Abstract
Background
No data are available about whether Coronavirus disease 2019 (COVID‐19) pandemic have led to changes in clinical profiles or results of exercise testing once the usual activity was reassumed, as well as if wearing a facemask has any impact on the tests. The aim of this study is to evaluate differences in the patients referred to exercise stress testing in the context of COVID‐19 pandemic and analyse the feasibility and results of these tests wearing a facemask.
Methods
We included all patients referred for an exercise test from 1 June to 30 September 2020 and compared them with the patients attended within the same period in 2019 before and after propensity score matching. All patients referred in 2020 wore a facemask.
Results
A total of 854 patients were included: 398 in the 2020 group and 456 in 2019. No significant differences in baseline characteristics of the patients were observed, with the exception of dyspnoea, which was nearly twice as high in 2020 as compared with 2019. Regarding the results of the tests, no differences were observed, with almost 80% of maximal tests, similar functional capacity and over a 20% of positive exercise tests in both groups. These results remained after propensity score matching.
Conclusion
COVID‐19 pandemic has not changed the clinical profile of patients referred to exercise testing. In addition, performing exercise testing wearing a facemask is feasible, with no influence in functional capacity and clinical results.
Keywords: COVID‐19, exercise testing, facemask, functional capacity, ischaemic cardiomyopathy
1. INTRODUCTION
At the end of 2019, a few cases of pneumonia of suspected viral origin were reported from the Chinese city of Wuhan. 1 A novel RNA virus from the family Coronaviridae 2 called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was identified as the cause of a new disease, COVID‐19. 3 The clinical presentation is quite variable, from no symptoms to severe presentation as acute respiratory distress syndrome and shock. 4 The fact that the disease may be asymptomatic or mildly symptomatic in a large proportion of patients, in addition to the robust capacity for human‐to‐human transmission and long incubation period facilitated the virus spread rapidly all over the world. 5 COVID‐19 led to significant global morbidity and mortality and, as a consequence, on March 11, 2020 it was declared as a pandemic by the World Health Organization (WHO). 6 In the absence of pharmacological treatment or vaccine, the governments adopted measures as social distancing and lock‐down, as well as promoted handwashing and wearing facemasks. 7 In addition, once the rate of COVID‐19 admissions declined, the healthcare systems had to face the enormous challenge of reorganized themselves and return to the normal activity in this novel scenario. 8 , 9 It has been recommended that all patients and health care workers wear a facemask, 10 especially in medical procedures that would be potentially aerosol‐generating, as it is the case of exercise stress testing. The problem is there is no evidence about how this could affect the results of the tests or if the clinical profile of the patients derived to an exercise test have changed during this time. 11
The aim of this study is to evaluate whether, once the normal care activity was reassumed, there are significant differences in the patients referred to exercise stress testing in the context of COVID‐19 pandemic and analyse the feasibility and results of these tests wearing a facemask.
2. METHODS
2.1. Patients
In Spain, it is mandatory to wear a facemask in all public spaces since 19 May, 2020. 12 By that time, the number of COVID‐19 admissions had declined, and we had already resumed our usual activity. For this reason, in this case‐control retrospective study we included all consecutive individuals aged 18 years or older who were referred for exercise echocardiography or exercise electrocardiogram tests to Complexo Hospitalario Universitario de A Coruña (A Coruña, Spain) from 1 June to 30 September 2020 and were compared with the patients attended within the same period in 2019. The facemasks (surgical or FFP2) had to cover the nose and mouth and were worn during the entire tests. All patients were asymptomatic, afebrile and without clinical suspicion of COVID‐19 disease.
This study was approved by the Comité de Ética de la Investigación de A Coruña‐Ferrol, our local research ethics committee.
Reporting of the study conforms to broad EQUATOR guidelines 13
2.2. Clinical data
Demographics, clinical variables and stress testing results were recorded in dedicated databases prospectively. A history of coronary artery disease (CAD) was defined as previous myocardial infarction as well as unstable and stable angina. Coronary revascularization was defined as previous percutaneous or surgical revascularization. Chest pain was classified as typical angina, probable angina and non‐ischaemic chest pain as previously described. 14 Whenever possible, ß‐blocker or other negative chronotropic therapies were discontinued for at least 48 hours before testing.
