Abstract
Adverse clinical outcomes related to SARS‐CoV‐2 infection among liver transplant (LTx) recipients remain undefined. We performed a meta‐analysis to determine the pooled prevalence of outcomes among hospitalized LTx recipients with COVID‐19. A database search of literature published between December 1, 2019, and November 20, 2020, was performed per PRISMA guidelines. Twelve studies comprising 517 hospitalized LTx recipients with COVID‐19 were analyzed. Common presenting symptoms were fever (71%), cough (62%), dyspnea (48%), and diarrhea (28%). Approximately 77% (95% CI, 61%‐93%) of LTx recipients had a history of liver cirrhosis. The most prevalent comorbidities were hypertension (55%), diabetes (45%), and cardiac disease (21%). In‐hospital mortality was 20% (95% CI, 13%‐28%) and rose to 41% (95% CI, 19%‐63%) (P < 0.00) with ICU admission. Additional subgroup analysis demonstrated a higher mortality risk in the elderly (>60‐65 years) (OR 4.26; 95% CI, 2.14‐8.49). There was no correlation in respect to sex or time since transplant. In summary, LTx recipients with COVID‐19 had a high prevalence of dyspnea and gastrointestinal symptoms. In‐hospital mortality was comparable to non‐transplant populations with similar comorbidities but appeared to be less than what is reported elsewhere for cirrhotic patients (26%‐40%). Importantly, the observed high case fatality in the elderly could be due to age‐associated comorbidities.
Keywords: COVID‐19, liver transplantation, SARS‐CoV‐2
Abbreviations
- ACE2 receptor
angiotensin‐converting enzyme 2 receptor
- ARDS
acute respiratory distress syndrome
- CAID
cirrhosis‐associated immune dysfunction
- CI
confidence interval
- CNI
calcineurin inhibitor
- COVID‐19
corona virus disease 2019
- ICU
intensive care unit
- IQR
interquartile range
- LTx
liver transplantation
- MELD
model for end‐stage liver disease
- MMF/MPA
mycophenolate mofetil/mycophenolic acid
- mTORi
mammalian target of rapamycin inhibitors
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- T2DM
type 2 diabetes mellitus
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is highly transmissible disease and entails significant mortality. The COVID‐19 pandemic has already affected over 89 million people globally, with a fatality rate of 2%‐6%. 1 The infection is of significant health concern in the elderly as well as populations with underlying comorbidities such as hypertension, diabetes mellitus, and chronic lung diseases. SARS‐CoV‐2 carries a higher risk of adverse outcomes in patients with specific disease states including chronic liver disease. SARS‐CoV‐2 virus can have higher adverse outcomes in patients with comorbidities, including chronic liver disease and liver transplant recipients owing to immune dysregulation, immunosuppressive state, and associated comorbidities. 2 , 3 , 4 , 5
Liver transplantation has been established as a life‐saving procedure for all forms of end‐stage liver disease; however, in the initial phase of the global COVID‐19 pandemic, most transplant centers were forced to restrict transplant activities not only due to the highly transmissible nature of the pathogen but also because of a heightened risk of severe disease in the immunocompromised individuals. 6 , 7 Hence, a better understanding of the disease process in this specific cohort needs to be pursued to allow for optimal organ and patient selection for liver transplantation as well as perioperative screening and immunosuppression management. Moreover, to optimize transplant timing, such concerns ought to be balanced between the impact of a SARS‐CoV‐2 infection in cirrhotic patients versus liver transplant recipients. Recent reports by two international collaborative registries (ie, the COVID‐Hep registry at COVID‐Hep.net and the SECURE‐cirrhosis registry at covidcirrhosis.web.unc.edu) included data from 103 cirrhotic patients from 18 countries. These databases reported a 95.2% hospitalization rate and overall in‐house mortality of 39.8%. The studies revealed a significant association between case fatality and Child‐Pugh class “C” as well as higher MELD score (model for end‐stage liver disease) with a dismal prognosis and overall mortality of 63.2%. 8
In this meta‐analysis, we summarize the existing literature pertaining to COVID‐19 in liver transplant recipients in order to determine the impact of the pathogen in this cohort. Furthermore, we highlight reported changes in immunosuppressive regimens and attempt to identify modifiable clinical factors associated with clinical outcomes and mortality. We also reviewed the existing literature to compare the morbidity and mortality between decompensated cirrhotic patients and liver transplant recipients.
2. METHODS
2.1. Search strategy
The initial systematic review was performed following registration in PROSPERO, an international database of prospectively registered systematic reviews (CRD42020191699). The search strategy was formulated as per the Cochrane Handbook for Systematic Reviews and reported as per the guidelines proposed by a meta‐analysis of observational studies in epidemiology (MOOSE). 9 A comprehensive electronic literature search was made using MeSH terms “COVID‐19” AND “liver transplantation”; “Coronavirus” AND “liver transplantation”; “COVID‐19” AND “liver transplantation” AND “mortality”; “COVID‐19” AND “liver transplantation” AND “Clinical outcomes”. We searched the following databases: MEDLINE, PubMed, EMBASE, MedRxiv, Cochrane, Crossref, Scopus, and clinical trial registries on November 10, 2020. Additionally, a manual search suing the free terms “2019 novel coronavirus”, “SARS‐CoV‐2”, “2019‐nCoV infection” was made for preprints, case reports, abstracts, and bibliographies to identify additional eligible studies. The final search was completed on November 20, 2020, and was not restricted by language or geography. After an initial screen of titles and abstracts, the full text of identified articles was search based upon previously established inclusion criteria.
2.2. Inclusion criteria
All observational studies available in the form of full‐text articles relating to COVID‐19 in liver transplantation were reviewed. All other publications, including editorials, reviews, and letters, were also excluded. Our outcomes of interest included clinical presentation, the severity of respiratory disease, hospital admission, intensive care unit admission, the need for mechanical ventilation, the incidence of ARDS (acute respiratory distress syndrome), presence of acute kidney injury, blood levels of lymphocytes, liver enzymes, serum bilirubin and inflammatory markers, modifications of the immunosuppressive regimen, whether other treatments were administered, prognosis as related to recovery, graft rejection, and mortality as reported in the reviewed literature. Wherever possible, group‐wise comparisons were made to determine mortality comparing the following factors: age (<60‐65 years vs ≥60‐65 years), time since LTx (<2 years vs ≥2 years), and sex (as reported).
2.3. Data extraction
Two separate reviewers, KJ and IR, independently screened the search results using a two‐stage method via a shared online platform. In the first stage, article titles and abstracts were scrutinized to exclude obviously ineligible studies. During the second stage, full texts or available limited text (eg, posters) were read and additional articles were excluded. In case of disagreement in article selection, matters were discussed until a consensus was achieved in collaboration with the senior author (PW). The Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines were used to direct the search and study selection (Figure 1). The data were extracted from the included studies and organized into a pre‐defined data set to generate central tendency (ie, mean or median) and dispersion (ie, 95% CI, IQR, or range). Whenever means and standard deviations of the analyzed variables were not available, the values were inputted from the available statistics (ie, median, IQR, or range). 10 , 11
FIGURE 1.
