Abstract
Ecological restoration should be regarded as a public health service. Unfortunately, the lack of quantitative linkages between environmental and human health has limited recognition of this principle. The advent of the COVID‐19 pandemic provides the impetus for further discussion. We propose ecological countermeasures as highly targeted, landscape‐based interventions to arrest the drivers of land use‐induced zoonotic spillover. We provide examples of ecological restoration activities that reduce zoonotic disease risk and a five‐point action plan at the human‐ecosystem health nexus. In conclusion, we make the case that ecological countermeasures are a tenet of restoration ecology with human health goals.
Keywords: ecological countermeasures, invasive alien species, land use‐induced spillover, landscape immunity, restoration ecology, zoonotic disease
Implications for Practice.
Ecosystem health directly affects human health and ecological restoration should, therefore, be regarded as a public health service.
Ecological countermeasures can be employed to prevent land use‐induced zoonotic spillover by fostering landscape immunity and reducing the risk of human exposure to wildlife‐transmitted pathogens.
Invasive alien species removal and the reintroduction of native plants are ecological countermeasures when undertaken to address zoonotic disease risks.
Interdisciplinary collaboration, mechanistic studies of land use‐induced spillover, the integration of ecological and health targets in policy frameworks, increases in zoonotic pathogen surveillance, and community engagement will help advance ecological countermeasures.
Restoration ecologists can promote the linkages between ecological and human health within the One Health and planetary health frameworks.
Introduction
Ecosystem health directly affects human health (Patz et al. 2004; Andrade et al. 2020) and should, therefore, serve as a powerful incentive for ecological restoration (Aronson et al. 2016). A growing interest in One Health (Gibbs 2014) and planetary health (Seltenrich 2018) initiatives (see Supplement S1) demonstrates that scientists and policymakers increasingly recognize that human and environmental condition are co‐regulators; human society is an aspect and influencer of ecological systems. Considerable work remains, however, before human health is fully regarded as an ecological service (Patz et al. 2004; Reaser et al. 2021). Breed et al. (2020) identify the lack of quantitative linkages between environmental and human health as a principal knowledge gap that limits understanding of ecological restoration as a public health service. They propose a five‐point action plan to elucidate ecological‐human health links and firmly establish the ecological restoration‐human health nexus. Elements of the action plan include collaborations and conversations, education and learning, defining causal links, monitoring restoration and health outcomes, and community ownership and stewardship. Later in this article, we build on this action plan from the perspective of zoonotic disease prevention.
Advent of the COVID‐19 pandemic (SARS‐CoV‐2 virus) provides impetus for further discussion and operationalization of ecological approaches to protecting human health. More than 70% of emerging zoonoses, infectious diseases that are transmitted from animals to humans, originate in wildlife (Jones et al. 2008). Although attention has rightfully been given to wildlife consumption and commerce as contributors to the risk of human exposure to zoonotic pathogens, it is important to note that wildlife trade is rooted in land use change (Can et al. 2019; Kolby 2020). Land use change is usually the primary driver of pathogen transmission from wildlife to humans (Patz et al. 2004; Gottdenker et al. 2014), a process known as zoonotic pathogen spillover (Plowright et al. 2017). This misunderstanding of ecologically originating processes and the realization that insufficient attention is being given to landscape management measures for pandemic prevention inspired Plowright et al. (2021) to call on biological and social scientists to investigate the mechanisms by which land use change drives zoonotic spillover into human populations (termed “land use‐induced spillover”), Reaser et al. (2021) to propose fostering landscape immunity (the ecological conditions that, in combination, keep pathogen populations in check and foster the immunological defenses of wild species within a particular ecosystem) as an approach to reducing spillover risk, and Reaser et al. (2021) to recommend priority actions for employing protected areas to safeguard human populations from future pandemics. Here, we expand on this new body of work by focusing on “ecological countermeasures” as a novel concept and technical approach to addressing land use‐induced spillover. Ecological countermeasures offer a social‐ecological approach to restoration ecology in which landscape‐focused interventions are motivated by the need to address a public health priority and are, ideally, implemented consistent with the eight principles that underpin ecological restoration (Gann et al. 2019; Table 1). Ecological countermeasures are complementary to the regulatory reforms need to improve biosecurity, including wildlife trade and other pathways of zoonotic pathogen spread.
Table 1.
The application of the principles underpinning ecological restoration (Gann et al. 2019) to ecological countermeasure performance standards. aPerformance standard feasibility needs to be considered contextually. Since every situation is unique, these standards are meant to serve as best management practice benchmarks rather than evaluation criteria for cross‐project comparison.
