Abstract
The COVID‐19 pandemic brought living donor kidney transplant programs across the United States to a near halt in March 2020. As programs have begun to reopen, potential donor candidates often inquire about their risk of a COVID‐19 infection and its potential impact on kidney function after donation. To address their concerns, we surveyed 1740 former live kidney donors at four transplant centers located in New York and Michigan. Of these, 839 (48.2%) donors responded, their mean age was 46 ± 12.5 years, 543 (65%) were females, and 611 (73%) were white. Ninety‐two donors (11%) had symptoms suggestive of a COVID‐19 infection with fever (48%) and fatigue (43%) being the most common. Among those with symptoms, 42 donors underwent testing and 16 tested positive. Testing was more common among donors with private insurance, and a positive test result was more common among young black donors. Only one donor surveyed required hospitalization and none required dialysis. Fourteen donors have recovered completely and two partially. Our survey highlights that a COVID‐19 infection in former donors results in a mild disease with good recovery. These data will be useful for transplant programs to counsel living donors who are considering kidney donation during this pandemic.
Keywords: COVID‐19, infection, living kidney donors, outcomes
1. INTRODUCTION
Coronavirus disease 19 (COVID‐19), caused by the novel SARS‐CoV‐2, was declared a national emergency on March 13, 2020. 1 This declaration led to an abrupt halt to the living donor kidney transplant (LDKT) surgery in most parts of the United States. During the week of March 24, 2020, over two‐thirds of the LDKT programs were fully suspended. 2 , 3 The LDKT is considered an “elective procedure” and was postponed to conserve hospital resources and also to protect the recipient and the donor from COVID‐19 infection. 3 As the initial surge of COVID‐19 infections subsided and the hospital resource have been replenished, elective surgeries including LDKTs have resumed. Living donor safety is of paramount importance for all programs. The American Society of Transplantation and American Society of Transplant Surgeons have put forth recommendations to guide programs to safely resume LDKT. 4 , 5 The recommendations include testing and self‐quarantining to ensure that neither the donor nor the recipient has asymptomatic COVID‐19 infection at the time of transplant surgery. However, currently there is no guidance available on how potential donors should be counseled on their future risk of infection with COVID‐19 and its potential implications on their renal function after donor nephrectomy. Not surprisingly, these questions are being raised by prospective donors especially given the evidence of risk among individuals with reduced kidney function. 6 , 7 , 8 , 9 The living donor evaluation includes extensive medical testing prior to donation resulting in a donor pool with relatively few comorbidities, if any, and therefore, former donors should not be at higher risk for severe infection than general population. However, there are no data to support such counseling. As a result, we surveyed prior donors who had underwent donor nephrectomy at four transplant centers located in the epicenters during early stages of the COVID‐19 pandemic: New York and Michigan. Herein, we report the responses of former donors as it relates to COVID‐19 infection, testing, and recovery.
2. MATERIAL & METHODS
2.1. Study participants and setting
All living kidney donors who donated between January 1, 2015, to March 31, 2020, at 4 transplant centers were contacted to inquire if they had contracted a COVID‐19 infection. The participating transplant centers were located in New York—Columbia University Irving Medical Center and Weill Cornell Medicine and Michigan—Henry Ford Hospital and University of Michigan Hospital. All donors with an available email address were contacted except those from Henry Ford Hospital who were contacted via phone instead (due to lack of email address). The protocol was approved by the local institutional regulatory board at each site.
2.2. Survey design
The survey instrument developed by the study investigators assessed key topics of interest and consisted of 17 questions (Appendix S1). Participants were asked if they developed symptoms suggestive of COVID‐19 infection (fever, sore throat, diarrhea, fatigue, loss of taste or smell), with additional follow‐up questions including when symptoms developed, whether the infection was confirmed via a nose or throat swab, when the test was performed, how they got infected, if they were hospitalized and whether the infection affected their kidney function or if they needed dialysis, and lastly if they have recovered from COVID‐19 infection. We also collected information on their education level, medical insurance, and work status.