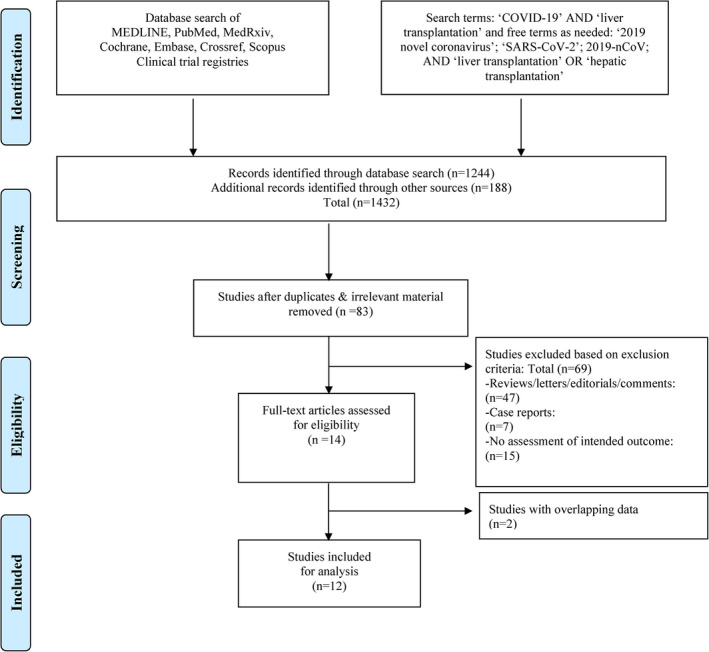
Search strategy and study selection used in this systematic review as per PRISMA protocol
Heterogeneity among included studies was investigated through I 2 statistics and designated as low if I2 was ≤ 25%, moderate if 25%‐75%, and high if I2 was ≥ 75%. 12 Due to the heterogeneity within and between the studies, a random‐effects model was chosen to compute the pooled prevalence (ie, effect size) with 95% confidence interval (CI). The software used for statistical analysis was STATA/SE 16 (Stata, College Station, TX). In case of a single arm “zero” event, 0.5 was added to the zero cells and the “metan” command was used. Whenever there was a “zero event” in both arms, the study was excluded from the analysis. The risk of bias for observational studies was evaluated via a quality analysis of included studies as per the guidelines suggested by the National Institutes of Health in the Quality Assessment Tool for Case Series Studies whenever applicable. 13 , 14
3. RESULTS
3.1. Search results
The primary literature search yielded a total of 83 articles matching our preliminary selection criteria. Of these, 69 were excluded as detailed in the PRISMA flowchart (Figure 1). Other articles such as case reports, reviews, letters to editor, opinions, and editorials were excluded as well (Table 1). Whenever we identified studies by the same authors containing overlapping data, only the study with highest number of cases was selected for inclusion. 15 , 16 A final total of 12 observational studies were included for data extraction (Figure 2). 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
TABLE 1.
Baseline attributes of post‐liver transplant patients in included studies
| Study | Country | Number | Sex (M/F) | Age (y) | Ethnicity | Comorbidities | Reason for transplantation | Medication (Immunosuppression + others) |
Time since liver transplant Median (range) |
|---|---|---|---|---|---|---|---|---|---|
| Becchetti et al 17 | Multiple | 57 | 40 (70%)/ 17 (30%) | 65 (57‐70) |
Caucasians: 53 (93%) Others: 4 (7%) |
HTN: 32 (56%) DM: 21 (37%) CAD: 7 (12%) Lung disease: 13 (23%) Obesity: 1 (17%) Cancer: 5 (9%) Smoking: 7 (12%) |
ESLD: 38 (68%) Liver tumor: 16 (29%) ALF: 4 (7%) Other: 9 (16%) |
CNI: 50 (88%) Prednisone: 10 (18%) MMF/MPA: 25 (44%) mTORi: 7 (12%) |
NA |
| Fernandez‐Ruiz et al 18 | Spain | 6 | 4 (67%)/ 2 (33%) | 72.5 (55.44‐80.89) | NA |
HTN: 3 (50%) DM: 4 (67%) CAD: 1 (17%) |
Cirrhosis: 4 (67%) HCC: 2 (33%) ALF: 1(17%) |
CNI: 2 (33%) Prednisone: 1 (17%) MMF/MPA: 2 (33%) mTORi: 3 (50%) |
11.6 (4.54‐21.46) y |
| Donato et al 21 | Italy | 8 | 6 (75%)/ 2 (25%) | 61 (55.11‐70.89) | NA |
CAD: 4 (50%) Lung disease: 4 (50%) |
NA |
CNI: 7 (87.5%) Prednisone: 2 (25%) MMF/MPA: 6 (75%) |
9.75 (4.19‐15.90) y |
| Yi et al 22 | United States | 4 | NA | NA | NA | NA | NA | NA | NA |
| Loinaz et al 23 | Spain | 19 | 14 (74%)/ 5 (26%) | 58 (57.55‐67.19) | NA |
HTN: 10 (52.6%) DM: 6 (31%) CAD: 7 (12%) Lung disease: 13 (23%) Obesity: 1 (17%) Cancer: 5 (9%) Smoking: 7 (12%) |
Cirrhosis: 17 (89.47%) HCC: 7 (36.81%) |
CNI: 8 (42.1%) Prednisone: 3 (15.8%) MMF/MPA: 10 (52.6%) mTORi: 4 (21.5%) |
6.91 (5.28‐12.15) y |
| Webb et al 24 | Multiple | 151 | 102 (68%)/ 49 (32%) | 60 (47‐66) |
African American: 16 (11%) Caucasian: 111 (74%) Hispanic: 6 (4%) Asian: 13 (8%) Others: 6 (3%) |
HTN: 63 (42%) DM: 65 (43%) CAD: 22 (15%) CVD: 3 (2%) Lung disease: 8 (6%) Obesity: 44 (29%) Cancer: 8 (5%) Smoking: 3 (2%) |
Cirrhosis: 105 (95%) PSC: 19 (13%) PBC: 6 (4%) Others: 20 (13%) |
CNI: 135 (89%) Prednisone: 67 (44%) MMF/MPA: 77 (51%) Aza: 13 (9%) mTORi: 7 (5%) |
5 (2‐11) y |
| Tschopp et al 25 | Switzerland | 5 | NA | NA | NA | NA | NA | NA | NA |
| Lee et al 26 | United States | 38 | 26 (68%)/ 12 (32%) | 63 (27‐81) |
African American: 5 (13%) Caucasian: 15 (39%) Hispanic: 14 (39%) Asian: 2 (5%) Others: 2 (5%) |
NA |
ESLD: 30 (79%) HCC: 7 (18%) ALF: 1 (3%) |
CNI: 38 (100%) Prednisone: 15 (39%) MMF/MPA: 19 (50%) mTORi: 1 (3%) |
3.8 (0.02‐28.2) y |
| Belli et al 27 | Multiple | 103 | 76 (74%)/ 27 (26%) | 65 (11‐82) | NA |
HTN: 52/101 (51%) DM: 41/101 (41%) CAD: 7/101 (7%) Lung disease: NA Obesity: 49/88 (56%) Cancer: NA Smoking: 13/101 (13%) |
NA | NA | NA |
| Colmenero et al 28 | Spain | 111 | 79 (71%)/ 32 (29%) | 65.34 ± 10.9 | NA |
HTN: 64/111 (58%) DM: 53/111 (48%) CAD: 22/111 (20%) Lung disease: 13/111 (12%) |
NA |
CNI: 72 (65%) Prednisone: 24 (22%) MMF/MPA: 57 (51%) mTORi: 23 (21%) |
8.8 (2.91‐14) y |
| Patrono et al 20 | Italy | 9 | 7 (78%)/ 2 (22%) | NA | NA | NA |
CNI: 9 (100%) Prednisone: 2 (22%) MMF/MPA: 5 (55%) mTORi: 2 (2%) |
||
| Bhoori et al 19 | Italy | 6 | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: ALF: Acute liver failure; Aza: Azathioprine; CAD: Coronary artery disease; CNI: calcineurin inhibitor; CVD: Cerebrovascular disease; DM: Diabetes mellitus; ESLD: End‐stage liver disease; F: Female; HCC: Hepatocellular carcinoma; HTN: Hypertension; M: Male; MMF: Mycophenolate mofetil; MPA: Mycophenolic acid; mTORi: mammalian target of rapamycin inhibitor; NA: Not available; PBC: primary biliary cholangitis; PSC: primary sclerosing cholangitis; Yrs: Years.
