Abstract
Background
Controversy exists regarding the drug selection in hypertension (HTN) management in patients with COVID‐19. This study aimed to compare the effects of losartan and amlodipine in patients with primary HTN and COVID‐19.
Methods
In this randomised clinical trial, hospitalised patients with COVID‐19 and primary HTN were enrolled in the study. One arm received losartan, 25 mg, twice a day and the other arm received amlodipine, 5 mg per day for 2 weeks. The main outcomes were compare 30‐day mortality rate and length of hospital stay.
Results
The mean age of patients treated with losartan (N = 41) and amlodipine (N = 39) was 67.3 ± 14.8 and 60.1 ± 17.3 years, respectively (P value = .068). The length of hospital stay in losartan and amlodipine groups was 4.57 ± 2.59 and 7.30 ± 8.70 days, respectively (P value = .085). Also, the length of ICU admission in losartan and amlodipine group was 7.13 ± 5.99 and 7.15 ± 9.95 days, respectively (P value = .994). The 30‐day mortality was two and five patients in losartan and amlodipine groups, respectively (P value = .241).
Conclusions
There was no priority in losartan or amlodipine administration in COVID‐19 patients with primary HTN in decreasing mortality rate, hospital and ICU length stay. Further studies need to clarify the first‐line anti‐HTN medications in COVID‐19.
What’s known
Hypertension is a major disease that increases the risk of acute respiratory failure, hospital admission and mortality rate among patients with COVID‐19.
Controversy exists regarding the drug selection in hypertension management in patients with COVID‐19.
What’s new
There was no priority in losartan or amlodipine administration in COVID‐19 patients with primary HTN in decreasing mortality rate.
There was no priority in losartan or amlodipine administration in COVID‐19 patients with primary HTN in decreasing hospital length stay.
There was no priority in losartan or amlodipine administration in COVID‐19 patients with primary HTN in decreasing ICU length stay.
1. INTRODUCTION
Several underlying medical conditions are associated with increasing the risk of COVID‐19 severity and are associated with a higher mortality rate. 1 , 2 , 3 Hypertension (HTN) is a major disease that increases the risk of acute respiratory failure, hospital admission and mortality rate among patients with COVID‐19. 4 , 5 It is a main co‐morbidity among patients with COVID‐19 and management of HTN in COVID‐19 is an essential for reduction of mortality and morbidity. In contrary, a recent hypothesis highlights no association between HTN treatment with RAAS inhibitors and unfavourable outcomes in COVID‐19. 6
The primary therapeutic strategy for the management and monitoring of HTN are some of renin‐angiotensin‐aldosterone system (RAAS) inhibiting molecules such as angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs). 7 , 8 , 9 The expression of angiotensin‐converting enzyme (ACE) II has been proposed to be increased by the activation of ACE inhibitors (ACEIs) and ARBs. Therefore, over the COVID‐19 pandemic, susceptibility to severe infection can be reduced. 10 , 11 Although it has been suggested the ACEIs counter the anti‐inflammatory effects of ACE2, direct inhibitory efficacy of ACE against the ACE2 has not been proved in experimental surveys. 12 , 13 Accordingly, there is a controversy in the successive use of ACEI/ARB in the patients with COVID‐19, which emphasises that ACEIs and ARBs may promote the ACE2 receptor expression in the animal trials and some others suggest these drug classes as an additional therapy for COVID‐19 treatment. 14 , 15 Therefore, it seems that the ARBs and ACEIs are two‐edged swards in COVID‐19 management and some studies were recommended CCBs as an alternative treatment in patients with HTN and COVID‐19. 10
An antagonist of angiotensin I type 1 receptor called losartan is considered as an effectively strong drug for the treatment of such cases. 16 , 17 Novel investigations suggest the maturation of dendritic cells, impairment of T‐helper 1 immune response can be impeded by losartan which eventually reduces the inflammatory procedures induced by angiotensin II. 18 , 19 Nevertheless, the losartan defensive mechanisms in acute lung injury have not yet been fully understood.
Beneficial or harmful effects of anti‐hypertension medications in patients with COVID‐19 and primary HTN are still unclear. On the contrary, there are controversy in best‐choice medication in patients with primary HTN and COVID‐19. Therefore, this study aimed to compare the effects of losartan and amlodipine in patients with COVID‐19 and primary HTN.