| Ecological Restoration Principle | Recommended Performance Standardsa |
|---|---|
| 1. Engages stakeholders |
* Project motivated by need to protect human health * Local community is made aware of the project need, Intent, and implications * Baseline data collection employs citizen science * Plan development and review involves public input * Project feasibility analysis evaluates community acceptance and recommends acceptable measures * Implementation, monitoring, and adaptive management are stakeholder inclusive * Local community has long‐term role as ecological/health stewards |
| 2. Draws on many types of knowledge |
* Baseline data addresse biological, ecological, geophysical, and social science parameters * Plan considers Traditional Ecological Knowledge, Local Ecological Knowledge, and other community‐specific factors * Observations and knowledge are routinely captured and integrated for adaptive management, utilizing peer‐learning networks and practitioner‐researcher collaborations |
|
3. Informed by native ecosystems, while considering environment change |
* Native community assessed, with emphasis on the dynamics of relevant microbes, vertebrate hosts, land use change and associated ecological conditions, and human activity patterns * Landscape immunity measures and goals considered from a wide range of spatio‐temporal perspectives |
| 4. Supports ecosystem recovery |
* Addresses key land use change drivers * Restores and maintains landscape immunity * Ecological structure and function fostering landscape immunity becomes self‐regulating |
| 5. Assessed against clear goals and objectives, using measurable indicators |
* Considers interventions that arrest zoonotic pathogen infection, shedding, and/or spillover by restoring ecological structure and function to achieve landscape immunity * Measures and monitors wildlife stress‐related/immunological biomarkers * Measures and monitors zoonotic pathogen prevalence and exposure/infection intensity * Measures and monitors human and domestic animal host proximity to wildlife hosts |
| 6. Seeks the highest level of recovery attainable |
* Goal is to recover and maintain landscape immunity by reestablishing ecosystem structure and function * Ultimately, project succeeds in preventing land use‐induced spillover |
| 7. Gains cumulative value when applied at large scales | * Reduces risk of disease outbreaks from local to pandemic scales |
| 8. Is part of a continuum of restoration activities | * Meets, complements, and provides return on investment for restoration activities with explicit conservation and/or sustainable development goals |
Countermeasures are generally regarded as actions taken to counteract a threat (Dictionary.com). In the military context, countermeasures involve the employment of devices and/or techniques to impair the operational effectiveness of enemy activity (DOD 2020). Medical countermeasures constitute life‐saving medicines and medical supplies used to diagnose, prevent, or treat conditions associated with chemical, biological, radiological, or nuclear (CBRN) threats, emerging infectious diseases, or natural disasters (https://www.cdc.gov/cpr/readiness/mcm.html). From an environmental perspective, countermeasures typically refer to site remediation and restoration activities undertaken to address contaminants (e.g. Fesenko & Howard 2012; Shuangchen et al. 2017).
For zoonotic disease outbreaks, countermeasures have largely focused on medical and veterinary interventions (Sokolow et al. 2019). We define ecological countermeasures as highly targeted, landscape‐based interventions to arrest one or more of the elements of land use‐induced spillover, particularly the environmental stressors that: (1) trigger increased exposure and susceptibility of wildlife to pathogen infection; (2) cause these animal hosts to shed viable pathogens in sufficient quantity to spill over to (infect) other susceptible hosts, including humans; and (3) then spread through the human population (‘the infect‐shed‐spill‐spread’ cascade; Plowright et al. 2021; Figs. 1 & 2 therein). Ideally, ecological countermeasures would be used to restore landscape immunity and/or reduce human exposure to wildlife‐transmitted pathogens (Fig. 1; see contextual overview in Reaser et al. 2021).
Figure 1.
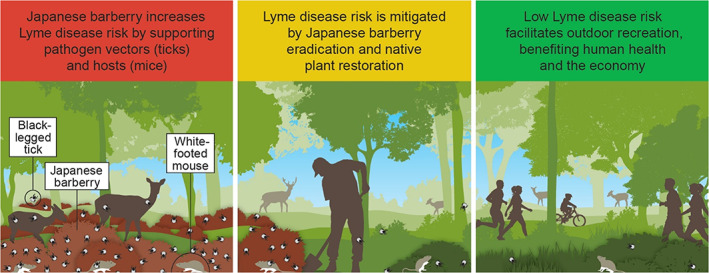
Ecological countermeasure for Lyme disease.
We provide a short list of geographically and taxonomically diverse examples of ecological restoration activities that reduce zoonotic disease risk and apply ecological countermeasure principles and practices to Breed et al.'s (2020) five‐point action plan. The case studies presented include measures to: (1) remove or otherwise control invasive alien plants and animals that magnify spillover risks; and (2) reintroduce or increase populations of native species to re‐establish habitat resources and trophic structure, thereby controlling pathogen prevalence and distribution.
Case Studies
Invasive alien plants may provide optimal habitat for zoonotic pathogens, hosts, and vectors; they tend to have long flowering durations, vigorous growth, and increase biomass as they spread, particularly in disturbed sites (Stone et al. 2018). The large‐scale removal of invasive alien plants that facilitate zoonotic spillover (e.g. via microclimate or trophic changes) can function as an ecological countermeasure when the goal is disease risk mitigation. In aquatic environments, there is a clear link between invasive alien plants, water stagnation, and the prevalence of mosquito‐borne diseases. Upon reviewing relevant literature, Stone et al. (2018) concluded that the control of invasive alien plants in aquatic environments could contribute to malaria risk mitigation. They highlight research priorities to integrate vector and invasive alien plant management in a synergistic fashion.