FIGURE 2.
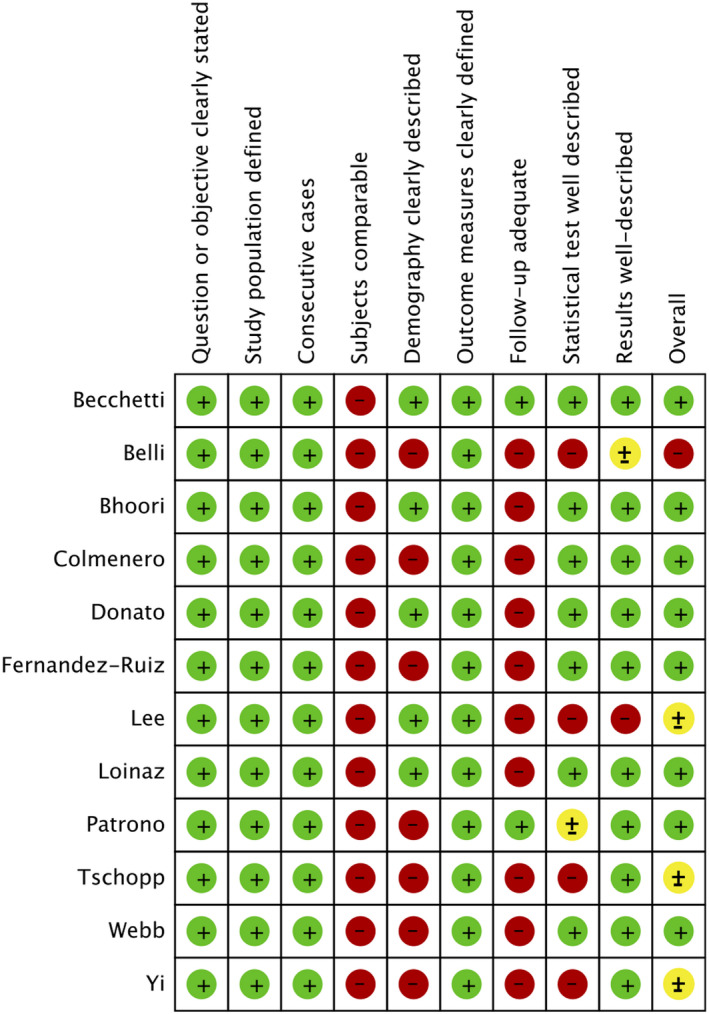
Quality assessment of included studies. (green—low risk of bias; yellow—unclear risk of bias; red—high risk of bias)
The pooled estimate of reported attributes of COVID‐19 in the LTx population was assessed through exploratory random‐effects analyses of proportion and effect size, and these are presented as percentages. The detailed results of the data analysis are tabulated in Tables 2 and 3. Twelve studies are included that reported a total of 517 eligible liver transplant recipients diagnosed with COVID‐19. The pooled estimate mean age of these patients was 63.58 years (95% CI 59.66‐67.48 years). The male population proportion was 70.87% (95% CI 68.25%‐73.50%). Approximately 77% (95% CI, 61%‐93%) of the patients had undergone liver transplantation in the setting of liver cirrhosis. The time from transplant to COVID‐19 diagnosis was defined in seven studies including 342 patients with a mean time after LTx of 8.89 years (95% CI, 6.60‐11.17 years). Overall, 118 of the identified recipients (80%) acquired COVID‐19 > 2 years after liver transplantation; only 29 patients (20%) were identified who presented to COVID infection within 2 years of liver transplantation. It should be noted that the time interval between development of initial symptoms to presentation was incompletely reported. Based on the available data, the pooled prevalence among Caucasian LTx recipients was 70% (95% CI 46%‐93%), African Americans 11% (95% CI 7%‐16%), and Latinos/Hispanics 5% (95% CI 2%‐8%).
TABLE 2.