2.3. Exercise stress testing
All patients underwent exercise stress testing on a treadmill. Blood pressure, heart rate and serial electrocardiograms were obtained at baseline and at each stage of the exercise protocol. An exercise electrocardiogram was considered positive in cases of horizontal or downsloping ST‐segment depression or elevation ≥ 1 mm at 80 ms after the J point, in patients with interpretable baseline electrocardiograms. The electrocardiograms were considered uninterpretable in the presence of left bundle branch block, pre‐excitation, paced rhythm, other repolarization abnormalities or treatment with digoxin. 15
Exercise echocardiography was performed by experienced echocardiographers and both, the practitioner and the nurse, wore a facemask (surgical or FFP2) during the entire test. Echocardiography images were acquired in three apical views (long axis, 4‐ and 2‐chambers) and two parasternal views (long and short axis) at baseline, at peak of exercise, and in the immediate post‐exercise period, as previously described. 16 Regional wall motion abnormalities were evaluated with a 16‐segment model of the left ventricle. Each segment was graded on a 4‐point scale (1 = normal wall motion, 2 = hypokinetic, 3 = akinetic and 4 = dyskinetic), and wall motion score index (WMSI) was calculated as the sum of scores divided by the number of segments visualized. The worst WMSI obtained at peak or post‐exercise imaging was recorded. Ischaemia was defined as the development of new or worsening wall motion abnormalities with exercise. Extension of myocardial ischaemia was classified based on the number of segments involved as localized (≤2 ischaemic segments) and extensive (>2 ischaemic segments).
A maximal test was defined as the achievement of at least 85% of the mean age predicted heart rate, otherwise the test was considered submaximal.
A positive exercise stress test was defined as either positive exercise electrocardiography or positive exercise echocardiography.
Functional capacity was expressed in metabolic equivalents (METs). Predicted peak METs where calculated using the Veterans Affairs referral model in men (predicted METs = 18 − 0.15 × age) and with the St. James model in women (predicted METs = 14.7 − 0.13 × age). 17 We calculated the ratio: (METs achieved/predicted METs) x 100. Patients achieving less than 100% of their age and gender predicted METs were classified as having exercise intolerance.
2.4. Statistical analysis
Categorical variables were reported as percentages and differences were evaluated with the chi‐square test and Fisher's exact test as appropriate. Continuous variables were expressed as mean ± standard deviation and were compared with the Student t test.
To assess the potential effect of wearing facemasks during exercise testing on estimated functional capacity, we used propensity score matching to adjust for differences in baseline characteristics with a matching ratio of 1:1 using nearest neighbour matching without replacement. The criterion for matching pairs used a calliper of width equal to 0.2 of the standard deviation of the logit of the propensity score. To assess the degree of the balance between the two matched groups, a standardized difference was computed for each explanatory factor, with a value of less than 0.1 indicating a good balance in the matched cohort. The matching was carried out with the following variables the basis of their clinical relevance: age, sex, personal history of diabetes mellitus, arterial hypertension, smoking, family and personal history of CAD, coronary revascularization, left bundle branch block, atrial fibrillation, typical angina, dyspnoea, beta‐blockers, nitrates, calcium channel blockers and renin–angiotensin‐aldosterone system blockers. In the propensity‐matched cohort, differences in proportions were compared using McNemar's test for correlated binary proportions, and differences in continuous variables were examined using the Wilcoxon signed ranks test.
Logistic regression was used to evaluate the association of facemask use with positivity of the tests, while lineal regression was employed to examine its association with exercise workload. We tested for interactions to assess whether these associations differed by the type of test performed.
Statistical analyses were performed using STATA® v.13 (STATA Corp., Texas, EE. UU.) and R version 4.0.3. A two‐sided P <.05 was considered to indicate statistical significance.
3. RESULTS
3.1. Baseline characteristics
In total, 854 patients were included: 398 between 1 June and 30 September of 2020 wearing a facemask and 456 in the same period of 2019 without a facemask. Table 1 shows the baseline characteristics of the patients. There was no significant difference in age, sex and cardiovascular risk factors. Personal history of CAD, coronary revascularization and treatment were also similar between groups. Exercise echocardiography was the most frequent test (62.3% in 2019% vs 60.3% in 2020; P = .5) and there were no significant differences in the proportion of patients derived from the emergency unit (34.2% vs 28.6% respectively, P = .08). Although the main purpose for testing was non‐ischaemic chest pain, with no difference between groups, dyspnoea nearly doubled the indication for testing in 2020 (12.7% vs 20.6%, P < .05).