2.3. Survey administration
Columbia, Cornell and the University of Michigan sent the survey to donors with a known email address through the Qualtrics Survey Software. The message in the email gave donors an option to opt out and, by answering the survey questions the donor consented to participate in the study. Two additional weekly reminders were sent to the non‐respondents. Henry Ford Hospital contacted their donors weekly, up to three times, to conduct the survey over the phone. All donors were given an option to opt out of the study at the time of the phone call. Donors were surveyed or contacted from June 5 through August 31, 2020. Each transplant center contacted the United Network for Organ Sharing (UNOS) to obtain information on death and/or need for chronic dialysis on the living donors included in the study during the pandemic (March 1, 2020, through October 1, 2020).
2.4. Statistical analysis
Responses to each survey question were described as percentages or means, as appropriate. To obtain percentages, we divided the number of “yes” responses by the total number of participants who responded to the question. For questions about symptoms where participants were asked to “select all that apply,” the presenting clinical feature was reported as percentages of donors with that symptom. Donors were divided into four groups based on symptoms and testing results (if taken): (a) asymptomatic, (b) symptomatic but did not undergo testing, (c) symptomatic with negative COVID‐19 testing, and (d) symptomatic with positive COVID‐19 testing. The differences in baseline demographics and responses between the four groups were assessed using ANOVA, chi‐square test, and Fisher exact tests as appropriate. All analyses were performed using SAS for windows version 9.4 (SAS Institute).
3. RESULTS
We contacted 1740 live kidney donors who donated at the four transplant centers between January 1 2015, through March 31, 2020. Of these, 841 donors responded and 2 opted out. The study consisted of 839 donors (Figure 1). The response rate varied at participating transplant centers and was as follows: Weill Cornell Medicine—348 out of 654 (45%), University of Michigan—190 out of 345 (55%), Columbia University Irving Medical Center—184 out of 489 (38%), and Henry Ford Hospital—114 out of 252 (45%). The mean of age of donors who were included in the study was 46 ± 12.5 years, 543 (65%) were females, 611 (73%) were white, 76 (9%) were blacks, 69 (8%) had hypertension, and 261 (31%) had donated between 2019 and 2020.
FIGURE 1.
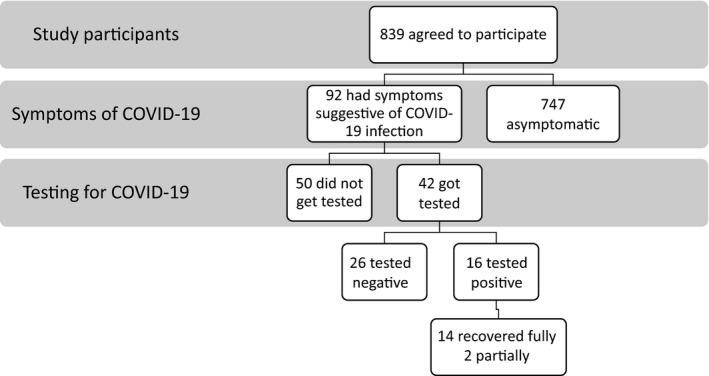
Distribution of the study participants based on symptoms and testing for COVID‐19 and recovery after infection
Of the 839 donors who completed the survey or were successfully contacted, 92 (11%) developed symptoms suggestive of a COVID‐19 infection. The symptoms and their frequency were fever (48%), fatigue (43%), sore throat (34%), shortness of breath (32%), loss of smell or taste (25%), and diarrhea (16%). Most of the donors developed symptoms in March and April (59 out of 92, 64%). The proportion of donors developing symptoms was similar across all four transplant centers ranging from 9% to 13% and did not vary by the year of donation.