Baseline clinical characteristics of liver transplant recipients with COVID‐19 in reviewed studies
| Study | Clinical presentation | Disease severity | Intensive care | Laboratory parameters | Serum markers | ||
|---|---|---|---|---|---|---|---|
| Median (range) | Significant decline n (%) | Median (range) | Significant change n (%) | ||||
| Becchetti et al 17 |
Fever: 44 (79%) Dyspnoea:26 (46%) Cough: 31 (54%) GIs: 18 (33%) |
Hospitalized 41 (72%) Radiographic evidence of pneumonia: 24/41 (59%) |
ARDS: 11/41 (27%) ICU: 4/41 (10%) MV: 4/41 (10%) |
WBC: 4500 (3330‐6000) / mm3 Platelet: 160x103 (91x103‐ 268x103) /mm3 Lymphocyte: 790 (400‐1100) / mm3 AST: 38 (25‐53) U/L ALT: 30 (20‐42) U/L ALP: 97 (74‐171) U/L |
NA |
Ferritin: 567 (171‐1194) ng/mL IL‐6:93 (59‐288) pg/L D‐dimer: 8.71 (3.8‐17.39) µg/mL |
NA |
| Fernandez‐Ruiz et al 18 |
Fever: 4 (67%) Dyspnea: 4 (67%) Cough: 4 (67%) GIs: 3 (50%) |
Hospitalized: 6 (100%) Radiological evidence of pneumonia: 4/6 (67%) |
ARDS: 2/6 (33%) AKI: 1/6 (33%) ICU: 1/6 (17%) MV: 1/6 (17%) |
NA | NA | NA | NA |
| Donato et al 21 |
Fever: 8 (100%) Dyspnea: 7 (87.5%) GIs: 1 (12.5%) |
Hospitalized: 5 (62.5%) Radiological evidence of pneumonia: 5/5 (100%) |
ARDS: NA AKI: NA ICU: 0/5 (0%) |
NA | NA | NA | NA |
| Yi et al 22 | NA |
Hospitalized: 2 (50%) Radiological evidence of pneumonia: NA |
ARDS: NA AKI: NA ICU: 1/2 (50%) |
NA | NA | NA | NA |
| Loinaz et al 23 |
Fever: 8 (42.1%) Dyspnea: 9 (47.4%) Cough: 16 (84.2%) |
Hospitalized: 14/19 (74%) Radiological evidence of pneumonia: 13/19 (68%) |
ARDS: NA AKI: 22/130 (17%) ICU: 2/14 (14.3%) MV: 1/14 (7.1%) |
NA | Lymphocytopenia (<1000/mm3): 11/14 (78.6%) | NA | CRP (>5 mg/dL): 9/14 (64.3%) |
| Webb et al 24 |
Resp: 83/149 (56%) GIs: 14/149 (9%) Resp & GIs: 31/149 (21%) |
Hospitalized: 124/151 (82%) |
ARDS: NA AKI: NA ICU: 43/124 (34.6%) MV: 30/124 (24%) |
NA | NA | NA | NA |
| Tschopp et al 25 | NA | Hospitalized: 5/5 (100%) |
ARDS: NA AKI: NA ICU: 0/5 (0%) MV: 0/5 (0%) |
NA | NA | NA | NA |
| Lee et al 26 |
Fever: 23 (61%) Dyspnea: 13 (34%) Cough: 21 (55%) GIs: 16 (42%) |
Hospitalized: 27/38 (71%) (data available 24) Radiological evidence of pneumonia: 22/24 (92%) |
ARDS: NA AKI: 13/24 (54%) ICU: 8/24 (33%) MV: 8/24 (33%) |
Lymphocyte: 600 (200‐5600) / mm3 AST: 31 (10‐1691) U/L ALT: 22 (5‐1578) U/L ALP: 131 (48‐1302) U/L |
NA |
Ferritin: 986 (36‐4677) ng/mL IL‐6:66.3 (12.5‐218) pg/L D‐dimer: 1.67 (0.27 ‐ 8.62) µg/mL |
NA |
| Belli et al 27 |
Fever: 71/102 (70%) Dyspnea: 35/102 (34%) Cough: 60/102 (59%) GIs: 24/102 (24%) |
Hospitalized: 83/103 (81%) Radiological evidence of pneumonia: 64/83 (77%) |
ARDS: NA AKI: NA ICU: 15/83 (18%) MV: 10/83 (15%) |
NA | NA | NA | NA |
| Colmenero et al 28 |
Fever: 83/111 (75%) Dyspnea: 46/111 (41%) Cough: 78/111 (70%) GIs: 38/111 (34%) |
Hospitalized: 96/111 (87%) Radiological evidence of pneumonia: 87/111 (78%) |
ARDS: 65/96 (68%) ICU: 12/96 (13%) MV: 9/12 (75%) AKI: NA |
Lymphocyte: 455 (275‐755) / mm3 | NA |
Ferritin: 847 (376‐1975) ng/mL D‐dimer: 1.05 (0.52‐3.29) µg/mL |
NA |
| Patrono et al 20 |
Fever: 7/9 (77%) Dyspnea: 1/9 (11%) Cough: 3/9 (33%) GIs: 3/9 (33%) |
Hospitalized: 9/10 (90%) Radiological evidence of pneumonia: 7/9 (77%) |
ARDS: NA ICU: NA MV: NA AKI: NA |
NA | NA | NA | NA |
| Bhoori et al 19 | NA |
Hospitalized: 3/6 (50%) Radiological evidence of pneumonia: NA |
ARDS: 3/6 (50%) AKI: NA ICU: 3/6 (50%) MV: 3/6 (50%) |
NA | NA | NA | NA |
Abbreviations: AKI: acute kidney injury; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ARDS: Acute respiratory distress syndrome; AST: aspartate aminotransferase; CRP: C‐reactive protein; GIs: Gastrointestinal symptoms; ICU: Intensive Care Unit; IL‐6: Interleukin 6; MV: Mechanical ventilation; NA: Not available; WBC: White blood count.
TABLE 3.
Outcomes in liver transplant recipients with COVID‐19 in reviewed studies
| Study | Modulation in immunosuppressive regimen | Other measures | Outcome | Remarks |
|---|---|---|---|---|
| Becchetti et al 17 |
|
|
|
|
| Fernandez‐Ruiz et al 18 |
|
|
|
|
| Donato et al 21 |
|
|
|
|
| Yi et al 22 |
|
|
|
|
| Loinaz et al 23 |
|
|
|
|
| Webb et al 24 |
|
|
|
|
| Tschopp et al 25 |
|
|
|
|
| Lee et al 26 |
|
|
|
|
| Belli et al 27 |
|
|
|
|
| Colmenero et al 28 |
|
|
|
|
| Patrono et al 20 |
|
|
|
|
| Bhoori et al 19 |
|
|
|
|
Abbreviations: CNI: calcineurin inhibitor; HCQ: Hydroxychloroquine; ICU: intensive care unit; IL‐6 antagonist: Interleukin‐6 antagonist; LT: liver transplantation; MMF: Mycophenolate mofetil; mTORi: mammalian target of rapamycin inhibitor; MV: Mechanical ventilation; NA: Not available; QTc: Corrected QT interval; yrs: years.
3.2. Comorbidities of liver transplant recipients with COVID‐19
We assessed the prevalence of various comorbidities outlined in the included studies. Type 2 diabetes mellitus (T2DM) was reported in eight studies; 211 patients out of 486 confirmed cases of COVID‐19 had T2DM with a pooled prevalence of 45% (95% CI, 38%‐53%). Hypertension was reported in eight studies; 251 patients among 486 COVID‐19 cases carried a diagnosis of hypertension with a pooled prevalence of 55% (95% CI, 47%‐64%). Further, seven studies, including 88 patients, had confirmed cardiac disease with a prevalence of 21% (95% CI, 13%‐30%). The prevalence of obesity among the indexed COVID‐19 cohort was 33% (95% CI, 11%‐56%). Six studies, 352 COVID‐19 patients, of which 42 had chronic lung disease patients with pooled prevalence of 14% (95% CI, 6%‐22%). The pooled prevalence of malignancy and smoking were both 11% (Table 4).