TABLE 1.
Baseline characteristics of the patients according to the time period and facemask use before and after propensity score matching
| Before adjusting for propensity score | After adjusting for propensity score | ||||||
|---|---|---|---|---|---|---|---|
| 2019 (without facemask) (n = 456) | 2020 (with facemask) (n = 398) | P | Standardized differences | 2019 (without facemask) (n = 318) | 2019 (without facemask) (n = 318) | Standardized differences | |
| Age (y) | 63.1 ± 13.4 | 64 ± 13.7 | .29 | 0.07 | 63.7 ± 13.4 | 63.1 ± 14.2 | 0.05 |
| Male, n (%) | 264 (57.9) | 227 (57) | .8 | 0.02 | 180 (56.7) | 181 (56.9) | 0.01 |
| Smoking, n (%) | 133 (29.2) | 101 (25.4) | .22 | 0.09 | 79 (24.8) | 82 (25.8) | 0.02 |
| Diabetes Mellitus, n (%) | 75 (16.5) | 59 (14.8) | .52 | 0.04 | 56 (17.6) | 46 (14.5) | 0.09 |
| Hypertension, n (%) | 216 (47.4) | 196 (49.3) | .58 | 0.04 | 151 (47.5) | 152 (47.8) | 0.01 |
| Hypercholesterolemia, n (%) | 234 (51.3) | 213 (53.5) | .52 | 0.04 | 165 (51.9) | 161 (50.6) | 0.03 |
| Family history of CAD, n (%) | 24 (5.3) | 23 (5.8) | .74 | 0.02 | 18 (5.7) | 21 (6.6) | 0.04 |
| History of CAD, n (%) | 82 (18) | 66 (16.6) | .59 | 0.04 | 49 (15.4) | 52 (16.4) | 0.03 |
| Coronary revascularization, n (%) | 63 (13.8) | 64 (16.1) | .21 | 0.06 | 44 (13.8) | 47 (14.8) | 0.03 |
| Atrial fibrillation, n (%) | 20 (4.4) | 25 (6.3) | .22 | 0.08 | 17 (5.3) | 16 (5) | 0.01 |
| LBBB, n (%) | 11 (2.4) | 11 (2.8) | .75 | 0.02 | 8 (2.5) | 8 (2.5) | 0 |
| Typical angina, n (%) | 47 (10.3) | 49 (12.3) | .36 | 0.06 | 34 (10.7) | 39 (12.3) | 0.05 |
| Dyspnoea, n (%) | 57 (12.5) | 82 (20.6) | .001 | 0.22 | 48 (15.1) | 46 (14.5) | 0.02 |
| Beta‐blockers, n (%) | 87 (19.1) | 87 (21.9) | .31 | 0.07 | 66 (20.8) | 65 (20.4) | 0.01 |
| Verapamil/diltiazem, n (%) | 5 (1.1) | 2 (0.5) | .46 | 0.06 | 4 (1.3) | 2 (0.6) | 0.07 |
| Nitrates, n (%) | 26 (5.7) | 20 (5) | .66 | 0.03 | 18 (5.7) | 16 (5) | 0.03 |
| ACE‐i/ARB, n (%) | 142 (31.1) | 161 (40.5) | .005 | 0.2 | 113 (35.5) | 116 (36.5) | 0.02 |
Abbreviations: ACE‐i, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CAD, coronary artery disease; LBBB, left bundle branch block; n, number.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Exercise stress testing results
The Bruce protocol was employed in most of the patients (83.4%), and there were no complications during the tests. Physical exhaustion was the most common indication for termination of exercise (91.5% in 2019% vs 90.7% in 2020, P = .7) and a similar percentage of patients presented exercise‐induced chest pain (9.2% vs 9.8% respectively, P = .77). Table 2 shows the tests results obtained in both groups. There were no significant differences in haemodynamic data except for peak systolic blood pressure and, consequently, in peak rate pressure product, higher in the non‐facemask group. Almost 80% of the tests were maximal, with no significant differences in both groups (79% in 2019% vs 77.1% in 2020, P = .52).