Similar opportunities are being identified for terrestrial environments, especially for tick‐borne disease management. Japanese barberry (Berberis thunbergii), a woody understory shrub, was introduced to the United States from Asia in 1875 for ornamental landscaping. It now invades a wide range of natural areas throughout much of the United States and eastern Canada (USDA 2020). Japanese barberry benefits at least two species that contribute to Lyme disease (Borrelia burgdorferi) spillover. Barberry infestations foster microclimates favorable to the proliferation of blacklegged ticks (Ixodes scapularis), a species known to transmit several zoonotic pathogens (Williams & Ward 2010) and nesting areas for white‐footed mice (Peromyscus leucopus), as well as other rodents that host B. burgdorferi (Linske et al. 2018). In barberry removal experiments, Williams and Ward (2010) found that intact barberry stands had 280 ± 51 adult blacklegged ticks/ha, which was significantly higher than for controlled (121 ± 17/ha) and no barberry (30 ± 10/ha) areas. Linske et al. (2018) found that management of barberry stands reduced contact opportunities between blacklegged ticks and white‐footed mice. They encouraged the eradication and control of the invasive shrub to reduce the number of B. burgdorferi‐infected blacklegged ticks.
Numerous animals that host or vector zoonotic pathogens have become widespread invasive alien species. Of these, various rodent species are among the highest risk invasive hosts, while several species of mosquitoes and ticks pose the greatest concern as invasive vectors capable of facilitating large‐scale disease outbreaks (Chinchio et al. 2020). However, lessor‐known animal species can also facilitate disease outbreaks of epidemic and pandemic proportions. Schistosomiasis is an infestation of parasitic flatworms (Schistosoma spp.) via aquatic snail hosts (e.g. invasive Biomphalaria straminea) that causes life‐threatening health conditions (e.g. anemia, liver failure, bladder cancer, and lasting cognitive impairment) in more than 250 million people, with nearly 800 million more at risk, in Africa, Asia, and South America (Sokolow et al. 2016). In Africa's Senegal River Basin, Sokolow et al. (2015) demonstrated that re‐introduction of river prawns indigenous to the west coast of Africa (Macrobrachium vollenhovenii) where dam construction blocked their annual migration could offer a sustainable, low‐cost form of snail control; when used in synergy with existing drug distribution campaigns, the prawns were able to reduce or locally eliminate the parasite. Re‐establishing trophic structure by restoring river prawns within these river systems could serve as a novel ecological countermeasure.
In addition to eradicating or controlling biota that act as spillover risk amplifiers, ecological countermeasures could be employed to augment key habitat resources under conditions of scarcity that stress wildlife hosts and/or drive them into human‐occupied areas for supplementation. In Bangladesh, bats (Pteropus medius) visit silver date palm trees (Phoenix sylvestris) tapped for sap collection. Bats lick the shaved area of the tree and sometimes urinate or defecate in the collection pots, contaminating the sap with Nipah virus (Luby et al. 2006; McKee et al. 2020). Although covering sap containers has reduced disease risk (Nahar et al. 2013), the ideal solution would be an ecological countermeasure that draws bat populations to food resources not shared with people (Mckee et al. 2020). In Australia, “population distancing” is being used to reduce Hendra virus spillover from bats (Pteropus alecto) to horses and subsequently humans, a process triggered by destruction of the bats' winter foraging habitat. When nutrient stressed due to loss of winter nectar resources, bat populations fragment, increase viral shedding, shift from natural landscapes into agro‐urban areas occupied by people and domestic animals. In these agricultural landscapes, horses become exposed to Hendra virus when feeding in the vicinity of fruit trees used by bats (Plowright et al. 2015; Plowright et al. 2016; Edson et al. 2019). Therefore, scientists have actively reached out to the farming community, encouraging them to keep unstabled horses in open pastures and away from trees in flower or fruit (Martin et al. 2015). A more socially acceptable and biologically meaningful risk mitigation approach would be to address the problem at its source; to draw the bats away from the agricultural landscape by restoring native habitat as an ecological countermeasure. Regeneration of winter‐flowering habitat via large‐scale native tree planting could potentially reverse these processes and reduce spillover events (P. Eby 2021, Griffith University. personal communication). Feasibility studies, including mechanistic modeling, are currently in progress (Plowright Grant, U.S. National Science Foundation #DEB‐1716698; DARPA PREEMPT program # D18AC00031).
Large‐scale tree planting has been popularized to meet biodiversity conservation, carbon sequestration, and other sustainable development goals (Bastin et al. 2019; Domke et al. 2020), although not without controversy (Veldman et al. 2019). We caution that such projects, when conducted in human‐occupied areas, might attract pathogen‐hosting wildlife to new food and habitat resources, thereby increasing the risk of human exposure to zoonotic pathogens. Under some circumstances, the societal costs of these large‐scale tree projects may outweigh the benefits. Reaser et al. (2021) call for donor agencies and other relevant institutions to proactively evaluate and further develop tree planting projects with zoonoses prevention services in mind. Ideally, these projects would be strategically harnessed as ecological countermeasures to prevent land use‐induced spillover.