2. METHODS
2.1. Study design
The current study was a prospective randomised clinical trial in order to compare the effects of losartan and amlodipine in primary HTN management of patients with COVID‐19. The study was approved by the Medical Ethical Committee of Tabriz University of Medical Sciences and was registered at Iranian Registry for Clinical Trials (IRCT ID: IRCT20180802040678N4) on 1 April 2020. Informed consent was obtained from patients before enrolment.
2.2. Study participants
Patients with COVID‐19 and primary HTN were recruited to the study in Imam Reza Hospital of Tabriz University of Medical Sciences in Tabriz, Iran, from 2 April 2020 to 30 June 2020.
Based on the COVID‐19 pneumonia prevention and control program (5th edition) publishing by the national health commission of world health organization (WHO) guidance, COVID‐19 was detected through the reverse transcription‐polymerase chain reaction (RT‐PCR) 20 (ICD code: U07.1).
Inclusion criteria were the following: age 18 years and older, patients with primary HTN with systolic blood pressure (SBP) level of 130‐140 mmHg and diastolic blood pressure (DBP) of 85‐90 mmHg who were managed by non‐pharmacological strategies or were newly diagnosed.
Exclusion criteria were pregnant and lactating patients, severe hepatic and renal failure, bilateral renal artery stenosis and patients with the history of uncontrolled HTN, and also patients showing losartan side effects such as cough exacerbation, increased potassium levels in blood and baseline, new anaemia, shock or reduction of blood pressure 90/60 mmHg or less, all had been excluded.
2.3. Randomisation
The patients were randomised (randomly assigned 1:1) according to inclusion and exclusion criteria and via block randomisation in both groups. Randomisation was done by a computer‐generated random number for the assignment of participants to the losartan or amlodipine arm. A researcher who was not involved in our survey conducted the allocation in order to maintain blinding. Till the achievement and assessment of all data, submitted cases who received drug administration and analysing the results remained blind via randomised and allocated processes.
2.4. Drug treatment
Besides standard treatment, supportive and symptomatic therapy in both groups, in losartan group patients was received 25 mg losartan (Actoverco, Karaj, Iran) tablets twice per day (before breakfast and after dinner) and in amlodipine group patients was received amlodipine besilate 5 mg (Actoverco, Karaj, Iran) per day at least for 14 days. In intubated patients, the drugs were continued using nasogastric tube. The study design is shown in Figure 1.
FIGURE 1.
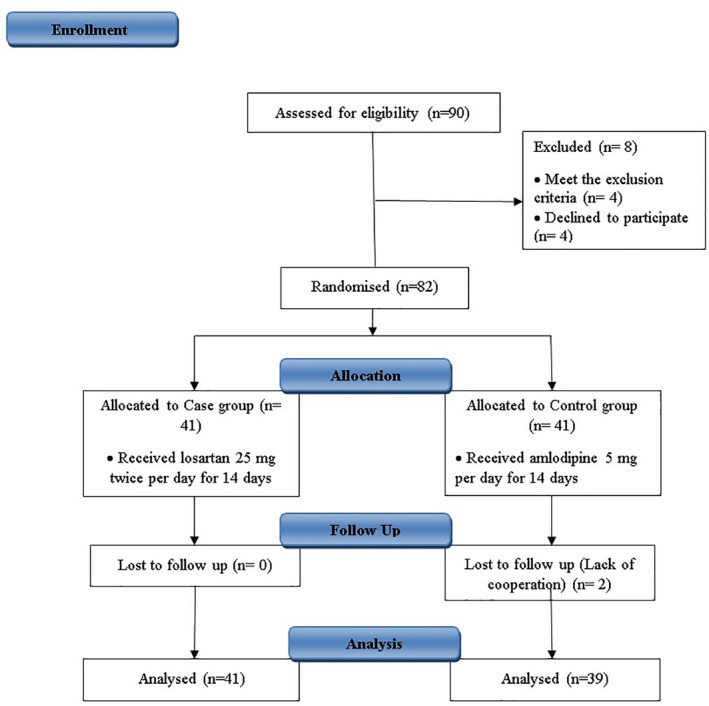
Study flow diagram
2.5. Data collection
In the primary examination by a pulmonologist, demographic data including age and sex, and also medical history or co‐morbidities were extracted. Furthermore, clinical characteristics were also obtained.