Of the 92 donors with symptoms, 42 (46%) got tested for COVID‐19 infection via a nasal or throat swab—among whom 16 tested positive. Of the 16 donors who tested positive, 10 tested positive in March and April (N = 10 out of 16, 62.5%). Table 1 compares demographic characteristics of donors across 4 groups (a) asymptomatic, (b) symptomatic but did not undergo testing, (c) symptomatic with negative COVID‐19 testing, and (d) symptomatic with positive COVID‐19 testing. Donors who developed COVID‐19 symptoms were more likely to be young. Testing for a COVID‐19 infection was more common among donors with private insurance (81% vs 66%; p = .02). Young black donors were more likely to have a confirmed COVID‐19 infection. Approximately half of the donors (50%) were unaware of how they contracted the virus (8 out of 16). The percentage of donors with a confirmed COVID‐19 infection was also similar across all four transplant centers and ranged from 1% to 3% and also by the year of donation.
TABLE 1.
Comparison of characteristics among donors grouped by symptoms of a COVID‐19 infection and testing results
| Asymptomatic | Symptomatic for a COVID‐19 infection | p‐Value | |||
|---|---|---|---|---|---|
| Not tested | Not tested | Tested | |||
| (n = 747) | (n = 50) | Positive (n = 16) | Negative (n = 26) | ||
| Age, years | 47 ± 13 | 41 ± 12 | 42 ± 13 | 45 ± 14 | .01 |
| Female gender (%) | 488 (65%) | 25 (50%) | 11 (69%) | 19 (73%) | .13 |
| Race | |||||
| White | 546 (73%) | 39 (78%) | 8 (50%) | 18(69%) | .04 |
| Black | 65 (9%) | 4 (8%) | 4 (25%) | 3 (12%) | |
| Others | 136 (18%) | 7 (14%) | 4 (25%) | 5 (19%) | |
| Education | |||||
| Some HS | 10 (1%) | 0 | 0 | 0 | .29 |
| HS | 79 (11%) | 4 (8%) | 3 (18%) | 0 | |
| Some College | 137 (18%) | 11 (22%) | 2 (13%) | 4 (15%) | |
| College | 230 (31%) | 22 (44%) | 4 (25%) | 9 (35%) | |
| Graduate/Professional | 282 (38%) | 10 (20%) | 7 (44%) | 12 (46%) | |
| Missing | 9 (1%) | 3 (6%) | 0 | 1 (4%) | |
| Insurance | |||||
| None | 35 (5%) | 3 (6%) | 0 | 1 (4%) | .02 |
| Medicaid | 42 (6%) | 10 (20%) | 2 (13%) | 0 | |
| Medicare | 80 (11%) | 4(8%) | 2 (12%) | 3 (12%) | |
| Private | 590 (78%) | 33 (66%) | 12 (75%) | 22 (84%) | |
| Working, yes | 532 (71%) | 35 (70%) | 11 (69%) | 20 (77%) | .80 |
| HTN, yes | 63 (8%) | 3(6%) | 1 (6%) | 2(8%) | .93 |
| Year of donation | |||||
| 2015 | 100 (13%) | 7 (14%) | 3 (19%) | 1 (4%) | .74 |
| 2016 | 114 (15%) | 13 (26%) | 3 (19%) | 3 (11%) | |
| 2017 | 120 (16%) | 7 (14%) | 3 (19%) | 5 (19%) | |
| 2018 | 176 (24%) | 10 (20%) | 5 (31%) | 8 (31%) | |
| 2019 | 183 (25%) | 11 (22%) | 2 (12%) | 8 (31%) | |
| 2020 | 54 (7%) | 2 (4%) | 0 | 1 (4%) | |
Data are presented as mean ± standard deviation & number of patients (%).
Abbreviations: HS, high school; HTN, history of hypertension.
Out of the 57 donors who underwent nephrectomy in 2020, three reported developing symptoms suggestive of a COVID‐19 infection. None of them had symptoms at their 1 month post‐donation visit. Among the donors with symptoms of a COVID‐19 infection and those who tested positive, only one donor required hospitalization and all others were managed at home. None of the donors reported deterioration in their kidney function or required dialysis. Fourteen donors with confirmed infection have recovered completely, and two have reported partial recovery. Communications with UNOS confirms that none of the donors eligible for this study died or initiated on chronic dialysis during the study period (March 1, 2020, through October 1, 2020).