TABLE 2.
Results of exercise tests according to the time period and facemask use
|
2019 (without facemask) n = 456 |
2020 (with facemask) n = 398 |
P | P after matching | |
|---|---|---|---|---|
| Resting systolic blood pressure, mm Hg | 131 ± 21 | 131 ± 20 | .89 | .3 |
| Peak systolic blood pressure, mm Hg | 187 ± 27 | 181 ± 29 | <.001 | .007 |
| Resting heart rate, bpm | 76 ± 15 | 77 ± 15 | .47 | .45 |
| Peak heart rate, bpm | 147 ± 24 | 147 ± 23 | .94 | .57 |
| Resting rate pressure product, ×103 mm Hg bpm | 10.1 ± 2.8 | 10.1 ± 2.7 | .65 | .93 |
| Peak rate pressure product, ×103 mm Hg bpm | 27.7 ± 6.4 | 26.8 ± 6.4 | .04 | .21 |
| Resting E/e’ septal | 12.5 ± 6.1 | 11.9 ± 5.1 | .4 | .62 |
| Peak E/e’ septal | 11.8 ± 4.3 | 12.1 ± 4.8 | .6 | .79 |
| Exercise workload, METs | 9.7 ± 3.7 | 9.5 ± 3.5 | .4 | .93 |
| Exercise workload predicted, METs | 7.8 ± 2.2 | 7.7 ± 2.3 | .31 | .57 |
| Positive Exercise stress test, n (%) | 93 (20.4) | 92 (23.1) | .34 | .49 |
| Positive echo criteria | 58 (22.8) | 45 (22.1) | .8 | .83 |
| Resting left ventricular ejection fraction, % | 59.5 ± 7 | 60.1 ± 5.4 | .28 | .47 |
| Peak left ventricular ejection fraction, % | 64.3 ± 11.3 | 65.3 ± 10.4 | .27 | .48 |
| Resting wall motion score index | 1.1 ± 0.2 | 1 ± 0.1 | .53 | .66 |
| Peak wall motion score index | 1.2 ± 0.3 | 1.2 ± 0.2 | .66 | .63 |
Abbreviations: bpm, beats per minute; MET, metabolic equivalent; n, number.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Over 20% of the patients had a positive exercise testing, without differences between groups. When considering the test performed, a similar proportion of positive exercise echocardiography (31% vs 33.8, P = .5) and exercise electrocardiography (2.9% vs 6.9%, P = .09) was observed. The severity of exercise‐induced wall motion abnormalities was similar, with almost two‐thirds of the positive echocardiograms showing extensive ischaemia (74.7% in 2019% vs 77.9% in 2020, P = .65).
Regarding functional capacity, the workload reached was similar in both groups. A percentage of predicted METs > 100% was observed in near 75% of the patients, although it was more frequent in women (87% vs 83%, P = .29) than in men (67.8% vs. 66.1%, P = .69).
After adjusting for propensity score, 318 patients remained in each group. Both groups were well balanced (Table 1). There were no significant differences in METs achieved (Figure 1), even after stratification by exercise test modality: 10.2 ± 4 vs 10.7 ± 3.6, P = .21 in exercise electrocardiography and 9.1 ± 3.4 vs 8.7 ± 3.3, P = .22 in exercise echocardiography. Figure 2 shows the proportion of positive exercise tests, with no significant differences between wearing a facemask or not. No association was observed between wearing facemask and METS achieved (P = .99) or proportion of positive exercise tests (P = .59). Finally, in a multivariate analysis we observed no interaction between the modality of exercise test and the use of facemask with the METs achieved (P = .26) or the proportion of positive exercise tests (P = .53).
FIGURE 1.