Beyond tree planting, we foresee using various other types of natural resource augmentation scenarios to complement and/or serve as an interim step in implementing countermeasures within a broad ecological restoration framework. Could the strategic use of feeding stations, artificial water sources, bird nest boxes, coverboards, sound and light features, electromagnetic fields, scented objects, or other introduced landscape features that attract or deter wildlife populations become part of the ecological countermeasures arsenal? Becker et al. (2018), summarizing the findings of a collection of scientific papers that investigate the influence of anthropogenic resources subsidies on host‐pathogen dynamics in wildlife, conclude that public education and adaptive management can contribute to “win–win” scenarios for feeding wildlife that optimize benefits for conservation, wildlife disease management, and human health. Nest boxes have been used to increase and expand populations of native barn owls (Tyto alba) to control non‐native rodent populations in agricultural and urban environments (Saufi et al. 2020). In Vermont, private landowners constructed more than 400 houses to attract native tree swallows (Tachycineta bicolor) for mosquito control. The Bird House Forest has not only greatly increased the swallow population, but it has also become a tourist destination drawing economic resources to the local community (https://www.atlasobscura.com/places/birdhouse-forest).
Action Plan
Breed et al. (2020) rightly point out that the “rising public costs of the global burden of disease must incentivize society to push toward a restorative culture, and away from a culture of ecological degradation.” They further note, however, that the specific process and mechanisms by which health benefits may be confirmed through ecological restoration require further elucidation. Their five‐point action plan is intended to seed further inquiry in this regard. We recognize ecological countermeasures as an opportunity to operationalize a restorative culture and, therefore, welcome their action plan as a timely unifying framework for defining and exploring resolution mechanisms. Here, we explicitly contextualize their conceptual action plan, providing an overview of needs and opportunities for further investigating land use‐induced spillover and establishing ecological countermeasures as a component of ecological restoration:
1. Collaborations and conversations. Waugh et al. (2020), as well as Amuai and Winkler (2020), recognize the need to regard One Health and planetary health as distinct yet complementary approaches to pandemic prevention. As these highly collaborative, multi‐disciplinary initiatives become better institutionalized, opportunities will increase to advance ecological countermeasures in concept and practice. For example, the U.S. Health and Human Services Centers for Disease Control (CDC) has been hosting One Health Zoonotic Disease Prioritization (OHZDP) workshops around the world to identify, prioritize, and develop collaborative action plans for diseases of greatest concern (https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/fact-sheet.html). The results of these workshops could be used to identify specific contexts and partnerships for ecological countermeasure implementation. Likewise, members of the scientific community dedicated to solving major societal challenges have been calling for planetary health approaches to preventing future pandemics (Brown & Horton 2020; O'callaghan 2020). A focus on land use‐induced spillover could take these high‐level conversations to the ground. Plowright et al. (2021) emphasize that studies of land use‐induced zoonotic spillover as an interdisciplinary priority is justified from technical perspectives, as well as strategic pragmatism. Gaps in our knowledge of land use influences on the infect‐shed‐spill‐spread cascade need to be addressed in situ in order to inform ecological countermeasures. Proactive partnerships between epidemiologists, immunologists, and ecologists will enable the rapid synthesis of ideas and approaches across disparate areas of technical investigation and practice (see Becker et al. 2020). Only by initiating conversations at the margins of these disciplinary boundaries can scientists develop the fit‐to‐context, restorative solutions urgently needed to prevent future pandemics.
2. Education and learning. Scientific understanding of land use‐induced spillover is in its infancy. Plowright et al. (2021) summarize information gaps for the factors driving the infect‐shed‐spill‐spread cascade. White and Razgour (2020) point out that, at least for mammals, the majority of published studies regarding anthropogenic land use change influences on zoonotic pathogen dynamics are reviews rather than primary empirical studies. These and other authors (e.g. Halliday et al. 2017) identify geographic, taxonomic, and additional biases in our current knowledge of zoonotic disease, while Watsa (2020) warns of the insufficient number and distribution of pathogen reference libraries. Although there has been an increase in investments for zoonotic pathogen discovery in understudied species and regions (e.g. PREDICT; https://www.ecohealthalliance.org/program/predict), the need remains to educate policymakers, funding agencies, and early career scientists on these information gaps to inspire the resources and sizable body of researchers needed to identify and employ ecological countermeasures. Ideally, an appropriate educational institution and donor will step forward to curate the emerging knowledge in an open‐access, interoperable database established and managed with rapid peer‐learning goals (also see Action 4).
3. Defining the causal links. Ultimately, untangling the causal relationships between land use change and zoonotic spillover will require the coupling of field‐based empirical studies that identify the parsimonious links with large‐scale experiments and dynamic mechanistic models (Plowright et al. 2008). Advances are being made across a wide range of taxa and contexts. For example, Süld et al. (2014) elucidate the complexity involved in identifying causal linkages between land use and zoonotic pathogen dynamics, tying the supplementary feeding sites of wild boar (Sus scrofa) in Northern Europe to the spread of several zoonotic diseases (including alveolar echinococcosis, trichinellosis, rabies, and sarcoptic mange) via the invasive raccoon dog (Nyctereutes procyonoides). In the St. Louis region of Missouri (U.S.A), Allan et al. (2010) demonstrated that Amur honeysuckle (Lonicera maackii), which is invasive in much of North America, increases human risk of exposure to ehrlichiosis, an emerging infectious disease caused by bacterial pathogens transmitted by the lone star tick (Amblyomma americanum). They observed that white‐tailed deer (Odocoileus virginianus), a preeminent tick host and pathogen reservoir, preferentially used areas invaded by honeysuckle, consequently leading to a considerably greater numbers of ticks infected with pathogens in honeysuckle‐invaded areas relative to adjacent honeysuckle‐uninvaded areas. They proposed honeysuckle eradication as tick‐borne disease intervention. Reaser et al. (2021; Supplemental Table 1) provide additional examples of research that mechanistically links land use to at least one component of the infect‐shed‐spill‐spread cascade. They call on scientists to expand the number of empirical studies of land use‐induced spillover for comparative purposes, as well as to identify ecological countermeasure options in specific contexts. Plowright et al. (2021; Supplementary Material) and Becker et al. (2020) review data gaps and provide examples of inquiry needs to advance such studies.
