Abstract
Background
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has generated a pandemic with alarming rates of fatality worldwide. This situation has had a major impact on clinical laboratories that have attempted to answer the urgent need for diagnostic tools, since the identification of coronavirus disease 2019 (COVID‐19). Development of a reliable serological diagnostic immunoassay, with high levels of sensitivity and specificity to detect SARS‐CoV‐2 antibodies with improved differential diagnosis from other circulating viruses, is mandatory.
Methods
An enzyme‐linked immunosorbent assay (ELISA) using whole inactivated virus cultured in vitro, was developed to detect viral antigens. WB and ELISA investigations were carried out with sera of convalescent patients and negative sera samples. Both analyses were concurrently performed with recombinant MABs to verify the findings.
Results
Preliminary data from 10 sera (5 patients with COVID‐19, and 5 healthy controls) using this immunoassay are very promising, successfully identifying all of the confirmed SARS‐CoV‐2‐positive individuals.
Conclusion
This ELISA appears to be a specific and reliable method for detecting COVID‐19 antibodies (IgG, IgM, and IgA), and a useful tool for identifying individuals which have developed immunity to the virus.
Keywords: COVID‐19, ELISA, native antigen, serological test, viral culture
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has generated a pandemic with alarming rates of fatality worldwide. This ELISA appears to be a specific and reliable method for detecting COVID‐19 antibodies (IgG, IgM and IgA), and provides a useful tool for identifying individuals which have developed immunity to the virus.

1. INTRODUCTION
On December 31st 2019, China notified the World Health Organization (WHO) of a novel coronavirus outbreak which was first observed a few weeks earlier in a patient presenting with severe respiratory disease in Wuhan. 1 In January 2020, the virus was identified as a novel coronavirus with more than 70% similarity with the severe acute respiratory syndrome coronavirus (SARS‐CoV), and it was officially named by the WHO as 2019 novel coronavirus (nCoV) or SARS‐CoV‐2. 1 Currently, SARS‐CoV‐2 has attributed to a worldwide pandemic resulting in over 40 million infections and 1 million deaths, with numbers constantly growing. 2
Coronaviruses are enveloped, non‐segmented, positive‐sense single‐stranded RNA viruses with a diameter of 60–140 nm, including the spike, with genome sizes ranging from 26 to 32 kilobases (the largest known viral RNA genome). 3 , 4 Coronaviruses have four structural proteins essential for virion assembly and viral transmission: the viral membrane containing the transmembrane (M) glycoprotein; the spike (S) glycoprotein; and the envelope (E) protein, surrounding a disordered or flexible, probably helical, nucleocapsid (N) protein. 4 , 5 Projections composed of S glycoprotein trimers protrude from the surface of the virus with an approximate length of 20 nm. The main difference of this new coronavirus compared to SARS‐CoV (which also belongs to the beta genera), appears to be localized in the S glycoprotein. 6
The symptoms of coronavirus disease 2019 (COVID‐19) appear after an incubation period ranging between 2 and 11 days and most commonly include fever, cough, fatigue, and dyspnea, often associated with lymphopenia. 7
Considering the evolution of the worldwide pandemic, an improved insight into the development of virus‐specific antibodies after SARS‐CoV‐2 infection is of high importance. To date, it is still unclear whether patients previously diagnosed with COVID‐19 develop immunity, and if this immunity remains. Therefore, serological investigations play an essential role to not only better understand the host response to the virus and provide more accurate estimations of the spread within the population, but they also have an impact on vaccine and therapy development. In addition, as we approach the colder seasons, a differential diagnosis which differentiates SARS‐CoV‐2 infections from other viral infections (coronavirus or not) that cause respiratory syndromes, is fundamental. 7 , 8
Due to the paucity of evidence regarding immunity and serology of SARS‐CoV‐2, well‐validated serological assays are urgently required. Thus, the aim of this study is to use inactivated SARS‐CoV‐2 viral cultures as an antigen source to characterize the whole viral antigen and produce serologic tests.