TABLE 4.
Summary statistics presented as pooled estimates of outcomes of interest
| Attributes | Events | Total | Studies | Pooled prevalence (95%CI) a |
|---|---|---|---|---|
| Random‐effects model | ||||
| Demographic variables | ||||
| Age (y) | NA | 502 | 9 | 63.58 (59.66‐67.48) |
| Male | 354 | 505 | 10 | 70.87 (68.25‐73.50) |
| Caucasian | 246 | 179 | 3 | 0.70 (0.46‐0.93) |
| African American | 189 | 21 | 2 | 0.11 (0.07‐0.16) |
| Latino/Hispanic | 189 | 20 | 2 | 0.05 (0.02‐0.08) |
| Asian | 189 | 10 | 2 | 0.05 (0.02‐0.08) |
| Comorbidities | ||||
| Diabetes | 211 | 486 | 8 | 0.45 (0.38‐0.53) |
| Hypertension | 251 | 486 | 8 | 0.55 (0.47‐0.64) |
| Cardiac disease | 88 | 472 | 7 | 0.21 (0.13‐0.30) |
| Chronic lung disease | 42 | 352 | 6 | 0.14 (0.06‐0.22) |
| Malignancy | 24 | 279 | 6 | 0.11 (0.02‐0.20) |
| Obesity | 71 | 200 | 6 | 0.33 (0.11‐0.56) |
| Smoking | 23 | 317 | 4 | 0.07 (0.00‐0.14) |
| Maintenance immunosuppression | ||||
| CNI | 252 | 291 | 8 | 0.86 (0.76‐0.95) |
| MMF/MPA | 144 | 288 | 7 | 0.50 (0.44‐0.56) |
| mTORi | 24 | 280 | 6 | 0.09 (0.03‐0.15) |
| Presenting parameters and symptoms | ||||
| Time since transplant (y) | NA | 342 | 7 | 8.89 (6.60‐11.17) |
| Fever | 240 | 350 | 8 | 0.71 (0.61‐0.81) |
| Cough | 213 | 342 | 7 | 0.62 (0.53‐0.72) |
| Dyspnea | 144 | 353 | 9 | 0.48 (0.36‐0.61) |
| GIs | 87 | 293 | 6 | 0.28 (0.20‐0.35) |
| Investigations | ||||
| Radiological evidence of pneumonia | 226 | 299 | 8 | 0.77 (0.69‐0.84) |
| Clinical management and outcome | ||||
| ARDS | 81 | 146 | 5 | 0.56 (0.26‐0.86) |
| ICU admissions | 95 | 417 | 12 | 0.22 (0.12‐0.32) |
| Mechanical ventilation | 67 | 322 | 10 | 0.24 (0.12‐0.36) |
| CNI withheld/reduced | 39 | 91 | 4 | 0.38 (0.09‐0.67) |
| MMF/MPA withheld/reduced | 28 | 55 | 5 | 0.60 (0.17‐0.90) |
| mTORi withheld/reduced | 7 | 14 | 3 | 0.50 (0.25‐0.76) |
| Increase/pulse steroid | 59 | 283 | 7 | 0.22 (0.13‐0.31) |
| Hydroxychloroquine | 248 | 441 | 8 | 0.58 (0.35‐0.82) |
| Lopinavir/ritonavir | 73 | 412 | 7 | 0.17 (0.07‐0.28) |
| Tocilizumab | 27 | 397 | 5 | 0.06 (0.01‐0.12) |
| Azithromycin | 125 | 407 | 5 | 0.41 (0.10‐0.73) |
| Hospital death | 87 | 411 | 11 | 0.20 (0.13‐0.28) |
| ICU death | 39 | 95 | 11 | 0.41 (0.19‐0.63) |
Abbreviations: ARDS: Acute respiratory distress syndrome; CNI: Calcineurin inhibitor; GIs: Gastrointestinal symptoms; ICU: Intensive care unit; MMF: Mycophenolate mofetil; MPA: Mycophenolic acid; mTORi: mammalian target of rapamycin inhibitor.
Pooled prevalence is measured as effect size (ES). Age is presented as a mean; the remaining variables are expressed as the proportion of individuals (ie, events) out of total available sample size based upon inclusion of index parameters. ES is explained as a percentage in the result section.
3.3. Clinical characteristics of liver transplant recipients with COVID‐19
The pooled estimate of presenting symptoms of COVID‐19 in the LTx population is presented in Table 4. Fever was the most common with a prevalence of 71% (95% CI, 61%‐81%), followed by cough in 62% (95% CI, 53%‐73%). Other common manifestations were dyspnea in 48%, and gastrointestinal symptoms in 28% of patients.
3.4. Clinical presentation, disease severity, and mortality among liver transplant recipients with COVID‐19
The pooled prevalence of pneumonia on imaging was 77% (95% CI, 69%‐84%). The pooled prevalence of complications such as ARDS and respiratory status requiring mechanical ventilation was 56% (95% CI, 26%‐86%) and 24% (95% CI, 12%‐36%), respectively (Figure 3A, B). The incidence of intensive care unit (ICU) admission was 22% (95%CI, 12%‐32%) (Figure 3C). The pooled prevalence of in‐hospital mortality rate was 20% (95%CI, 13%‐28%) (Figure 3D) and was significantly lower as compared to the mortality for patients admitted to the ICU which was 41% (95% CI, 19%‐63%) (P < 0.00) (Figure 3E).
FIGURE 3.
