19. 88 , 89 Ruxolitinib another JAK1/JAK2 inhibitor has been used in patients with advanced HER2‐negative breast cancer with increased CRP, obtaining favourable change in health‐related quality of life. 90 Other JAK inhibitors such as tofacitinib and GSK2586184 have been reported as decreasing CRP drugs. 91
Several other drugs that decrease circulating CRP concentrations have been reported. In this context, beta‐blockers may affect CRP concentrations. 92 , 93 Inhibitors of cyclooxygenase (celecoxib, rofecoxib), 94 platelet aggregation (clopidogrel, abciximab) 95 and, ACE (ramipril, captopril, fosinopril), 96 decreasing lipid agents (statins, ezetimibe, fenofibrate), 97 antioxidants (α‐Tocopherol), 98 Ang II receptor blockers (valsartan, irbesartan, olmesartan, telmisartan), 99 and anti‐diabetic agents (rosiglitazone and pioglitazone) 100 represent some of the drugs that decrease CRP levels (Figure 3).
FIGURE 3.
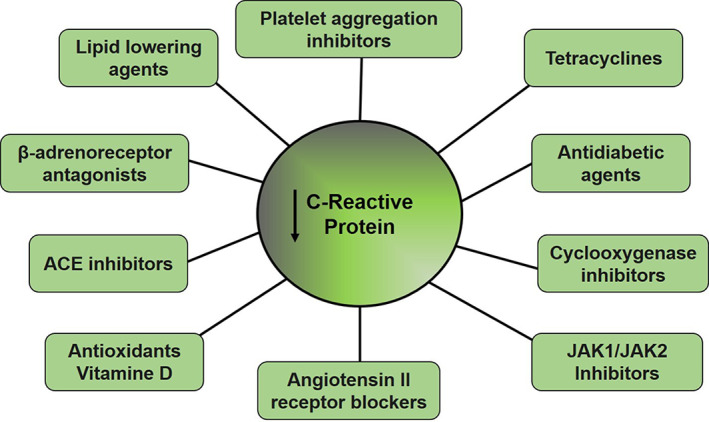
Pharmaceuticals with the ability to reduce levels of C‐reactive protein
8. CONCLUSION
During Covid‐19, SARS‐CoV‐2 can alter the renin‐angiotensin system and induce increased Ang II activity, which induces CRP production with subsequent tissue damage and increased severity of Covid‐19. The use of drugs that act by reducing CRP production represents a reasonable therapeutic approach that should be tested in controlled clinical trials.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept, design, interpretation and literature search: Jesus A. Mosquera‐Sulbaran. Literature search, figure illustration and interpretation: Adriana Pedreañez. Literature search, drafting of the manuscript and interpretation: Yenddy Carrero and Diana Callejas. All authors agreed to manuscript publication.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Paul Griffiths, Professor of Virology, University College London, for reviewing, suggestions and editing. We also thanks to Instituto de Investigaciones Clinicas Dr. Americo Negrette, Universidad del Zulia. Maracaibo, Venezuela.