4. Monitoring restoration and health outcomes. There are timely opportunities to include “ecological restoration for human health” targets within ecological policy and management frameworks, such as those being negotiated under the Convention on Biological Diversity toward a 2050 benchmark (https://www.cbd.int/conferences/post2020#). With regard to land use‐induced spillover, targets should prioritize zoonotic pathogens surveillance and monitoring, especially given pending shifts in species' geography due to globalization and climate change. Although pathogen surveillance is often viewed as relevant to the biodiverse tropics, the need is valid for temperate and polar regions as well. For example, in Ireland, Nally et al. (2016) discovered a novel serovar of pathogenic Leptospira associated with the invasive greater white‐toothed shrew (Crocidura russula). As a complement to decentralized zoonotic pathogen libraries, Watsa (2020) proposes a publicly accessible, centralized, curated system for monitoring zoonotic pathogens. Although she presents the GISAID (global initiative on sharing all influenza data) EpiFlu repository (https://www.gisaid.org/) as an example of a disease‐focused public database that could be expanded to include other zoonoses, we would prefer to see zoonotic pathogen data directly or inter‐operably incorporated into GBIF (Global Biodiversity Information Facility; http://gbif.org) to allow for co‐analysis with pathogen host and vector distributions, geographic mapping tools, and land use data. With a view toward enabling the early detection and rapid response to the introduction of harmful biological organisms, Reaser et al. (2020b) envision a national invasive alien species framework for the United States that is applicable globally. This proposal and a complementary paper by Wallace et al. (2020) provide guidance for accessing and analyzing invasive alien species information, of which zoonotic disease data is a component. In combination, such information, when available in an open source and inter‐operable manner, would enhance our capacity to generate ecological countermeasures, thereby mitigating zoonotic disease risk. In order to facilitate adaptive management, the monitoring and evaluation of ecological countermeasures should be standard practice.
5. Community ownership and stewardship. Halliday et al. (2020) observe that public health interventions often fail due to a lack of attention to their social, cultural, and historical contexts and poor engagement of the people they are designed to benefit. They note that effective community participation has been crucial for successful control of Ebola in West Africa, rinderpest eradication, and the success of many neglected tropical disease programs. Due to land use and ethical sensitivities, community trust and engagement is key to ecological countermeasure development and acceptance. Island Conservation is effectively supporting the Floreana Island community in their efforts to eradicate rodents and cats that pose public health risks and are barriers to ecological restoration. As voracious predators, feral cats and non‐native rodents are two of the most significant threats to island biodiversity, particular endemic species. In addition, both pose disease risks to native wildlife and human populations. For example, cats are the host for Toxoplasma gondii, which causes the disease toxoplasmosis, to which most warm‐blooded animals, including humans, are susceptible. The presence of rodents in and around human habitation can lead to an increased risk of toxoplasmosis, lymphocytic clorio‐meningitis, Plague, leptospirosis, hantavirus, and salmonellosis, for example. Although the risk of disease spread associated with COVID‐19 has delayed project implementation (K. Campbell 2020, Island Conservation, personal communication), a feasibility study that explicitly notes the benefits of ecological restoration to these health concerns has already assessed social, legal, and environmental acceptability of the invasive alien species removal plans. The findings indicate that the plan is feasible within the region and calls for periodic re‐evaluation of social acceptance as results are received from processes to engage the community (Island Conservation 2013). The large‐scale tree planting effort proposed in southern Australia as an ecological countermeasure for Hendra virus mitigation would be community‐based (Plowright, personal communication). Gaddy (2020) provides insights into the application of local knowledge in emerging infectious disease research that is applicable to ecological countermeasures. Where relevant, Traditional Ecological Knowledge (TEK), Local Ecological Knowledge (LEK), and a wide range of other forms of stakeholder engagement should be incorporated into these initiatives (Table 1). Ultimately, community understanding and acceptance is fundamental to mitigating the environmental stressors that drive land use‐induced spillover—currently and into the future—so that public health goals are achieved. Fisher et al. (2020) make the case that ecological restoration is most effective when approached from a social‐ecological perspective and synthesize key insights from the field of social‐ecological ecosystems research that are applicable to ecological restoration. They conclude their analyses by suggesting two cross‐cutting research priorities focused on social‐ecological restoration: first, to conduct post hoc comparisons of different restoration projects; and, second, to establish “living labs” that facilitate social‐ecological restoration. The development and implementation of ecological countermeasures are well‐suited to meet both of these objectives.