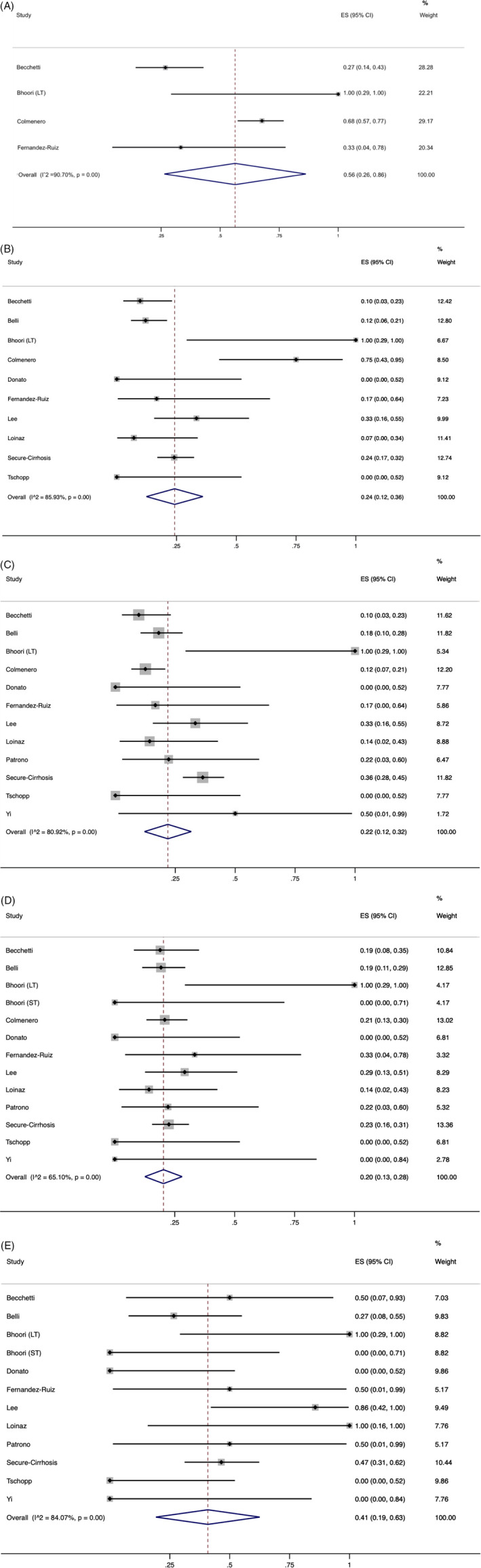
A, Pooled prevalence of intensive care admission in liver transplant recipients diagnosed with COVID‐19. The red dashed line represents the overall effect size of the studies (0.22) and prevalence of 22%. The edges of the blue diamond represent 95% confidence intervals (0.12‐0.32). ES = Effect size. B, Pooled prevalence of acute respiratory distress syndrome in liver transplant recipients diagnosed with COVID‐19. The red dotted line represents the overall effect size of the studies (0.56) and prevalence of 56%. The edges of the blue diamond represent 95% confidence intervals (0.26‐0.86). ES = Effect size; C, Pooled prevalence of mechanical ventilation requirement in liver transplant recipients diagnosed with COVID‐19. The red dashed line represents the overall effect size of the studies (0.24) and prevalence of 24%. The edges of the blue diamond represent 95% confidence intervals (0.12‐0.36). ES = Effect size. D, Pooled prevalence of hospital mortality in liver transplant recipients diagnosed with COVID‐19. The red dotted line represents the overall effect size of the studies (0.20) and prevalence of 20%. The edges of the blue diamond represent 95% confidence intervals (0.13‐0.28). ES = Effect size. E, Pooled prevalence of intensive care mortality in liver transplant recipients diagnosed with COVID‐19. The red dashed line represents the overall effect size of the studies (0.41) and prevalence of 41%. The edges of the blue diamond represent 95% confidence intervals (0.19‐0.63). ES = Effect size
3.5. Immunosuppression regimen and other drug management in liver transplant recipients with COVID‐19
The available data from the included studies revealed that calcineurin inhibitors were used for maintenance immunosuppression in 86% (95% CI, 76%‐95%) of reported patients and were withheld or reduced in 38% (95% CI, 9%‐67%). Similarly, mycophenolate mofetil/mycophenolic acid (MMF/MPA) was a part of the immunosuppressive regimen in 50% (95% CI, 44%‐56%) of reported patients and modified in 60% (95% CI, 17%‐90%). Additionally, 9% (95% CI, 3%‐15%) of patients were on mammalian target of rapamycin inhibitors (mTORi) and of these 50% (95% CI, 25%‐76%) of patients had dose modification (Table 4).
Increased doses or pulsed steroids were administered in 22% (95% CI, 13%‐31%) of patients, presumably with intent to modulate the cytokine syndrome by controlling pulmonary hyperinflammation. Similarly, the pooled prevalence of patients who received hydroxychloroquine and tocilizumab was 58% (95% CI, 35%‐82%) and 6% (95% CI, 1%‐12%), respectively (Table 4).
3.6. Group‐wise comparison of mortality
We analyzed the available mortality data in terms of age (<60‐65 years vs ≥60‐65 years), sex/ gender (as reported), and time from LTx at presentation (<2 years vs ≥2 years). The COVID‐19 related deaths were significantly associated with older age ≥ 60‐65 years (OR 4.26; 95%CI, 2.14‐8.49). However, no increased risk of mortality was observed with respect to time since transplant (OR 3.07; 95%CI, 0.65‐14.46) or sex/gender (as reported) (OR 1.05; 95%CI, 0.62‐1.80) (Figure 4A‐C).
FIGURE 4.
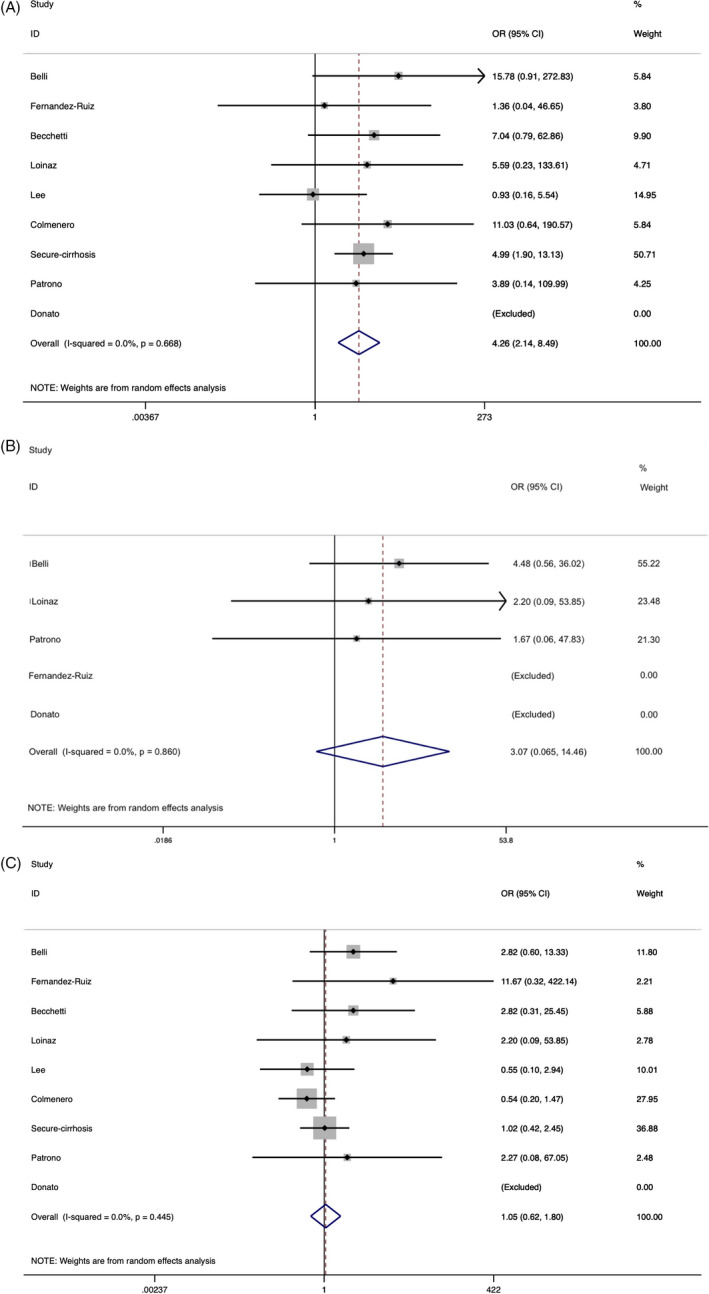
A, Forest plot representing odds ratio (OR) of COVID‐19 related death in liver transplant recipients in age group ≥ 60‐65 years vs <60‐65 years while comparing the weight of the studies in the meta‐analysis. The diamond shows higher risk ≥ 60‐65 year old group following analysis (red dashed line represents OR of 4.26). The edges of the blue diamond represent 95% confidence intervals (2.14‐8.49). B, Forest plot representing odds ratio (OR) of COVID‐19 related death in liver transplant recipients in late post‐transplant period group (>2 years) in contrast to early post‐transplant period (≤2 years) while comparing the weight of the studies in the meta‐analysis. The diamond shows the odds ratio in late post‐transplant group 3.07 and is represented by the red dotted line. The edges of the blue diamond represent 95% confidence intervals (0.65‐14.46). C, Forest plot representing the proportion of COVID‐19 related death in liver transplant recipients in the female population in contrast to the male population while comparing the weight of the studies in the meta‐analysis. The diamond shows no increased risk between the groups; odds ratio 1.05 is represented by the red dashed line, and the edges of the blue diamond represent 95% confidence intervals (0.62‐1.80)
4. DISCUSSION
The pathophysiology of COVID‐19 includes uncontrolled replication of the highly aggressive pathogen SARS‐CoV‐2 and dysregulation of the host immune response. 29 , 30 , 31 SARS‐CoV‐2 primarily affects the respiratory tract and pulmonary parenchyma. More recently, gastrointestinal and hepatic involvement have been reported in 14%‐53% of infected patients manifesting as derangements of liver function with rates as high as 58%‐78% in seriously ill COVID‐19 patients. 31
Hepatic manifestations of SARS‐CoV‐2 infection may relate to the higher expression of ACE2 receptors in cholangiocytes. Notably, patients with liver cirrhosis experience cirrhosis‐associated immune dysfunction (CAID) with altered inflammatory response and may be more susceptible to an aggressive clinical course of COVID‐19. 32 , 33 , 34 Moreover, COVID‐19 infection in the setting of chronic liver disease may further exacerbate the underlying condition and lead to hepatic decompensation and acute on chronic liver failure. 4 , 35 In LTx recipients, immunosuppression following liver transplantation may increase the likelihood of SARS‐CoV‐2 infection.
Management of patients with cirrhosis in the current pandemic is very challenging. However, recent studies have demonstrated the benefits of restoring liver function by transplantation, which can reduce the mortality risk to that of the general population. 15 , 24 , 36 The associated risk among cirrhotic patients with COVID‐19 was detailed in several studies and is noted to rise sharply with decompensated cirrhosis and in patients with Child‐Pugh Score C. 36 , 37 , 38 Marjot et al studied mortality outcomes of COVID‐19 in 386 cirrhotic patients. The reported mortality of those in the ICU was 59.2% and even higher among those who needed invasive ventilation. 36 Further studies are required to elucidate the impact of SARS‐CoV‐2 infection in cirrhotic patients versus LTx recipients.