In all cases, chest computed tomography (CT) scan was done, and before commencing the interview, all laboratory information were collected.
2.6. Primary and secondary outcomes
In this study, the primary outcomes were comparison of 30‐days mortality and length of hospital stay between groups. The secondary outcomes were disease severity assessment, needs to intubation, laboratory and clinical parameters change. Disease severity was assessed by sequential organ failure assessment (SOFA) respiratory score. The SOFA assessment is used to assess of critical patients to determine the extent of organ function or rate of failure. Total score is calculated by a SOFA calculator. Total scores range are from 0 to 24, with higher scores indicating greater chance of mortality. 21
2.7. Statistical analysis
The normal distribution of variables was evaluated using the Kolmogorov‐Smirnov test. Qualitative and normally distributed quantitative variables were displayed as numbers (percentages) and mean ± standard deviation, respectively. Paired t test was utilised to compare the differences between variables before and after the drug consumption. Chi‐squared or independent sample t‐test was also used for differences between groups. P value < .05 was considered statistically significant. Data were analysed using SPSS, 24.0 (SPSS Inc, Chicago, IL).
3. RESULTS
3.1. Characteristics of participants
A total of 82 patients with COVID‐19 and primary HTN were included in the study. Finally, 41 (mean age 67.3 ± 14.8, 53.7% men) were in the losartan group and 39 (mean age 60.1 ± 17.3, 53.8% men) in the amlodipine group were analysed. There was no significant age (P value = .068) and sex (P value = .232) difference between the two groups. Baseline characteristics of patients are summarised in the Table 1. The blood pressure, pulse rate, respiratory rate, body temperature and O2 saturation of patients are shown in the Table 1.
TABLE 1.
Baseline characteristics of patients
| Variable | Losartan | Amlodipine |
|---|---|---|
| Age (y) | 67.3 ± 14.8 | 60.1 ± 17.3 |
| Gender (n) | ||
| Male | 22 (53.7%) | 19 (46.3%) |
| Female | 21 (53.8%) | 18 (46.2%) |
| Smoking (n) | 5 (12.2%) | 6 (15.4%) |
| Medical history (n) | ||
| Diabetes mellitus | 11 | 8 |
| Cardiovascular diseases | 8 | 7 |
| COPD/Asthma | 5 | 7 |
| Hyperlipidaemia | 4 | 3 |
| Imaging findings (n) | ||
| Ground‐glass opacity | 27 (65.9%) | 31 (79.5%) |
| Consolidation | 7 (17.1%) | 5 (12.8%) |
| Mix pattern | 7 (17.1%) | 3 (7.7%) |
3.2. Primary outcomes
Of the patients in the losartan group, 39 (95.1%) were survived and 2 (4.9%) were died. In addition, eight patients (19.5%) were intubated in this group. In the amlodipine group, 34 patients (87.18%) were discharged and 5 patients (12.82%) were died. Also, nine patients (23.08%) were intubated in this group. Morewise, the mean duration of hospitalisation in losartan group was 4.57 ± 2.59 while the mean duration of hospitalisation in amlodipine group was 7.30 ± 8.70 days, that shows more days hospitalisation in controls (P value = .085). Also the length of ICU admission in losartan group was 7.13 ± 5.99 days, while it was 7.15 ± 9.95 days in the amlodipine group that shows more length of ICU admission in amlodipine group (P value > .05). Comparison of outcomes is shown in Table 2.
TABLE 2.
Disease severity, length of admission and mortality in two groups
| Variables | Group | Mean ± SD | P value |
|---|---|---|---|
| SOFA score, d | Baseline | ||
| Losartan | 3.08 ± 1.35 | .954 | |
| Amlodipine | 3.74 ± 2.21 | ||
| At Discharge | |||
| Losartan | 2.42 ± 1.17 | .084 | |
| Amlodipine | 4.26 ± 3.71 | ||
| Length of admission, d | Losartan | 4.57 ± 2.59 | .085 |
| Amlodipine | 7.30 ± 8.69 | ||
| Length of ICU admission, d | Losartan | 7.13 ± 5.99 | .994 |
| Amlodipine | 7.15 ± 9.95 | ||
| 30‐d mortality | Losartan (n) | ||
| Cure | 39 | .241 | |
| Death | 2 | ||
| Amlodipine (n) | |||
| Cure | 34 | ||
| Death | 5 |
3.3. Secondary outcomes
Characteristics of patients before and after intervention in both groups including cell blood counts, electrolyte profiles, liver and kidney function tests, inflammatory parameters and blood gas analysis are shown in Table 3.