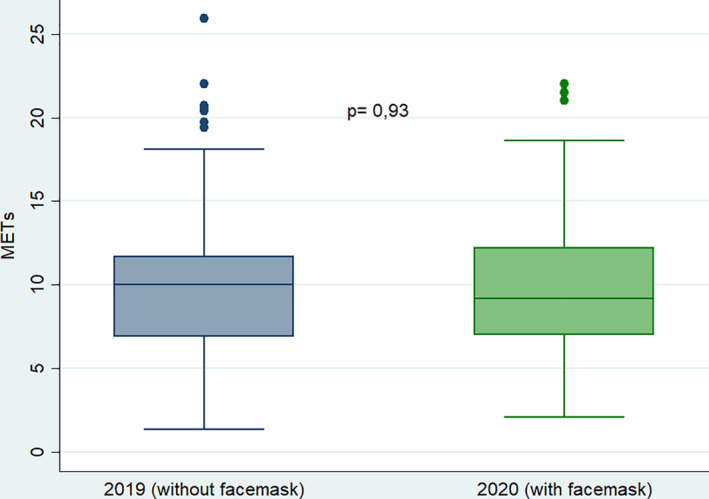
Box‐plot graph representing the functional capacity of both groups, expressed in metabolic equivalents (METs)
FIGURE 2.
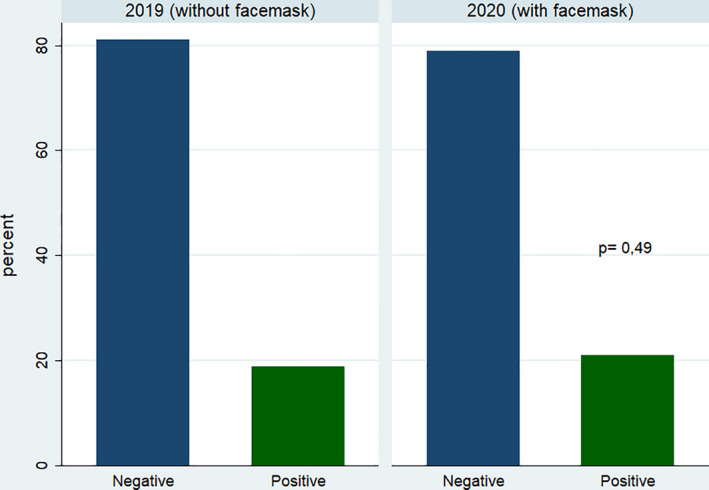
Bar chart representing the proportion of positive and negative exercise tests in both groups
4. DISCUSSION
To our knowledge, this is the first study that evaluates the clinical profile and the results of the patients referred to exercise testing in the new clinical scenario of COVID‐19 pandemic once the usual activity was reassumed.
Despite the global impact and changes that the COVID‐19 pandemic has entailed in medical care and specifically in cardiovascular imaging units, our study shows no significant differences in baseline characteristics of patients referred for exercise stress testing and the results of the tests were comparable to the same period in 2019, except for a significant increase in patients referred for dyspnoea in the 2020 group. We think this finding can be explained by the fact of wearing a facemask, mandatory in our country since the end of May. Wearing a facemask has been associated with discomfort and difficulty in breathing 18 which could have led to an increase of medical consultation for this reason. The discomfort has been related to neurological reactions due to increase temperature of the inspired air or associated psychological factors such as claustrophobia and anxiety caused by the facemask. 19 Other fact that could have influenced was the consensus document published by the Heart Failure Association of the European Society of Cardiology in 2019 that includes exercise echocardiography as part of the diagnostic algorithm of heart failure with preserve ejection fraction. 20
The main findings of our study were, first, to confirm that there were no differences in the proportion of positive exercise testing between both groups, with a rate of positive tests that is in concordance with the rates currently observed. 21 During the peak of the COVID‐19 pandemic in early 2020, most of non‐urgent medical attention and diagnostic test were deferred to reduce SARS‐CoV‐2 transmission among patients and health care workers, following the scientific societies recommendations. 22 , 23 In addition, a marked decline in acute cardiovascular hospitalizations was observed 24 with a worrying near 50% reduction in ST‐segment elevation myocardial infarction (STEMI) admission. 25 Because of this, it would have been reasonable to think that the reduced access to diagnostic tests would lead to a high burden of undiagnosed cardiovascular disease and a rebound of clinical activity at the expense of urgent testing. The period of our study started once the rate of new COVID‐19 admissions and deaths had declined in our area and we had already resumed our usual care activity. However, we have not observed an increase in test requests made from the emergency department, nor a greater positivity or severity in the results.
