To the Editor:
Primary hemophagocytic lymphohistiocytosis (pHLH) is a frequently fatal disease characterized by excessive cytokine‐mediated immune cell activation associated with homozygous mutations of genes encoding proteins important for perforin‐mediated cytolysis (PRF1, UNC13D, STX11, RAB27A, and STXBP2). 1 Recommended treatment, based on the HLH‐2004 trial, consists of chemotherapy with dexamethasone and etoposide followed by hematopoietic stem cell transplant (HSCT). The estimated 5‐year survival rate is only 59%, 2 supporting a need for alternative approaches. Anticytokine therapy to treat pHLH such as the recently FDA‐approved anti‐interferon‐gamma antibody emapalumab 3 or the previously reported interleukin 1 (IL‐1) receptor antagonist anakinra 4 , 5 , 6 appear to be associated with decreased morbidity and mortality.
Herein we report a patient with pHLH resulting from a homozygous frame‐shift mutation of the pHLH gene encoding syntaxin 11 (STX11). He achieved remission with anakinra monotherapy, but subsequently developed an acute SARS‐CoV‐2 infection responsive to increased anakinra dosing. Excessive proinflammatory cytokine release has been implicated in both pHLH 7 and severe coronavirus disease‐2019 (COVID‐19) infection. 8 , 9 However, the effect of concomitant SARS‐CoV‐2 in children with pHLH has not been reported. 10
A 29‐month‐old North Indian male presented with a 2‐week history of persistent fever, decreased oral intake, and cough. On admission, he was febrile, ill‐appearing, and in moderate respiratory distress, with a large right‐sided pleural effusion and hepatosplenomegaly. He had pancytopenia, hypofibrinogenemia, hypertriglyceridemia, hyperferritinemia, and an elevated D‐dimer (Table S1).
Before a genetic diagnosis and based on a previously described stepwise approach to diagnose and treat pediatric secondary HLH (sHLH), 3 , 5 he was started on anakinra (10 mg/kg/day divided every 6 hours) on day 3 of admission. The soluble IL‐2 receptor alpha chain (sCD25) serum level was noted to be elevated, and he was diagnosed with HLH according to HLH‐04 criteria. 12 There was no evidence of malignancy, rheumatologic disease, or active infections. A SARS‐CoV‐2 PCR swab was negative on day 5.
There was a rapid improvement (Figure 1) and within 1 week, the ferritin decreased by more than 50%, suggestive of an anakinra response, 5 and his fever, hypofibrinogenemia, and thrombocytopenia resolved. Anakinra was weaned to every 8 hours, and he was discharged home. We identified a homozygous frame‐shift mutation of the STX11 gene (p.Gln230Alafs*125). The patient eventually achieved remission on anakinra monotherapy (defined in HLH‐04). 2
FIGURE 1.
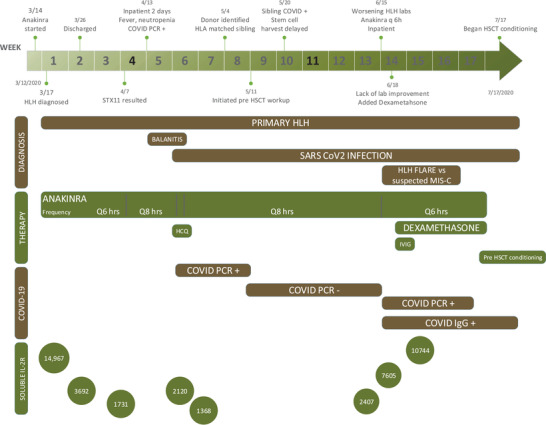
Timeline representing the clinical course, therapy escalation, and weaning based on the patient's clinical response and other important milestones in his disease course from diagnosis to hematopoietic stem cell transplant (HSCT)
While awaiting an HSCT, he had two disease reactivations, 2 which were managed by anakinra escalation. The first febrile episode (neutropenia, elevated sCD25) was 4 weeks later (Table S1). Anakinra was empirically increased to every 6 hours. At this time, he had a positive SARS‐CoV‐2 PCR swab. He responded well, and after 5 days anakinra was weaned to every 8 hours.
Eight weeks after the SARS‐CoV‐2 infection, he again presented with fever, neutropenia, thrombocytopenia, hypertriglyceridemia, and elevated sCD25. Anakinra was increased back to every 6 hours. Due to lack of improvement and concern for multi‐inflammatory syndrome of childhood (MIS‐C), 11 intravenous immunoglobulin and dexamethasone (at 10 mg/m2/day) were added after 72 hours. He responded well to the combination therapy and achieved clinical response after 1 week. He remained in remission on anakinra and dexamethasone until he started HSCT conditioning. He received an HSCT from his HLA‐identical brother who is heterozygous for the STX11 mutation. The patient is currently fully engrafted, has no complications, and continues to remain in remission 3 months after his HSCT.