TABLE 3.
Clinical and laboratory findings before and after the intervention
| Variables | Losartan group (n = 41) | Amlodipine group (n = 39) | P b |
|---|---|---|---|
| Systolic blood pressure (mmHg) | |||
| Baseline | 132.24 ± 4.22 (130‐141) | 133.41 ± 3.81 (130‐139) | .287 |
| At discharge | 114.16 ± 10.19 (101‐139) | 109.62 ± 9.74 (99‐130) | .103 |
| P a | <.001 | <.001 | — |
| Diastolic blood pressure (median of day), (mmHg) | |||
| Baseline | 86.55 ± 2.81 (85‐100) | 86.86 ± 2.64 (85‐97) | .642 |
| In discharge | 72.28 ± 7.59 (63‐90) | 72.14 ± 7.51 (62‐94) | .925 |
| P a | <.001 | .077 | — |
| Pulse rate (n) | |||
| Baseline | 93.8 ± 15.791 (62‐130) | 87.79 ± 14.944 (58‐120) | .113 |
| In discharge | 87.86 ± 10.497 (60‐105) | 84.38 ± 9.584 (64‐105) | .218 |
| P a | .020 | .658 | — |
| Respiratory rate (n) | |||
| Baseline | 22.12 ± 7.295 (10‐55) | 22.46 ± 5.281 (16‐38) | .832 |
| In discharge | 15.31 ± 4.516 (8‐26) | 17.29 ± 1.961 (14‐20) | .032 |
| P a | .001 | .002 | — |
| Body temperature (°C) | |||
| Baseline | 36.741 ± 1.7671 (26.5‐39) | 37.024 ± 0.4771 (36‐38.2) | .405 |
| In discharge | 36.511 ± 0.6098 (34.3‐39) | 36.571 ± 0.1678 (36.5‐37.2) | .660 |
| P a | .820 | <.001 | — |
| O2 saturation (%) | |||
| Baseline | 86.49 ± 8.62 (60‐96) | 87.52 ± 11.089 (40‐96) | .664 |
| In discharge | 91.65 ± 5.453 (72‐96) | 94.11 ± 2.158 (90‐99) | .020 |
| P a | .010 | .019 | — |
| White blood cell count (n)/µL | |||
| Baseline | 8807.32 ± 4675.435 (3300‐22300) | 8186.21 ± 3567.184 (2700‐15400) | .602 |
| In discharge | 23 269.57 ± 67 747.78 (1100‐333000) | 12 936.84 ± 18 713.49 (5100‐89000) | .524 |
| P a | .331 | .238 | — |
| Neutrophil (%) | |||
| Baseline | 77.5 ± 12.4308 (55‐100) | 76.79 ± 9.2999 (57.3‐93) | .787 |
| In discharge | 82.687 ± 8.7714 (63.7‐96.8) | 77.372 ± 15.5331 (41.6‐96) | .206 |
| P a | .171 | .934 | — |
| Lymphocyte (%) | |||
| Baseline | 17.32 ± 11.1105 (4.5‐40.1) | 17.703 ± 8.4144 (2‐35.2) | .876 |
| In discharge | 11.813 ± 8.2195 (0.7‐33.4) | 15.422 ± 12.768 (2.1‐51.3) | .279 |
| P a | .018 | .415 | — |
| Platelet (n)/µL | |||
| Baseline | 208 012 ± 77 957 (94000‐474000) | 217 276 ± 83 963 (84000‐437000) | .637 |
| In discharge | 216 166 ± 83 766 (95000‐400000) | 234 052 ± 94 862 (87000‐424000) | .516 |
| P a | .865 | .243 | — |
| Haemoglobin (g/dL) | |||
| Baseline | 12.676 ± 2.1436 (8.8‐18.1) | 12.862 ± 2.0491 (7.5‐15.9) | .716 |
| In discharge | 11.761 ± 2.2259 (8‐15.7) | 12.032 ± 2.5151 (8.3‐15.8) | .714 |
| P a | .219 | .029 | — |
| MPV | |||