Multiple small studies have already reported that the severe clinical impact of COVID‐19 infection in LTx recipients is seen in the setting of ARDS, admission to ICU, the need for mechanical ventilation, severe hepatic injury (reflected in increased serum liver enzymes and bilirubin in association with reduced albumin), and in the presence of comorbidities. 7 , 39 , 40 , 41 , 42
The findings of this systematic review and meta‐analysis were based on a large number of hospitalized liver transplant recipients with diagnosis of COVID‐19. The data analysis revealed a pooled hospital mortality of 20% (95% CI, 13%‐28%) in LTx recipients with confirmed COVID‐19 and could be secondary to the higher burden of comorbidities and was in line with the observed case fatality in the general population with similar comorbidities (11%‐55%). 43 , 44 , 45 , 46 The observed finding can also be explained because of the increasing age and age‐related morbidity, which were implicated as important attributes for increased case fatality of 18.7% for patients between 60 and 69 years of age and of 35.8% for patients of 70 and 79 years of age. 47 The mortality was lower than reported among cirrhotic liver disease patients with COVID‐19, for whom outlined mortality was 26%‐40%; this strongly correlated with a higher Child‐Pugh class and a higher MELD score. 8 , 36 , 48 , 49 , 50 , 51
The current meta‐analysis included twelve studies with a relatively high number of hospitalized liver transplant patients with COVID‐19 from diverse geographical regions. The most commonly outlined symptoms among the hospitalized liver transplant cohort with COVID‐19 were fever (71%), cough (62%), dyspnea (48%), and gastrointestinal symptoms (28%). Here, the prevalence of fever and cough was comparable to the report published by WHO‐China joint commission involving 55,924 confirmed cases of SARS‐CoV‐2 infection in general population. According to the report, fever (87.9%) was the most common presenting symptom, followed by cough (67.7%) and sputum production (33.4%), while dyspnea and gastrointestinal symptoms were in 18.6% and 3.7% patients, respectively. 52
The evidence from recent studies has underscored that the higher prevalence of comorbidities are associated with increased disease severity, higher ICU admission, severe deoxygenation, mechanical ventilation requirement, or death from COVID‐19 in the general population. 53 , 54 A meta‐analysis involving 14 studies and 29 909 COVID‐19 patients reported 1,445 deaths and outlined factors of significant association with mortality including old age (pooled OR 4.59), male gender (pooled OR 1.50), hypertension (pooled OR 2.70), cardiovascular disease (pooled OR 3.72), T2DM (pooled OR 2.41), lung disease (pooled OR 3.53), and malignancy (pooled OR 3.04). 55 A similar pattern was shown in a propensity score‐matched analysis conducted by international registries (COVID‐Hep and SECURE‐Cirrhosis) including 151 LTx recipients where they implicated old age and comorbidities as attributes of increased mortality. 24
Despite the fact that the management of liver cirrhosis patients in the current pandemic is very challenging and there are no explicit guidelines available regarding the reduction or withdrawal of immunosuppressive agents in transplant recipients affected by SARS‐CoV‐2 infection. Our meta‐analysis has importantly addressed the current practice of immunosuppression management in the setting of COVID infection in LTx recipients. Certainly, all transplant centers have considered an approach to reduce the immunosuppressants level to limit the viral replication while avoiding the potentiation of rejection. In the studies included in this analysis, a variable percentage of hospitalized subjects had one or more of their immunosuppressants withdrawn; the most frequently withheld/reduced agent was MMF/MPA in 60% (95% CI, 17%‐90%). Steroid doses were increased or administered in a pulsed fashion in 22% (95% CI, 13%‐31%) of patients. The approach has dual value as COVID‐19 induces an inflammatory state which engenders ARDS development, and immunosuppression and anti‐inflammatory drugs such as high dose steroids could be useful in controlling and preventing the cytokine storm along with ensuring adequate immunosuppression following withdrawal/reduction of antimetabolites and CNIs. 56 , 57 However, further studies are required to discern whether comorbidities and immunosuppressed state are associated with a higher incidence and severity of infection against the plausible role of the immunosuppressed state in limiting cytokine syndrome induced inflammatory state. Caution needs to be exercised in managing post‐liver transplant patients because once infected with SARS‐CoV‐2, they may remain infectious for a longer duration due to higher viral titers and a prolonged replication period. 35
There are certain limitations to this meta‐analysis. First, the studies we analyzed were retrospective reports which have their inherent design limitations. Second, the data were heterogenous with particularly wide variations in rates of hospitalization and ICU admission. Third, we used a random‐effects model for data analysis and the results require cautious interpretation due to high heterogeneity of outcomes. Nevertheless, despite these limitations, we believe this meta‐analysis can help further the understanding of the impact of COVID‐19 among hospitalized LTx recipients. The strengths of our analysis include the comprehensive nature of the literature review which aims to include all relevant studies and represents a large volume of patient data that facilitated the estimation of associated potential risk and mortality.