Mosquera‐Sulbaran JA, Pedreañez A, Carrero Y, Callejas D. C‐reactive protein as an effector molecule in Covid‐19 pathogenesis. Rev Med Virol. 2021;31(6):e2221. 10.1002/rmv.2221
REFERENCES
- 1. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID‐19 and the liver. J Hepatol. 2020;73:1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RodOviedo‐ Trespalacios JEO, Cortes‐Ramirez J. A brief‐review of the risk factors for covid‐19 severity. Rev Saude Publica. 2020;54:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo X, Zhou W, Yan X, et al. Prognostic value of C‐reactive protein in patients with COVID‐19. Clin Infect Dis. 2020:ciaa641. Online ahead of print. 10.1093/cid/ciaa641. [DOI] [Google Scholar]
- 5. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bannaga AS, Tabuso M, Farrugia A, et al. C‐reactive protein and albumin association with mortality of hospitalised SARS‐CoV‐2 patients: a tertiary hospital experience. Clin Med. 2020;20:463‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Potempa LA, Rajab IM, Hart PC, Bordon J, Fernandez‐Botran R. Insights into the use of C‐reactive protein as a diagnostic index of disease severity in COVID‐19 infections. Am J Trop Med Hyg. 2020;103:561‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tillet WS, Francis T. Serological reactions in pneumonia with a non‐ protein somatic fraction of Pneumococcus. J Exp Med. 1930;52:561‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Med Assoc. 2001;286:327‐334. [DOI] [PubMed] [Google Scholar]
- 10. Boras E, Slevin M, Alexander MY, et al. Monomeric C‐reactive protein and Notch‐3 co‐operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69:165‐179. [DOI] [PubMed] [Google Scholar]
- 11. Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, Volankis JE. Testosterone and IL‐6 requirements for human C‐reactive protein gene expression in transgenic mice. J Immunol. 1998;160:5294‐5299. [PubMed] [Google Scholar]
- 12. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta‐analysis of peripheral C‐reactive protein, inetleukin‐6 and tumour necrosis factor‐α. Mol Psychiatry. 2016;21:642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hage FG, Szalai AJ. C‐reactive protein gene polymorphisms, C‐reactive protein blood levels and cardiovascular disease risk. J Am Coll Cardiol. 2007;50:1115‐1122. [DOI] [PubMed] [Google Scholar]
- 14. Du Clos TW, Mold C. C‐reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261‐277. [DOI] [PubMed] [Google Scholar]
- 15. Healy B, Freedman A. Infections. Br Med J. 2006;332:838‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kingsley A, Jones V. Diagnosing wound infection: the use of C‐reactive protein. Wounds UK. 2008;4:32‐46. [Google Scholar]
- 17. Volanakis JE. Human C‐reactive protein: expression structure and function. Mol Immunol. 2001;38:189‐197. [DOI] [PubMed] [Google Scholar]
- 18. Du Clos TW. Function of C‐reactive protein. Ann Med. 2000;32:274‐278. [DOI] [PubMed] [Google Scholar]
- 19. Cillóniz C, Torres A, Garcia‐Vidal C, et al. The value of C‐reactive protein‐to‐lymphocyte ratio in predicting the severity of SARS‐CoV‐2 pneumonia. Arch Bronconeumol. 2020;S0300‐2896:30277‐30285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torzewski J, Heigl F, Zimmermann O. First‐in‐Man: case report of selective C‐reactive protein apheresis in a patient with SARS‐CoV‐2 infection. Am J Case Rep. 2020;21.e925020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92:856‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Tao Z‐W, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID‐19. Ann Clin Microbiol Antimicrob. 2020;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poggiali E, Zaino D, Immovilli P, et al. Lactate dehydrogenase and C‐reactive protein as predictors of respiratory failure in CoVID‐19 patients. Clin Chim Acta. 2020;509:135‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu F, Li L, Xu MD, et al. Prognostic value of interleukin‐6, C‐reactive protein, and procalcitonin in patients with COVID‐19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID‐19 patients reveals IL‐6 and IL‐10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu Z, Cai TZ, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamada T, Wakabayashi M, Yamaji T, et al. Value of leukocytosis and elevated C‐reactive protein in