| Baseline | 10.131 ± 1.2455 (7.9‐14.2) | 13.116 ± 16.6717 (8.4‐930 | .381 |
| In discharge | 10.659 ± 1.482 (8.6‐14.5) | 15.213 ± 22.6369 (8.5‐97) | .450 |
| P a | .152 | .905 | — |
| RDW | |||
| Baseline | 14.237 ± 2.3369 (10‐20.4) | 14.208 ± 2.2692 (11.2‐21.80) | .933 |
| In discharge | 14.682 ± 2.4521 (11.5‐19.9) | 14.362 ± 1.6661 (11.9‐16.5) | .689 |
| P a | .726 | .622 | — |
| Creatinine (mg/dL) | |||
| Baseline | 2.9637 ± 7.90698 (0.6‐47) | 2.8993 ± 8.3426 (0.55‐46) | .974 |
| In discharge | 2.7632 ± 7.37937 (0.69‐38) | 3.9 ± 10.73347 (0.6‐48) | .679 |
| P a | .993 | .474 | — |
| Urea (mg/dL) | |||
| Baseline | 38.691 ± 17.9744 (1.1‐86) | 44.272 ± 46.2101 (0.9‐199) | .541 |
| In discharge | 45.241 ± 24.947 (1.3‐93) | 55.272 ± 48.8724 (1.2‐206) | .403 |
| P a | .263 | .588 | — |
| Sodium (mEq/L) | |||
| Baseline | 138.43 ± 3.071 (133‐148) | 136.86 ± 3.193 (128‐142) | .069 |
| In discharge | 139.9 ± 3.145 (136 −146) | 138.26 ± 3.619 (129‐143) | .133 |
| P a | .807 | .314 | — |
| Potassium (mEq/L) | |||
| Baseline | 4.187 ± 0.4328 (3.2‐4.9) | 4.269 ± 0.4878 (3.4‐5.2) | .467 |
| In discharge | 4.129 ± 0.4014 (3.2‐4.6) | 4.184 ± 0.7198 (2.5‐5.5) | .761 |
| P a | .056 | .705 | — |
| Calcium (mg/dL) | |||
| Baseline | 7.4514 ± 2.86195 (1.05‐10.1) | 8.0415 ± 2.14416 (0.89‐9.8) | .371 |
| In discharge | 7.4017 ± 2.90453 (1.03‐9.4) | 8.7375 ± 0.51624 (7.6‐9.5) | .071 |
| P a | .856 | .224 | — |
| Magnesium (mg/dL) | |||
| Baseline | 2.145 ± 0.531 (1.3‐4.1) | 1.985 ± 0.4213 (1.2‐2.7) | .182 |
| In discharge | 2.505 ± 0.7153 (1.6‐4.2) | 2.119 ± 0.2257 (1.8‐2.5) | .036 |
| P a | .040 | .333 | — |
| Phosphate (mg/dL) | |||
| Baseline | 2.611 ± 0.7328 (1.4‐4.4) | 2.733 ± 0.8195 (1.3‐4.4) | .536 |
| In discharge | 2.689 ± 0.5005 (1.9‐3.5) | 2.5 ± 0.6047 (0.9‐3.4) | .343 |
| P a | .772 | .685 | — |
| Aspartate aminotransferase (U/L) | |||
| Baseline | 39.51 ± 35.08 (9‐168) | 31.07 ± 14.684 (11‐58) | .237 |
| In discharge | 40.21 ± 28.913 (10‐110) | 31.67 ± 17.975 (13‐75) | .325 |
| P a | .184 | .592 | — |
| Alanine aminotransferase (U/L) | |||
| Baseline | 27.73 ± 14.689 (11‐67) | 24.64 ± 15.863 (9‐87) | .421 |
| In discharge | 30.68 ± 12.641 (10‐51) | 22.93 ± 12.792 (11‐60) | .087 |
| P a | .796 | .783 | — |
| Alkaline phosphatase (U/L) | |||
| Baseline | 205.62 ± 124.161 (32‐729) | 326.14 ± 524.88 (69‐2610) | .320 |
| In discharge | 175.26 ± 52.668 (101‐3190) | 170.2 ± 71.033 (75‐374) | .820 |
| P a | .577 | .584 | — |
| Fasting blood sugar (mg/dL) | |||