Budgetary limitations are often raised as a barrier to implementing new priorities and plans. However, the impacts of COVID‐19 and growing concerns about pandemics‐in‐the making (e.g. Nipah virus, McKee et al. 2020; Constable 2021) have already led to calls for substantially more funding to be committed to zoonotic disease research and response (e.g. Dobson et al. 2020), and the public is unlikely to accept “budgetary limitations” as an excuse for policy dismal. Common sense points to preventive actions as far more cost‐efficient than zoonoses response measures, especially as diseases move from localized to pandemic scales, leaving a wake of human suffering and economic damages to be felt for generations. Our action plan provides guidance for setting budgetary priorities. However, it should be noted that many of the opportunities to address land use‐induced spillover by advancing a restorative culture do not require substantial new financial inputs. Rather, they simply reflect a need to use existing investments more wisely. For example, many of the recommendations we provide necessitate changes in the administration of standard operating procedures and best management practices rather than the establishment of new programs. Other suggestions, such as coupling human health with conservation targets, will enable greater returns on investment for existing initiatives across sectors. Finally, we emphasize that one of the key tenets of a restorative culture must be a transition to investments in life‐generative rather than life‐depleting norms across all aspects of civil society.
Conclusion
We believe that, as a tenet of ecological restoration, ecological countermeasures could become standard operating procedures for zoonotic disease prevention and response. Since land use‐induced spillover scenarios are contextually unique, there is a need for a large, diverse, adaptable toolkit to mitigate zoonotic disease emergence and transmission. To date, efforts to mitigate zoonotic disease risk have largely focused on the control of specific pathogen, host, and vector species, including pathways of pathogen spread. This has proven inadequate under many scenarios; the scale of the problem substantially outsizes response willingness and capacity. Ecological countermeasures can serve as landscape‐based approaches to meeting human health priorities.
Although ecological restoration does not always provide direct, quantifiable benefits to human health, it would do so under the rubric of ecological countermeasures. The application of ecological countermeasures could be particularly valuable when there is a need to improve cost‐efficiencies and efficacy; where, for example, highly vulnerable human populations live in poverty and there are few resources, coordinating mechanisms, and adequately trained professionals to apply large‐scale medical and veterinary interventions in perpetuity. Such situations may also be prone to a lack of public trust in personally oriented government interventions but acceptable of landscape‐oriented approaches. Overall, ecological countermeasures may be more “public friendly” and provide substantial returns on investment for projects explicitly focused on zoonotic disease mitigation, while magnifying the returns on investment for natural resources projects with other primary goals (e.g. climate change mitigation). We welcome social scientists to collaborate on site‐specific analyses of community attitudes regarding ecological countermeasure, cost‐benefits, and economic efficiencies.
Furthermore, we agree with Meyerson et al. (2009) that there is a need for a comprehensive approach to biosecurity that considers ecological perspectives. Since zoonotic pathogens may be moved intentionally as well as unintentionally, we propose that ecological countermeasures are viewed, prioritized, and institutionalized as landscape‐scale interventions to safeguard civil society. The United Nations Decade on Ecosystem Restoration (https://www.decadeonrestoration.org/) provides a timely platform for furthering these concepts and prioritizing the necessary work ahead through multi‐lateral agreements and national policies.
Supporting information
Supplement S1. Glossary of terms
Acknowledgments
We thank Robyn Egloff for help developing Figure 1, as well as Stephen Murphy, Valter Amaral, and an anonymous reviewer for comments that helped improve the manuscript. RKP was supported by NSF DEB‐1716698, DARPA PREEMPT D18AC00031, and the USDA NIFA Hatch 1015891. For CABI, AW acknowledges core support from various agencies (https://www.cabi.org/about-cabi/who-we-work-with/key-donors/). The authors declare that they do not have a conflict of interest.
Author contributions: RKP, PJH, JKR developed the concept of ecological countermeasures; JKR drafted the manuscript with input from all other authors; JKR, RKP developed the figure and table; JKR, RKP edited and revised the document.
Coordinating Editor: Stephen Murphy
Contributor Information
Jamie K. Reaser, Email: jamiekreaser@gmail.com.
Raina K. Plowright, Email: raina.plowright@montana.edu.