The other significant finding was to verify the feasibility of performing exercise testing with facemask, with no differences in the proportion of maximal testing or in functional capacity, considering the METs achieved. Conventional routes of transmission of SARS‐CoV‐2 consist of respiratory aerosols, droplets and direct contact 26 so, wearing of facemask and hand hygiene have become the main measures to mitigate the disease transmission. 27 Exercise tests are considered potentially aerosol‐generating procedures, because of deep breathing and/or coughing during exercise. Due to this potential for aerosol generation, all patients and healthcare workers should consider wearing surgical facemasks as a minimum requirement. 22 If available, to perform rapid antigen tests for SARS‐CoV‐2 to each patient before the exercise test could be a good approach to reduce transmission in addition to the other requirements. During the time of our study, the rapid test were not available at our hospital, but all the physicians that performed the exercise tests followed the personal protective recommendations 10 and no infection by SARS‐CoV‐2 was detected in no member of our team in the periodic serologic tests performed.
Regarding functional capacity, in a recent study, Fikenzer et al observed a reduction in cardiopulmonary exercise capacity in 12 healthy volunteers. Each participant performed three incremental exertion cardiopulmonary exercise tests, one without mask, one with surgical mask and one with FFP2/N95 mask, showing a significant reduction in functional lung parameters as well as maximum oxygen consumption in both facemask groups. 28 However, it appears that there could be inconsistencies in the reported data, suggesting that measurement errors and not wearing facemask affected the results. 29 Another study compared the exercise capacity with and without facemask during a 6 minute walking test, with no differences in walked distance but with an increased in perceived dyspnoea. 30
4.1. Limitations
Our study has several limitations that must be considered for its interpretation. Firstly, the limitations inherent to an observational single‐centre study. Although propensity score analysis was applied, we cannot rule out the possibility that referral bias or local changes in clinical management might account for at least part of the results.
Secondly, maybe the period of the study is too short to detect differences in clinical profile of the patients referred for exercise testing, although we think that it is representative of what happened in the cardiovascular imaging laboratories once the normal activity was reassumed. In our hospital, the use of alternative non‐invasive imaging modalities for the detection of coronary artery disease in patients capable of exercising is limited, so referral for exercise echocardiography might be considered a surrogate for referral of these patients for non‐invasive imaging techniques.
Finally, all tests were performed using at least surgical facemask, but we do not know the number of patients who wore a surgical facemask or FFP2/N95 face mask. However, no differences were observed in the functional capacity regarding the modality of facemask in another study 28 and we think that our population is representative of current clinical practice.
5. CONCLUSION
COVID‐19 pandemic has not changed the clinical profile of patients referred to exercise testing comparing with the same period in 2019. In addition, we conclude that performing an exercise testing wearing a facemask is feasible, with no differences in functional capacity and clinical results.
CONFLICT OF INTEREST
Nothing to disclosure.
AUTHOR CONTRIBUTIONS
CBC, ABM and JP contributed to the conception or design of the work, analysis, interpretation of data for the work and drafted the manuscript. DLV, MQG, AVC, DMR, JYW, MPP and JMVR contributed to analysis, interpretation of data and critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Barbeito‐Caamaño C, Bouzas‐Mosquera A, Peteiro J, et al. Exercise testing in COVID‐19 era: Clinical profile, results and feasibility wearing a facemask. Eur J Clin Invest. 2021;51:e13509. 10.1111/eci.13509
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phan LT, Nguyen TV, Luong QC, et al. Importation and human‐to‐human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872‐874. 10.1056/NEJMc2001272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. OMS . WHO Director‐General’s opening remarks at the media briefing on COVID‐19 ‐ 11 March 2020. WHO Dir Gen speeches [Internet]. 2020 [cited 2020 Nov 15];(March):4. https://www.who.int/director‐general/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19–‐11‐march‐2020. Accessed November 30, 2020.
- 7. MacIntyre CR, Wang Q. Physical distancing, face masks, and eye protection for prevention of COVID‐19. Lancet. 2020;395(10242):1950‐1951. https://linkinghub.elsevier.com/retrieve/pii/S0140673620311831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. https://linkinghub.elsevier.com/retrieve/pii/S0735109720346374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiménez‐Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID‐19. Cardiology. 2020;145(8):481‐484. https://www.karger.com/Article/FullText/509181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hung J, Abraham TP, Cohen MS, et al. ASE statement on the reintroduction of echocardiographic services during the COVID‐19 pandemic. J Am Soc Echocardiogr. 2020;33(8):1034‐1039. https://linkinghub.elsevier.com/retrieve/pii/S0894731720303138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fersia O, Bryant S, Nicholson R, et al. The impact of the COVID‐19 pandemic on cardiology services. Open Hear. 2020;7(2):e001359. 10.1136/openhrt-2020-001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobierno de España . Boletón Oficial del Estado. Boletín Of del Estado no142. 2020;61561‐61567.