| Baseline | 116.71 ± 57.067 (16‐274) | 120.65 ± 40.873 (72‐224) | .755 |
| In discharge | 111.56 ± 33.703 (84‐202) | 148.15 ± 53.769 (95‐252) | .059 |
| P a | .271 | .037 | — |
| C‐reactive protein (mg/L) | |||
| Baseline | 17.97 ± 19.075 (0‐50) | 14.35 ± 16.94 (0‐44) | .438 |
| In discharge | 19 ± 21.839 (0‐50) | 12.25 ± 17.261 (0‐42) | .435 |
| P a | .483 | .697 | — |
| Erythrocyte sedimentation rate (mm/h) | |||
| Baseline | 33 ± 22.368 (2‐90) | 43.64 ± 31.48 (2‐94) | .182 |
| In discharge | 23.44 ± 12.156 (1‐40) | 46.2 ± 36.622 (4‐96) | .241 |
| P a | .320 | .596 | — |
| Lactate dehydrogenase (U/L) | |||
| Baseline | 587.21 ± 253.774 (0‐1108) | 585.22 ± 212.013 (264‐1027) | .976 |
| In discharge | 657.37 ± 383.675 (160‐1407) | 529.43 ± 285.616 (256‐1100) | .482 |
| P a | .094 | .238 | — |
| Pa O2 (mmHg) | |||
| Baseline | 46.565 ± 23.6733 (11.9‐100) | 43.109 ± 20.3456 (15‐86) | .576 |
| In discharge | 59.4 ± 21.6214 (31‐109) | 44.946 ± 22.255 (21‐100) | .084 |
| P a | .17 | .652 | — |
| Pa Co2 (mmHg) | |||
| Baseline | 46.522 ± 14.5597 (21.9‐87.1) | 40.579 ± 11.0142 (25‐71) | .062 |
| In discharge | 45.66 ± 8.3316 (32‐64.5) | 40.711 ± 8.5917 (25‐59) | .058 |
| P a | .90 | .216 | — |
| HCO3 (mEq/L) | |||
| Baseline | 25.935 ± 5.6337 (16‐44.3) | 23.528 ± 5.1165 (13‐36) | .078 |
| In discharge | 26.487 ± 4.5823 (16‐35) | 23.724 ± 4.6604 (16‐35.8) | .058 |
| P a | .29 | .707 | — |
| PH | |||
| Baseline | 7.3643 ± 0.04879 (7.25‐7.47) | 7.3603 ± 0.05809 (7.28‐7.49) | .763 |
| In discharge | 7.3642 ± 0.06536 (7.1‐7.46) | 7.3706 ± 0.06566 (7.23‐7.49) | .745 |
| P a | .41 | .855 | — |
Based on paired Student's t tests.
Based on independent t test.
In the losartan group, the mean admission‐ and discharge‐time SOFA score were 3.08 ± 1.35 and 2.42 ± 1.17, respectively (P value = .002). In the amlodipine group, the mean admission‐ and discharge‐time SOFA score was 3.74 ± 2.21 and 4.26 ± 3.71, respectively (P value = .326). The comparison of these groups highlighted no significant difference in disease severity between groups at discharge time (P value = .084).
3.4. Drug safety
We did not found adverse effects or symptoms with the losartan and amlodipine groups that were related to these medications administration.
4. DISCUSSION
The results of our study suggest that there were no significant difference in mortality rate, length of hospital stay, need to intubation between patients with primary HTN and COVID‐19 treated with losartan and amlodipine. Moreover, all patients were achieved to targeted blood pressure.