LITERATURE CITED
- Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL (2010) Invasive honeysuckle eradication reduces tick‐borne disease by altering host dynamics. Proceedings of the National Academy of Sciences 107:18523–18527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuai JH, Winkler AS (2020) One Health or planetary health for pandemic prevention‐ authors' reply. The Lancet 396(12):1882–1883 [DOI] [PubMed] [Google Scholar]
- Andrade A, Zambrana‐Torrelio C, Vasseur L, Nelson C, Carver S, Convery I (2020) Rewilding for human health. Ecologist. https://theecologist.org/2020/jul/03/rewilding-human-health [Google Scholar]
- Aronson JC, Blatt CM, Aronson TB (2016) Restoring ecosystem health to improve human health and well‐being: physicians and restoration ecologists unite in a common cause. Ecology and Society 21:39 [Google Scholar]
- Bastin J, Finegold Y, Garcia C, Mollicone D, Rezende M, Routh D (2019) The global tree restoration potential. Science 365:76–79 [DOI] [PubMed] [Google Scholar]
- Becker DJ, Albery GF, Kessler MK, Lunn TJ, Falco CA, Czirják GÁ, Martin LB, Plowright RK (2020) Macroimmunology: the drivers and consequences of spatial patterns in wildlife immune defence. Journal of Animal Ecology 89:972–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Hall RJ, Forbes KM, Plowright RK, Altizer S (2018) Anthropogenic resource subsidies and host – parasite dynamics in wildlife. Philosophical Transactions of the Royal Society B 373:20170086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breed MF, Cross AT, Wallace K, Bradby K, Flier E, Goodwin N, et al. (2020) Ecosystem restoration: a public health intervention. EcoHealth Online. 10.1007/s10393-020-01480-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Horton R (2020) A planetary health perspective on COVID‐19: a call for papers. The Lancet 395:1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can OE, D'Cruze N, MacDonald DM (2019) Dealing in deadly pathogens: taking stock of the legal trade in live wildlife and risks to human health. Global Ecology and Conservation 17:ee00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchio E, Crotta M, Romeo C, Drewe JA, Guitian J, Ferrari N (2020) Invasive alien species and disease risk: an open challenge in public and animal health. PLoS Pathology 16:e1008922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable H (2021) The other virus that worries Asia. British Broadcasting, 11 January 2021. https://www.bbc.com/future/article/20210106-nipah-virus-how-bats-could-cause-the-next-pandemic [Google Scholar]
- Dobson AP, Pimm SL, Hanna L, Kaufman L, Ahumada JA, Ando A, et al. (2020) Ecology and economics for pandemic prevention. Science 369:379–381 [DOI] [PubMed] [Google Scholar]
- DOD (Department of Defense) (2020) DOD Dictionary of military and associated terms. https://www.jcs.mil/Portals/36/Documents/Doctrine/pubs/dictionary.pdf
- Domke GM, Oswalt SN, Walters BF, Morin RS (2020) Tree planting has the potential to increase carbon sequestration capacity of forests in the United States. Proceedings of the National Academy of Sciences 117:24649–24651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson D, Peel AJ, Huth L, Mayer DG, Vidgen ME, McMichael L, et al. (2019) Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto). Epidemiology & Infection 147:e240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko SV, Howard BJ (2012) Environmental countermeasures and restoration. Pages 9–29.In: Meyers RA (ed), Encyclopedia of sustainability science and technology. Springer, New York: [Google Scholar]
- Fisher J, Riechers M, Loos J, Martin‐Lopez B, Temperton VM (2020) Making the UN Decade on Ecological Restoration a social‐ecological endeavour. Trends in Ecology & Evolution 36:20–21 [DOI] [PubMed] [Google Scholar]
- Gaddy H (2020) Using local knowledge in emerging infectious disease research. Social Science & Medicine 258:113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann GD, McDonald T, Walder B, Arronson J, Nelson CR, Johnson J, et al. (2019) International principles and standards for the practice of ecological restoration. Restoration Ecology 27:S1–S46 [Google Scholar]
- Gibbs (2014) The evolution of One Health: a decade of progress and challenges for the future. Vet Record 174:85–91 [DOI] [PubMed] [Google Scholar]
- Gottdenker NL, Streicher DG, Faust CL, Carroll CR (2014) Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth 11:619–632 [DOI] [PubMed] [Google Scholar]
- Halliday JEB, Hampson K, Hanley N, Lemo T, Sharp JP, Haydon DT, Cleaveland S (2017) Driving improvements in emerging disease surveillance through locally relevant capacity strengthening. Science 357:146–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday JEB, Hampson K, Hanley N, Lembo T, Sharp JP, Haydon DT, Cleaveland S (2020) Driving improvements in infectious disease surveillance through locally relevant capacity strengthening. Science, 357:146–148. 10.1126/science.aam8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolby, J (2020) To prevent the next pandemic, it's the legal wildlife trade we should worry about. National Geographic. https://www.nationalgeographic.com/animals/2020/05/to-prevent-next-pandemic-focus-on-legal-wildlife-trade/(accessed 7 May 2020).