predicting severe coronavirus 2019 (COVID‐19): a systematic review and meta‐analysis. Clin Chim Acta. 2020;509:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahu BR, Kampa RK, Padhi A, Panda AK. C‐reactive protein: a promising biomarker for poor prognosis in COVID‐19 infection. Clin Chim Acta. 2020;509:91‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Groot RJ. Structure, function and evolution of the hemagglutinin‐esterase proteins of corona and toroviruses. Glycoconj J. 2006;23:59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367:1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581:221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res 2015;202:120‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin‐ and caveolae independent endocytic pathway. Cell Res 2008;18:290‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin‐(1‐7) is an endogenous ligand for the G protein‐coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258‐8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167‐176. [DOI] [PubMed] [Google Scholar]
- 40. Kim JM, Heo HS, Ha YM, et al. Mechanism of Ang II involvement in activation of NF‐κB through phosphorylation of p65 during aging. Age (Dordr). 2012;34:11‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott AJ, O'Dea KP, O'Callaghan D, et al. Reactive oxygen species and p38 mitogen‐activated protein kinase mediate tumor necrosis factor α‐converting enzyme(TACE/ADAM‐17) activation in primary human monocytes. J Biol Chem. 2011;286:35466‐35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour‐necrosis factor‐alpha from cells. Nature. 1997;385:729‐733. [DOI] [PubMed] [Google Scholar]
- 43. Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour‐necrosis factor‐alpha. Nature. 1997;385:733‐736. [DOI] [PubMed] [Google Scholar]
- 44. Xu J, Sriramula S, Xia H, et al. Clinical relevance and role of neuronal AT1 receptors in ADAM17‐mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121:43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9:757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang G, Tan Z, Zhou L, et al. Effects of angiotensin II receptor blockers and ACE (Angiotensin‐Converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID‐19 and hypertension: a single‐center retrospective study. Hypertension. 2020;76:51‐58. [DOI] [PubMed] [Google Scholar]
- 47. Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24:21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. C‐reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 2009;8:3885‐3892. [DOI] [PubMed] [Google Scholar]
- 49. Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C‐reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930‐1932. [DOI] [PubMed] [Google Scholar]
- 50. Devaraj S, Singh U, Jialal I. The evolving role of C‐reactive protein in atherothrombosis. Clin Chem. 2009;55:229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C‐reactive protein on human endothelial cells. Circulation. 2000;102:2165‐2168. [DOI] [PubMed] [Google Scholar]
- 52. Calabró P, Chang DW, Willerson JT, Yeh ET. Release of C‐reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112‐1113. [DOI] [PubMed] [Google Scholar]
- 53. Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C‐reactive protein gene by interleukin‐6. J Biol Chem. 1996;271:9503‐9509. [DOI] [PubMed] [Google Scholar]
- 54. Potempa LA, Siegel JN, Fedel BA, Potempa RT, Gewurz H. Expression, detection and assay of a neoantigen (Neo‐CRP) associated with a free, human C‐reactive protein subunit. Mol Immunol. 1987;24:531‐541. [DOI] [PubMed] [Google Scholar]
- 55. Khreiss T, József L, Potempa LA, Filep JG. Opposing effects of C‐reactive protein isoforms on shear‐induced neutrophil‐platelet adhesion and neu‐trophil aggregation in whole blood. Circulation. 2004;110:2713‐2720. [DOI] [PubMed] [Google Scholar]
- 56. Du Clos TW. Pentraxins: structure, function and role in inflammation. ISRN Inflamm. 2013;2013.379040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ji SR, Wu J, Zhu L, et al. Cell membranes and liposomes dissociate C‐reactive protein (CRP) to form new, biologically active structural intermediate: mCRPm. FASEB J. 2007;21:284‐294. [DOI] [PubMed] [Google Scholar]
- 58. Thiele JR, Habersberger J, Braig D, et al. Dissociation of pentameric to monomeric C‐reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti‐inflammatory strategy. Circulation. 2014;130:35‐50. [DOI] [PubMed] [Google Scholar]