It is a major challenge to change or continue anti‐HTN medications in patients with HTN and COVID‐19. A recent retrospective study found that no association between ARBs taking by patients with COVID‐19 and no association between ARBs taking and poorer in‐hospital outcomes. 22
It should be considered that there was 7 years difference in the mean age of patients in the groups and it may be a notable factor in evaluating the mortality, morbidity and severity of COVID‐19. Because older ages accompanying with severe presentations of COVID‐19. 23
In animal models of ARDS and SARS, recombinant ACEII can protect the body from lung injuries. In a retrospective review performed on 539 hospitalised patients suffering from an infection, it has been demonstrated that this trend continues. The risk of pneumonia and mortality rate is reduced by the in‐hospital use of ACEI or ARB. 24 Moreover, according to a recent study on Japanese population, older age was an important factor to a worse prognosis in COVID‐19 patients, and ACEIs/ARBs could be beneficial for the prevention of confusion in COVID‐19 patients with HTN. 25
In a study by Liu et al, it has been reported that followed by COVID‐19 infection plasma angiotensin II concentration is expected to be elevated considerably. 26 However, ACEI/ARB efficacy on COVID‐19‐associated results has not been completely understood yet. Moreover, it is proposed that in comparison with ACEI, ARB can be more effective in the attenuation of death in patients with chronic obstructive pulmonary disease (COPD). 27 , 28
Using ACEI and ARB drugs to manage hypertensive patients with COVID‐19 has always been challenging. These drugs are responsible for the increase of ACEII, a cellular receptor of COVID‐19 that is needed for the viral infiltration into the host. 29 Highly expression of ACE can be observed in the cell membrane of vascular endothelial cells, and more prominent it can be seen in the lungs. 30
The correlation between ACEI/ARB pathway and the COVID‐19 mortality rate may result from the co‐morbidities and in‐hospital medications. Previously, it has been suggested that low levels of potassium may be a marker of unopposed angiotensin II. 31 , 32 Thus, the link between antihypertensive drugs and coronavirus can be defined as low levels of potassium known as hypokalaemia. However, further investigations are required to approve the link between these three factors. Potassium level was reduced more significantly in patients who used losartan in the present study, also the reduction of potassium level in the amlodipine group was less than cases and not significant.
Final responses to angiotensin II in an organ can be reduced by losartan, an angiotensin II antagonist with a selective, competitive task. This drug is constantly advised for patients with high blood pressure who are afflicted to diabetic nephropathies. 33 Physiological impacts of angiotensin II such as the secretion of aldosterone are neutralised by this antihypertensive drug which can increase the activation of plasma renin because of low levels of angiotensin II.
The results of a new study show that losartan suppresses polarised Th1/Th17‐mediated inflammatory responses. 34 One of the novels discovered strategies is damaging the Th1 and Th17 response results from losartan acute lung injury induced by lipopolysaccharides.
A recent study retrospective study found using amlodipine in HTN treatment in patients with COVID‐19 were associated with improvement in mortality rate and critical condition of patients. 35 Therefore, amlodipine safety in COVID‐19 patients was in line with our results.
The presented study has some limitations. The small sample size especially small group of patients with the critical condition and short‐term follow up were the limitations of this single‐centre study. Also, all of the patients were Iranian; therefore, the findings might not be generalised in different ethnicity. Possible confounding factors not otherwise accounted for this study was another limitation.
5. CONCLUSIONS
In conclusion, there was no priority in losartan or amlodipine administration in COVID‐19 patients with primary HTN. Further studies need to clarify the first‐line anti‐hypertension medications in COVID‐19. Further studies are required to advise losartan as a safe treatment in patients with COVID‐19 and primary HTN.
DISCLOSURE
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENT
We would like special thank to Tuberculosis and Lung Disease Research Center of Tabriz University of Medical Sciences. This work was supported by COVID‐19 grant from the Tabriz University of Medical Sciences (Grant number: 65204).