- Island Conservation (2013) Floreana Island ecological restoration: rodent and cat eradication feasibility analysis version 6.0. Island Conservation, Santa Cruz, California: https://www.cbd.int/doc/lifeweb/Ecuador/images/FeasibilityAnalysis.pdf [Google Scholar]
- Linske MA, Williams SC, Ward JS, Stafford KC 3rd (2018) Indirect effects of Japanese barberry infestations on white‐footed mice exposure to Borrelia burgdorferi . Environmental Entomology 47:795–802 [DOI] [PubMed] [Google Scholar]
- Luby SP, Rahman M, Hossain MJ, Blum L, Husain MM, Gurley E, et al. (2006) Foodborne transmission of Nipah virus, Bangladesh. Emerging Infectious Disease 12:1888–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Plowright R, Chen C, Kault D, Selleck P, Skerratt LF (2015) Hendra virus survival does not explain spillover patterns and implicates relatively direct transmission routes from flying foxes to horses. Journal of General Virology 96:1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee CD, Islam A, Luby SP, Salje H, Hudson PJ, Plowright RK, Gurley ES (2020) The ecology of Nipah virus in Bangladesh: a nexus of land use change and opportunistic feeding behavior in bats. bioRxiv. 10.1101/2020.11.30.404582v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson FA, Meyerson LA, Reaser JK (2009) Biosecurity from the ecologist's perspective: developing a more comprehensive approach. International Journal of Risk Assessment and Management 12:147–160 [Google Scholar]
- Nahar N, Mondal UK, Sultana R, Hossain MJ, Khan MSU, Gurley ES, Oliveras E, Luby SP (2013) Piloting the use of indigenous methods to prevent Nipah virus infection by interrupting bats' access to date palm sap in Bangladesh. Health Promotion International 28:378–386 [DOI] [PubMed] [Google Scholar]
- Nally JE, Arent Z, Bayles DO, Hornsby RL, Gilmore C, Regan S, McDevitt AD, Yearsley J, Fanning S, McMahon BJ (2016) Emerging infectious disease implications of invasive mammalian species: the greater white‐toothed shrew (Crocidura russula) is associated with a novel serovar of pathogenic Leptospira in Ireland. PLoS Neglected Tropical Disease 10:e0005174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'callaghan C (2020) Why is planetary health the solution to prevent COVID‐19. Barcelona, Spain: ISGlobal Barcelona Institute for Global Health; https://www.isglobal.org/en/healthisglobal/‐/custom‐blog‐portlet/‐por‐que‐la‐salud‐planetaria‐es‐la‐solucion‐para‐evitar‐crisis‐como‐la‐covid‐19‐/6112996/0 (accessed 16 December 2020) [Google Scholar]
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, et al. (2004) Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives 112:1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Reaser JK, Locke H, Woodley SJ, Patz JA, Becker D, et al. (2021) Understanding land use‐induced spillover: a call to action to safeguard environmental, animal, and human health. The Lancet Planetary Health 5. https://dx.doi.org/10.1016/S2542-5196(21)00031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Llyod‐Smith JO (2017) Pathways to zoonotic spillover. Nature Reviews Microbiology 15:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O (2016) Transmission or within‐host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Neglected Tropical Diseases 10:e0004796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, et al. (2015) Ecological dynamics of emerging bat virus spillover. Proceedings of the Royal Society B: Biological Sciences 282(1798):20142124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE (2008) Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment 6:420–429 [Google Scholar]
- Reaser JK, Tabor GM, Becker D, Muruthi P, Witt A, Woodley SJ, et al. (2021) Land use‐induced spillover: priority actions for protected and conserved area managers. PARKS, The International Journal of Protected Areas and Conservation 27 (Special Issue). https://dx.doi.org/10.2305/IUCN.CH.2021PARKS-27SI.en [Google Scholar]
- Reaser JK, Hunt BE, Ruiz‐Aravena M, Tabor GM, Patz JA, Becker D, et al. (2021) Reducing land use‐induced spillover risk by fostering landscape immunity: policy priorities for conservation practitioners. Preprint: https://ecoevorxiv.org/7gd6a/ [Google Scholar]
- Reaser JK, Guala GF, Simpson A, Morisette JT, Fuller P (2020b) A national invasive species information framework. Biological Invasions 22:21–36 [Google Scholar]
- Saufi S, Ravindran S, Hamid NH, Abidin CMRZ, Ahmad H, Ahmad AH, Salim H (2020) Diet composition of introduced barn owls (Tyto alba javanica) in urban area in comparison with agriculture settings. Journal of Urban Ecology 6:juz025 [Google Scholar]
- Seltenrich N (2018) Down to earth: the emerging field of planetary health. Environmental Health Perspectives 126:072001‐1‐072001‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuangchen M, Jin C, Kunling J, Lan M, Sijie Z, Kai W (2017) Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renewable and Sustainable Energy Reviews 73:225–235 [Google Scholar]
- Sokolow SH, Nova N, Peplin KM, Peel AJ, Pulliam JRC, Manlove K, et al. (2019) Ecological interventions to prevent and manage zoonotic pathogen spillover. Philosophical Translations of the Royal Society B 374:20180342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, Lafferty KD, Kuris AM, Rickards C, de Leo GA (2016) Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Neglected Tropical Diseases 10:e0004794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow SH, Huttinger E, Jouand N, Hsieh MH, Lafferty KD, Kuris AM, et al. (2015) Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proceedings of the National Academy of Sciences 114:E7028–E7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Witt ABR, Walsh GC, Foster WA, Murphy ST (2018) Would the control of invasive alien plants reduce malaria transmission? A review. Parasites and Vectors 11:76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süld K, Valdmann H, Laurimaa L, Soe E, Davison J, Saarma U (2014) An invasive vector of zoonotic disease sustained by anthropogenic resources: the raccoon dog in Northern Europe. PLoS One 9:e96358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman JW, Aleman JC, Alvarado ST, Anderson TM, Archibald S, Bond WJ, et al. (2019) Comment on “The global tree restoration potential”. Science 366:eaay7976 [DOI] [PubMed] [Google Scholar]
- Wallace R, Bargeron CT, Reaser JK (2020) Enabling decisions that make a difference: guidance for improving access to and analysis of invasive species information. Biological Invasions 22(10):37–45 [Google Scholar]
- Watsa M (2020) Rigorous wildlife disease surveillance. Science 369:145–147 [DOI] [PubMed] [Google Scholar]
- Waugh C, Lam SS, Sonne C (2020) One Health or planetary health for pandemic prevention? The Lancet. 396:P1882. 10.1016/S0140-6736(20)32387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Razgour O (2020) Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land‐use change. Mammal Review 50:336–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Ward JS (2010) Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environmental Entomology 39:1911–1921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement S1. Glossary of terms