4. DISCUSSION
Our study reports that the rate of confirmed COVID‐19 infection in former living kidney donors is low, 2%. It is possible that the true infection rate may be higher as over half the donors with symptoms did not get tested (50 out of 92), possibly due to the lack of availability of universal testing early during the pandemic and lack of private insurance. Similar to the general population, fever was the most common presenting feature and diarrhea was less common. 10 Most of the donors with COVID‐19 infection were managed at home. No participants reported deterioration in kidney function, and all donors have recovered fully or partially. The milder course experienced by the living donor population may be in part due to the young age, and minimal comorbidities in the donor population. None of the donors who donated in 2020 contracted COVID‐19 infection due to hospitalization for nephrectomy.
Individuals without a private health insurance were less likely to get tested for COVID‐19 infection. Similar to the general population, black and young donors were more likely to develop COVID‐19 infection. 11 , 12 This could be related to socio‐economic factors leading to limited ability to engage in physical distancing and access to health care. There was no difference in the proportion of donors working through the pandemic and development of COVID‐19 infection or symptoms. It is possible that younger donors would have contracted the virus from community.
The strengths of our study are the good response rate of 48%, the sampling of the population from transplant centers with large living donor volumes located in the epicenter of the pandemic and our ability to ascertain outcomes in non‐respondents from UNOS/OPTN data. The proportion of donors with symptoms suggestive of a COVID‐19 infection and those with confirmed infection were similar across all transplant centers and therefore is reflective of entire study population and not skewed by any particular center. Limitations of our study are that not every donor with symptoms was tested and the donors who did not respond to our survey were more likely to be younger black males, and both could lead to our underreporting of the prevalence COVID‐19 infection in former live kidney donors. We do not have access to actual test reports and therefore rely on the accuracy of self‐reporting, particularly as it relates to the impact on renal function. Since most of our donors were managed at home and had mild disease, we believe that acute kidney injury is less likely to occur in this population. Lastly, our results could be affected by recall bias but the donors were queried within 6 months of onset of the pandemic. Give the public awareness around COVID‐19 infection, it would be hard for an individual to forget that they had symptoms or confirmed infection.
In summary, our small study confirms mild COVID‐19 infections in former living kidney donors with good recovery. Among those infected, none reported deterioration in their kidney function. Findings from this study can be used by the transplant community to counsel living donors who are considering kidney donation during this pandemic. While living donors do not seem to have severe from COVID‐19 infection, the decision to continue living donor transplants should be based on institutional policies and local prevalence.
CONFLICT OF INTEREST
None.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work would not have been possible without the help of the living donor coordinators at each of the transplant center.
Doshi MD, Tsapepas D, Prashar R, et al. COVID‐19 infection in former living kidney donors. Clin Transplant. 2021;35:e14230. 10.1111/ctr.14230
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/; 2020.
- 2. Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lentine KL, Vest L, Schnitzler MA, et al. Survey of U.S. living kidney donation and transplant practices in the COVID‐19 era. Kidney Int Rep. 2020.5(11):1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Society of Transplantation (AST) . 2019‐nCoV (Coronavirus): Recommendations and Guidance for Organ Donor Testing. Updated May 19, 2020. Available at Accessed Updated May 19, 2020; 2020.
- 5. American Society of Transplant Surgeons (ASTS) . Re‐engaging Organ Transplantation in the COVID‐19 Era. June 5, 2020. Accessed.
- 6. Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID‐19. Kidney Int. 2020;98(1):209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JR, Silberzweig J, Akchurin O, et al. Characteristics of acute kidney injury in hospitalized COVID‐19 patients in an urban academic medical center. Clin J Am Soc Nephrol. 2020. 10.2215/CJN.07440520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nimkar A, Naaraayan A, Hasan A, et al. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from Covid‐19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):687‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harhay M, Klassen A, Fleetwood J, et al. Living organ donor perspectives on organ donation during the COVID‐19 pandemic. J Am Soc Nephrol. 2020;Kidney Week Edition(31):277. [Google Scholar]
- 10. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID‐19 pandemic – United States, may‐august 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID‐19 on black communities. Ann Epidemiol. 2020;47:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