In summary, the clinical presentation of COVID‐19 in LTx recipients resembles that reported for the general population with the exception of a higher prevalence of dyspnea and gastrointestinal symptoms. As the COVID‐19 pandemic evolves, transplant surgeons and hepatologists must consider the role of liver transplant and the potential increased risk of infection in the immunocompromised host while also acknowledging the defined mortality risk of untreated decompensated cirrhosis. Further studies are warranted to better understand the impact of SARS‐CoV‐2 on liver transplant recipients and to formulate specific management algorithms which take into account co‐morbidities, modifications of immunosuppression, and the personalized nature of post‐transplant follow‐up care.
CONFLICTS OF INTEREST
None of the authors reported any conflicts of interest, including no specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
AUTHOR CONTRIBUTIONS
PW and KJ involved in concept/design. KJ and IR involved in data acquisition. KJ, JSP, TB, and PW involved in data analysis/interpretation. KJ, JSP, IR, PJB, and FV drafted the article. PW, TB, DD, RNB, JF, KJ, IR, FV, JSP, and PJB critically revised the article. PW, KJ, IR, FV, TB, DD, RNB, JF, JSP, and PJB approved the article.
Jayant K, Reccia I, Virdis F, et al. COVID‐19 in hospitalized liver transplant recipients: An early systematic review and meta‐analysis. Clin Transplant. 2021;35:e14246. 10.1111/ctr.14246
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang J‐F, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID‐19. Am J Transplant. 2020;20(7):1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang C, Shi L, Wang F‐S. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Fan J‐G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in EpidemiologyA proposal for reporting. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 10. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 13. National Institutes of Health . Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies; 2014. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools
- 14. Ma L‐L, Wang Y‐Y, Yang Z‐H, Huang D, Weng H, Zeng X‐T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol. 2020;5(7):643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heimbach JK, Taner T. SARS‐CoV‐2 infection in liver transplant recipients: collaboration in the time of COVID‐19. Lancet Gastroenterol Hepatol. 2020;5(11):958‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becchetti C, Zambelli MF, Pasulo L, et al. COVID‐19 in an international European liver transplant recipient cohort. Gut. 2020;69(10):1832‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patrono D, Lupo F, Canta F, et al. Outcome of COVID‐19 in liver transplant recipients: a preliminary report from Northwestern Italy. Transpl Infect Dis. 2020;22(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donato MF, Invernizzi F, Lampertico P, Rossi G. Health status of patients who underwent liver transplantation during the coronavirus outbreak at a large center in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18(9):2131‐2133.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID‐19 and solid organ transplantation at a US high‐volume transplant center. Transplantation. 2020;104(11):2208‐2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loinaz C, Marcacuzco A, Fernández‐Ruiz M, et al. Varied clinical presentation and outcome of SARS‐CoV‐2 infection in liver transplant recipients: Initial experience at a single center in Madrid, Spain. Transpl Infect Dis. 2020;22(5):e13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS‐CoV‐2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20(10):2876‐2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee BT, Perumalswami PV, Im GY, et al. COVID‐19 in liver transplant recipients: an initial experience from the US epicenter. Gastroenterology. 2020;159(3):1176‐1178.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belli LS, Duvoux C, Karam V, et al. COVID‐19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5(8):724‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol. 2021;74(1):148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ong J, Young BE, Ong S. COVID‐19 in gastroenterology: a clinical perspective. Gut. 2020;69(6):1144‐1145. [DOI] [PubMed] [Google Scholar]
- 30. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helmy KY, Katschke KJ, Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124(5):915‐927. [DOI] [PubMed] [Google Scholar]
- 33. Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182(2):526‐533. [DOI] [PubMed] [Google Scholar]
- 34. Morita K, Fukuda Y, Nakano I, Katano Y, Hayakawa T. Peripheral lymphocyte subsets vary with stage of hepatitis C virus‐associated liver disease. Hepatogastroenterology. 2005;52(66):1803‐1808. [PubMed] [Google Scholar]
- 35. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID‐19 and the liver. J Hepatol. 2020;73(5):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2020; 10.1016/j.jhep.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73(3):566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology. 2020;72(3):1102‐1108. [DOI] [PubMed] [Google Scholar]
- 39. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID‐19. SN Compr Clin Med. 2020;2(8):1069‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller LE, Bhattacharyya R, Miller AL. Diabetes mellitus increases the risk of hospital mortality in patients with Covid‐19. Medicine (Baltimore). 2020;99(40):e22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbu MG, Thompson RJ, Thompson DC, Cretoiu D, Suciu N. The impact of SARS‐CoV‐2 on the most common comorbidities–a retrospective study on 814 COVID‐19 deaths in Romania. Front Med. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol. 2020;73(5):1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID‐19 patients with pre‐existing cirrhosis: a multicentre cohort study. Gut. 2020;2020:321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee YR, Kang MK, Song JE, et al. Clinical outcomes of coronavirus disease 2019 in patients with pre‐existing liver diseases: a multicenter study in South Korea. Clin Mol Hepatol. 2020;26(4):562‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bajaj JS, Garcia‐Tsao G, Biggins SW, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut. 2020;2020‐322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. WHO, Aylward, Bruce (WHO); Liang W (PRC) . Report of the WHO‐China Joint Mission on Coronavirus Disease. 2019 (COVID‐19); 2020.
- 53. Yang J, Zheng YA, Gou XI, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Virol. 2020;127:104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID‐19) infection: a systematic review and meta‐analysis of observational studies. Aging Male. 2020:1‐9. [DOI] [PubMed] [Google Scholar]
- 56. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID‐19: a double‐edged sword? Lancet. 2020;395(10230):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