- 59. Du Clos TW. Function of C‐reactive protein. Ann Med. 2000;32:274‐278. [DOI] [PubMed] [Google Scholar]
- 60. Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19:503‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mihlan M, Blom AM, Kupreishvili K, et al. Monomeric C‐reactive protein modulates classical complement activation on necrotic cells. FASEB J. 2011;25:4198‐4210. [DOI] [PubMed] [Google Scholar]
- 62. Braig D, Nero TL, Koch HG, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017;8:14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaplan MH, Volanakis JE. Interaction of C‐reactive protein complexes with the complement system I. Consumption of human complement associated with the reaction of C‐reactive protein with pneumococcal C‐polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135‐2147. [PubMed] [Google Scholar]
- 64. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C‐reactive protein in health and disease. J Clin Invest. 1993;91:1351‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kobayashi S, Inoue N, Ohashi Y, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C‐reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398‐1404. [DOI] [PubMed] [Google Scholar]
- 66. Ryu J, Lee CW, Shin JA, et al. FcγRIIa mediates C‐reactive protein‐induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovasc Res. 2007;75:555‐565. [DOI] [PubMed] [Google Scholar]
- 67. Kim Y, Ryu J, Ryu MS, et al. C‐reactive protein induces G2/M phase cell cycle arrest and apoptosis in monocytes through the upregulation of B‐cell translocation gene 2 expression. FEBS Lett. 2014;588:625‐631. [DOI] [PubMed] [Google Scholar]
- 68. Torzewski M, Rist C, Mortensen RF, et al. C‐reactive protein in the arterial intima role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094‐2099. [DOI] [PubMed] [Google Scholar]
- 69. Guyton KZ, Xu Q, Holbrook NJ. Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP‐1 element. Biochem J. 1996;314:547‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blaschke F, Bruemmer D, Yin F, et al. C‐reactive protein induces apoptosis in human coronary vascular smooth mus‐cle cells. Circulation. 2004;110:579‐587. [DOI] [PubMed] [Google Scholar]
- 71. Khreiss T, József L, Hossain S, Chan JS, Potempa LA, Filep JG. Loss of pen‐tameric symmetry of C‐reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem. 2002;277:40775‐40781. [DOI] [PubMed] [Google Scholar]
- 72. Gershov D, Kim S, Brot N, Elkon KB. C‐Reactive protein binds to apop‐totic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response implications for systemic autoimmunity. J Exp Med. 2000;192:1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Devaraj S, Venugopal S, Jialal I. Native pentameric C‐reactive protein displays more potent pro‐atherogenic activities in human aortic endothelial cells than modified C‐reactive protein. Atherosclerosis. 2006;184:48‐52. [DOI] [PubMed] [Google Scholar]
- 74. Verma S, Wang CH, Li SH, et al. A self‐fulfilling prophecy C‐reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913‐919. [DOI] [PubMed] [Google Scholar]
- 75. Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C‐reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439‐1441. [DOI] [PubMed] [Google Scholar]
- 76. Singh U, Devaraj S, Vasquez‐Vivar J, Jialal I. C‐reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43:780‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mosquera‐Sulbaran J, Hernandez‐Fonseca H. 91. Tetracycline and viruses: a possible treatment for COVID‐19? Antiviral Res. 2020:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tilakaratne A, Soory M. Antioxidant response of osteoblasts to doxycycline in an inflammatory model induced by C‐reactive protein and interleukin‐6. Infect Disord ‐ Drug Targets. 2014;14:14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang N, Tian X, Chen Y, et al. Low dose doxycycline decreases systemic inflammation and improves glycemic control, lipid profiles, and islet morphology and function in db/db mice. Sci Rep. 2017;7:14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meijer CA, Le Haen PAA, van Dijk RA. Activator protein‐1 (AP‐1) signalling in human atherosclerosis: results of a systematic evaluation and intervention study. Clin Sci (Lond). 2012;122:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mosorin M, Juvonen J, Biancari F, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double‐blind, placebo‐controlled pilot study. J Vasc Surg. 2001;34:606‐610. [DOI] [PubMed] [Google Scholar]