Nouri‐Vaskeh M, Kalami N, Zand R, et al. Comparison of losartan and amlodipine effects on the outcomes of patient with COVID‐19 and primary hypertension: A randomised clinical trial. Int J Clin Pract. 2021;75:e14124. 10.1111/ijcp.14124
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, AS, upon reasonable request.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nouri‐Vaskeh M, Alizadeh L. Fecal transmission in COVID‐19: a potential shedding route. J Med Virol. 2020;92:1731‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nouri‐Vaskeh M, Khalili N, Sharifi A, et al. Clinical characteristics of fatal cases of COVID‐19 in Tabriz, Iran: an analysis of 111 patients. Adv J Emerg Med. 2020;5:e12. [Google Scholar]
- 6. Drager LF, Pio‐Abreu A, Lopes RD, Bortolotto LA. Is hypertension a real risk factor for poor prognosis in the COVID‐19 pandemic? Curr Hypertens Rep. 2020;22:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imanpour H, Rezaee H, Nouri‐Vaskeh M. Angiotensin 1–7: a novel strategy in COVID‐19 treatment. Adv Pharm Bull. 2020;10:488‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30:160‐164. [DOI] [PubMed] [Google Scholar]
- 9. Kovell LC, Ahmed HM, Misra S, et al. US hypertension management guidelines: a review of the recent past and recommendations for the future. J Am Heart Assoc. 2015;4:e002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicin L, Abplanalp WT, Mellentin H, et al. Cell type‐specific expression of the putative SARS‐CoV‐2 receptor ACE2 in human hearts. Eur Heart J. 2020;41:1804‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin‐converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel AB, Verma A. COVID‐19 and angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323:1769‐1770. [DOI] [PubMed] [Google Scholar]
- 14. Zeinalian M, Salari‐Jazi A, Jannesari A, Khanahmad H. A potential protective role of losartan against coronavirus‐induced lung damage. Infect Control Hosp Epidemiol. 2020;41:752‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerging Microbes Infect. 2020;9:757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L, Qiu HB, Yang Y, Wang L, Ding HM, Li HP. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide‐induced acute lung injury in rat. Arch Biochem Biophys. 2009;481:131‐136. [DOI] [PubMed] [Google Scholar]
- 18. Jurewicz M, McDermott DH, Sechler JM, et al. Human T and natural killer cells possess a functional renin‐angiotensin system: further mechanisms of angiotensin II‐induced inflammation. J Am Soc Nephrol. 2007;18:1093‐1102. [DOI] [PubMed] [Google Scholar]
- 19. Hoch N, Guzik T, Chen W, et al. Regulation of T‐cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208‐R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. WHO . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19); 2020. https://www.who.int/publications/i/item/report‐of‐the‐who‐china‐joint‐mission‐on‐coronavirus‐disease‐2019‐(covid‐19)
- 21. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707‐710. [DOI] [PubMed] [Google Scholar]
- 22. Soleimani A, Kazemian S, Karbalai Saleh S, et al. Effects of angiotensin receptor blockers (ARBs) on in‐hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID‐19. Am J Hypertens. 2020;33:1102‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mallapaty S. The coronavirus is most deadly if you are older and male ‐ new data reveal the risks. Nature. 2020;585:16‐17. [DOI] [PubMed] [Google Scholar]
- 24. Henry C, Zaizafoun M, Stock E, Ghamande S. Impact of angiotensin‐converting enzyme inhibitors and statins on viral pneumonia. Baylor Univ Med Center Proc. 2018;31:419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuzawa Y, Ogawa H, Kimura K, et al. Renin‐angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai CC, Wang YH, Wang CY, Wang HC, Yu CJ, Chen L. Comparative effects of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:867‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nouri‐Vaskeh M, Sharifi A, Khalili N, Zand R, Sharifi A. Dyspneic and non‐dyspneic (silent) hypoxemia in COVID‐19: Possible neurological mechanism. Clin Neurol Neurosurg. 2020;198:106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Igase M, Kohara K, Nagai T, Miki T, Ferrario CM. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertens Res. 2008;31:553‐559. [DOI] [PubMed] [Google Scholar]
- 30. Clarke NE, Turner AJ. Angiotensin‐converting enzyme 2: the first decade. Int J Hypertens. 2012;2012:307315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin‐angiotensin‐aldosterone system alterations. Circ Res. 2015;116:960‐975. [DOI] [PubMed] [Google Scholar]
- 32. Kozhuharov N, Goudev A, Flores D, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. 2019;322:2292‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheetham C, Collis J, O'Driscoll G, Stanton K, Taylor R, Green D. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in non‐insulin‐dependent diabetes. J Am Coll Cardiol. 2000;36:1461‐1466. [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Zhang PS, Yu Q, et al. Losartan inhibits conventional dendritic cell maturation and Th1 and Th17 polarization responses: Νovel mechanisms of preventive effects on lipopolysaccharide‐induced acute lung injury. Int J Mol Med. 2012;29:269‐276. [DOI] [PubMed] [Google Scholar]
- 35. Solaimanzadeh I. Nifedipine and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID‐19. Cureus. 2020;12:e8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, AS, upon reasonable request.