In the treatment of pHLH, the recent shift toward the use of biologic agents offers promise in disease control with minimal morbidity. 3 While anakinra has shown to be effective in the management of sHLH, malignancy‐associated HLH, 4 , 5 , 13 and macrophage activation syndrome (MAS), 14 , 15 there is only one prior report on its use in pHLH as part of a multiagent regimen. 6
Herein, we highlight the successful management of pHLH using anakinra monotherapy. Our patient achieved disease remission with rapid improvement in clinical and laboratory parameters. Before treatment, the percentage of CD107a+ natural killer cell numbers was low (5%), but the mean channel fluorescence CD107a expression was low normal similar to patients with pHLH and the identical STX11 mutation. 16 Perhaps, this explains his presentation at age 2 versus early infancy.
While awaiting HSCT, he developed an acute SARS‐CoV‐2 infection, which could have exacerbated the pHLH due to increased hyperinflammation. 17 , 18 , 19 However, increasing the frequency of anakinra 14 alone was adequate to mitigate inflammation. Additionally, when he presented with possible MIS‐C, successful breakthrough management was facilitated with anakinra, adjunctive dexamethasone, and immunoglobulin. 11 He remained in remission with this therapy until he underwent successful HSCT.
The standard chemotherapy‐based approach for HLH is suboptimal due to excessive associated morbidity and mortality. The definitive therapy for pHLH is HSCT. However, a suitable stem cell transplant donor search can be time‐consuming. An effective less toxic therapy would permit a safe bridge to HSCT. The successful use of anakinra in this case supports further evaluation of such a bridging therapy in pHLH. A trial with a tiered approach that will introduce less toxic, effective agents as first‐line therapy while reserving more morbid therapies for refractory disease is being planned.
As the use of alternative HSCT donors is becoming increasingly safe, the importance of a less morbid bridging modality cannot be overemphasized. Anakinra and other anticytokine therapies should be evaluated so that the goal of rapid diagnosis‐to‐transplantation can be more effectively realized as the standard of care.
CONFLICT OF INTEREST
Carolyn Fein Levy owns stock in Pfizer. Randy Q. Cron has the following disclosures: Tofacitinib for sJIA Adjudication of MAS Committee Chair, Pfizer, 2017‐present; strategies for transitioning one therapy to another, Consultant, Novartis, Houston, TX, 2019; changing treatment landscape for MAS/sHLH advisory board, SOBI, Atlanta, GA, 2019; “The Cytokine Storm Syndrome: What clinicians need to know” WebMD funded by Sobi, 2020; “A Tale of Two Storms: COVID‐19 and the Cytokine Storm” WebMD funded by Sobi 2020; “COVID‐19 from Cytokine Signature to Cytokine Storm” WebMD funded by Sobi 2020; Co‐Principal Investigator on an ongoing investigator‐initiated clinical trial to study anakinra in a randomized, double‐blinded placebo‐controlled trial for treating hemophagocytic lymphohistiocytosis in children and adults (funded by SOBI).
Supporting information
Supporting Information
Anshul Vagrecha and Hiren B. Patel contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schulert GS, Cron RQ. The genetics of macrophage activation syndrome. Genes Immun. 2020;21(3):169‐181. [DOI] [PubMed] [Google Scholar]
- 2. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long‐term results of the cooperative HLH‐2004 study. Blood. 2017;130(25):2728‐2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811‐1822. [DOI] [PubMed] [Google Scholar]
- 4. Bami S, Vagrecha A, Soberman D, et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2020;67:e28581. [DOI] [PubMed] [Google Scholar]
- 5. Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72(2):326‐334. [DOI] [PubMed] [Google Scholar]
- 6. Kim SR, Kissoon‐Larkin T, Horn B, Elder M. Anakinra as an agent to control hemophagocytic lymphohistiocytosis in Griscelli type 2. Pediatr Blood Cancer. 2019;66(12):e27997. [DOI] [PubMed] [Google Scholar]
- 7. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aricò M, Danesino C, Pende D, Moretta L. Pathogenesis of haemophagocytic lymphohistiocytosis. Br J Haematol. 2001;114(4):761‐769. [DOI] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS‐CoV‐2 and hyperinflammation in pediatric COVID‐19: version 1. Arthritis Rheumatol. 2020;72(11):1791‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henter JI, Horne A, Aricó M, et al. HLH‐2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 13. Divithotawela C, Garrett P, Westall G, Bhaskar B, Tol M, Chambers DC. Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor ‐ anakinra. Respirol Case Rep. 2016;4(1):4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahn PJ, Cron RQ. Higher‐dose anakinra is effective in a case of medically refractory macrophage activation syndrome. J Rheumatol. 2013;40(5):743‐744. [DOI] [PubMed] [Google Scholar]
- 15. Parisi F, Paglionico A, Varriano V, Ferraccioli G, Gremese E. Refractory adult‐onset Still disease complicated by macrophage activation syndrome and acute myocarditis: a case report treated with high doses (8 mg/kg/d) of anakinra. Medicine (Baltimore). 2017;96(24):e6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macartney CA, Weitzman S, Wood SM, et al. Unusual functional manifestations of a novel STX11 frameshift mutation in two infants with familial hemophagocytic lymphohistiocytosis type 4 (FHL4). Pediatr Blood Cancer. 2011;56(4):654‐657. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID‐19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381‐1382. [DOI] [PubMed] [Google Scholar]
- 19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2(7):e393‐e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