- 82. Payne JB, Golub LM, Stoner JA, et al. The effect of subantimicrobial‐dose‐doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double‐masked, placebo‐controlled clinical trial. J Am Dent Assoc. 2011;142:262‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jing T, Wang H, Srivenugopal KS, et al. Conditional expression of type 2 angiotensin II receptor in rat vascular smooth muscle cells reveals the interplay of the angiotensin system in matrix metalloproteinase 2 expression and vascular remodeling. Int J Mol Med. 2009;24:103‐110. [DOI] [PubMed] [Google Scholar]
- 84. Santisteban MM, Ahmari N, Carvajal JM, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shi P, Grobe JL, Desland FA, et al. Direct pro‐inflammatory effects of prorenin on microglía. PLoS One. 2014;9.e92937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Febvre‐James M, Lecureur V, Fardel O. Potent repression of C‐reactive protein (CRP) expression by the JAK1/2 inhibitor ruxolitinib in inflammatory human hepatocytes. Inflamm Res. 2020;69:51‐62. [DOI] [PubMed] [Google Scholar]
- 88. Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib, a drug with potential effect to prevent SARS‐COV‐2 from entering target cells and control cytokine storm induced by COVID‐19. Int Immunopharmacol. 2020;86.106749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Keystone EC, Taylor PC, Drescher E, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis. 2015;74:333‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O'Shaughnessy J, DeMichele A, Ma CX, et al. A randomized, double‐blind, phase 2 study of ruxolitinib or placebo in combination with capecitabine in patients with advanced HER2‐negative breast cancer and elevated C‐reactive protein, a marker of systemic inflammation Breast Cancer. Res Treat. 2018;170:547‐557. [DOI] [PubMed] [Google Scholar]
- 91. De Vries LCS, Ludbrook VJ, Hicks KJ, D'Haens GR. GSK2586184, a JAK1 selective inhibitor, in two patients with ulcerative colitis. BMJ Case Rep. 2017;2017:bcr2017221078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Palmas W, Ma S, Psaty B, Goff DC, Darwin C, Graham‐Barr R. Antihypertensive medications and C‐reactive protein in the multi‐ethnic study of atherosclerosis. Am J Hyperten. 2007;20:233‐241. [DOI] [PubMed] [Google Scholar]
- 93. Jenkins NP, Keevil BG, Hutchinson IV, Brooks NH. Beta‐blockers are associated with lower C‐reactive protein concentrations in patients with coronary artery disease. Am J Med. 2002;112:269‐274. [DOI] [PubMed] [Google Scholar]
- 94. Kim SB, Kim SH, Chang JW, et al. Effects of cebecoxib on high‐sensitivity C‐reactive protein in chronic peritoneal dialysis patients. Ren Fail. 2004;26:381‐384. [DOI] [PubMed] [Google Scholar]
- 95. Woodward M, Lowe GD, Francis LM, Rumley A, Cobbe SM. CADET Study Investigators. A randomized comparison of the effects of aspirin and clopidogrel on thrombotic risk factors and C‐reactive protein following myocardial infarction: the CADET trial. J Thromb Haemost. 2004;11:1934‐1940. [DOI] [PubMed] [Google Scholar]
- 96. Koulouris S, Symeonides P, Triantafyllou K, et al. Comparison of the effects of ramipril versus telmisartan in reducing serum levels of high‐sensitivity C‐reactive protein and oxidized low‐density lipoprotein cholesterol in patients with type 2 diabetes mellitus. Am J Cardiol. 2005;95:1386‐1388. [DOI] [PubMed] [Google Scholar]
- 97. Sacks FM. High‐intensity statin treatment for coronary heart disease. J Am Med Assoc. 2004;291:1132‐1134. [DOI] [PubMed] [Google Scholar]
- 98. Devaraj S, Jialal I. Alpha‐tocopherol supplementation decreases serum C‐reactive protein and monocyte interleukin‐6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790‐792. [DOI] [PubMed] [Google Scholar]
- 99. Ruilope LM, Malacco E, Khder Y, Kandra A, Bonner G, Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST Study. Clin Ther. 2005;27:578‐587. [DOI] [PubMed] [Google Scholar]
- 100. Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679‐684. [DOI] [PubMed] [Google Scholar]