- 13. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 14. Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1(2):574‐575. https://linkinghub.elsevier.com/retrieve/pii/S073510978380093X [DOI] [PubMed] [Google Scholar]
- 15. Bouzas‐Mosquera A, Peteiro J, Broullón FJ, et al. Impact of electrocardiographic interpretability on outcome in patients referred for stress testing. Eur J Clin Invest. 2012;42(5):541‐547. 10.1111/j.1365-2362.2011.02615.x [DOI] [PubMed] [Google Scholar]
- 16. Peteiro J, Bouzas‐Mosquera A, Broullon FJ, Garcia‐Campos A, Pazos P, Castro‐Beiras A. Prognostic value of peak and post‐exercise treadmill exercise echocardiography in patients with known or suspected coronary artery disease. Eur Heart J. 2010;31(2):187‐195. 10.1093/eurheartj/ehp427 [DOI] [PubMed] [Google Scholar]
- 17. Kim ESH, Ishwaran H, Blackstone E, Lauer MS. External prognostic validations and comparisons of age‐ and gender‐adjusted exercise capacity predictions. J Am Coll Cardiol. 2007;50(19):1867‐1875. https://linkinghub.elsevier.com/retrieve/pii/S0735109707025697 [DOI] [PubMed] [Google Scholar]
- 18. Roberge RJ, Kim J‐H, Benson SM. Absence of consequential changes in physiological, thermal and subjective responses from wearing a surgical mask. Respir Physiol Neurobiol. 2012;181(1):29‐35. 10.1016/j.resp.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 19. Samannan R, Holt G, Calderon‐Candelario R, Mirsaeidi M, Campos M. Effect of face masks on gas exchange in healthy persons and patients with COPD. Ann Am Thorac Soc. 2020. 10.1513/AnnalsATS.202007-812RL [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pieske B, Tschöpe C, De Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297‐3317. [DOI] [PubMed] [Google Scholar]
- 21. Bouzas‐Mosquera A, Peteiro J, et al. Trends in referral patterns, invasive management, and mortality in elderly patients referred for exercise stress testing. Eur J Intern Med. 2015;26(10):787‐791. 10.1016/j.ejim.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 22. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr. 2020;33(6):648‐653. https://linkinghub.elsevier.com/retrieve/pii/S0894731720302091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han Y, Zeng H, Jiang H, et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID‐19 epidemic. Circulation. 2020;141(20):E810‐E816. 10.1161/CIRCULATIONAHA.120.047011 [DOI] [PubMed] [Google Scholar]
- 24. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;76(3):280‐288. https://linkinghub.elsevier.com/retrieve/pii/S0735109720353936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez‐Leor O, Cid‐Álvarez B, Ojeda S, et al. Impact of the COVID‐19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82‐89. https://www.recintervcardiol.org/en/?option=com_content&view=article&id=355&catid=61 [Google Scholar]
- 26. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asadi S, Cappa CD, Barreda S, et al. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10(1):15665. 10.1038/s41598-020-72798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fikenzer S, Uhe T, Lavall D, et al. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin Res Cardiol. 2020;109(12):1522‐1530. 10.1007/s00392-020-01704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hopkins SR, Stickland MK, Schoene RB, Swenson ER, Luks AM. Effects of surgical and FFP2, N95 face masks on cardiopulmonary exercise capacity: the numbers do not add up. Clin Res Cardiol. 2020;109(12):1605‐1606. 10.1007/s00392-020-01748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Person E, Lemercier C, Royer A, Reychler G. Effet du port d’un masque de soins lors d’un test de marche de six minutes chez des sujets sains. Rev Mal Respir. 2018;35(3):264‐268. 10.1016/j.rmr.2017.01.010 [DOI] [PubMed] [Google Scholar]