2. MATERIAL AND METHODS
2.1. Cell culture and virus production
Vero E6 cell lines (African green monkey kidney epithelial cells, ATCC® CRL‐1586™) were cultured in Dulbecco's modified Eagle's medium (DMEM; Bio‐Concept, Salem New Hampshire, USA) supplemented with 10% fetal bovine serum (FBS; Gibco™, Thermo Fisher, Waltham, MA, US). Vero E6 cells were cultured to an optimum monolayer confluence and infected with SARS‐CoV‐2 2019‐nCoV/Italy‐INMI1, the first viral strain isolated in Italy in January 2020 of which the complete sequence was submitted to GenBank (ID: MT066156) and is available on GISAID website (BetaCoV/Italy/INMI1‐isl/2020: EPI_ISL_410545). SARS‐CoV‐2 2019‐nCoV/Italy‐INMI1 was cultured in a DMEM medium supplemented with 5% FBS to a low specific Multiplicity Of Infection (MOI) of 0.05 and incubated at 37°C for 46–50 h in roller bottles (surface of 850 cm2). A single batch virus which was cultured without fetal serum to better analyze the electrophoretic pattern of the proteins was also produced.
SARS‐CoV‐2 isolation and culturing were performed by trained personnel in a Biosafety Level 3 Laboratory (BSL‐3).
2.2. Viral titration and plaque assay
Vero E6 cells were seeded into 24‐well plates 72/24 h before infection with tenfold serial diluted SARS‐CoV‐2 and incubating for a further 1 h at 37°C. After the absorption phase, 1 ml of DMEM supplemented with 5% FBS was added and the plates were incubated for 48 h at 37°C. After 48 h, the cytopathic effect (CPE) was checked and the viral titers calculated according to the Reed and Muench method. CPE was evaluated by microscopy, identifying cell rounding and detachment in monolayer cells. The Median Tissue Culture Infectious Dose (TCID50/ml), the residual CPE of virus in Vero E6 cells, was also determined at this time‐point (Figure S1A).
For viral titer analysis, plaque assay protocol remained the same until the absorption phase. Subsequently, 1 ml of medium consisting of 1.6% carboxymethylcellulose (Sigma‐Aldrich, St. Louis, Missouri, USA) and DMEM 2X, was added. The plate was incubated for 72 h at 37°C and then stained with crystal violet solution to count and calculate the viral titer (PFU/ml). Plaque assays, 72 h post‐infection, resulted in clear and easy quantifiable plaques (Figure S1B, S1C).
2.3. BPL virus inactivation
A virus inactivation protocol utilizing beta‐propiolactone (BPL), a commonly used reagent for viral inactivation in vaccine preparations, 9 , 10 , 11 , 12 was adopted and optimized. After thawing, virus batches were inactivated with BPL (Natalex, Warsaw, Poland) and 0.1% [v/v] of BPL was added in 3 consecutive additions to ensure inactivation. For each cycle of BPL treatment, viral suspension was incubated at room temperature (RT) for 3 h, shaken, and then incubated for a further 2 h at 37°C. Viral inactivation was verified by CPE assay, the absence of CPE and TCID50/ml <1 × 101.5 confirmed inactivation. In cases of incomplete inactivation, protocol stated that treatment with 0.1% BPL should be repeated and checked; however, although it was evident that after the first addition the virus was completely inactivated, due to safety concerns, BPL was added three times (Table S1). As an assay positive control, a live virus with a 104–105 TCID50/ml, was added to Vero E6 cells, and the CPE after 48/72 h, and the TCID50/ml titer were measured.
2.4. Virus purification
Each batch of inactivated virus was concentrated and purified by centrifugation. Batches were centrifuged at 10,000 g to remove cell debris and concentrated by ultracentrifugation at 100,000 g. As inactivated viruses are also present in debris pellets, the pellets were ultrasonically broken‐down and collected in a phosphate‐buffered saline solution (PBS, pH 7.2–7.8). Batches were stored at −80°C.
2.5. Viral protein quantification
Protein levels in the virus stock were quantified using the Bradford assay, as previously described. 13 The viral protein and a twofold serial dilution of a protein standard (bovine serum albumin, Sigma‐Aldrich) in PBS were mixed with Coomassie Plus (Bradford) solution (Thermo Fisher Scientific, Waltham, MA, US) and incubated at RT for 10 min. Absorbance was measured at 595 nm and used to calculate protein concentration (Spectrophotometer, Amersham, BioSciences, Buckinghamshire, United Kingdom).
2.6. Western blotting
Proteins were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) according to Laemmli protocol, 14 using a 10% (w/v) Pre‐Cast gel (Bio‐Rad, Hercules, CA, US). Samples were prepared in a Laemmli buffer with or without β‐mercaptoethanol, for reducing and non‐reducing conditions, respectively. Protein detection was performed by the silver stain system, using Pierce™ Silver Stain Kit (Thermo Fisher Scientific). Proteins were then electroblotted onto a nitrocellulose membrane with 0.2 µm pores (Bio‐Rad). The nitrocellulose was then blocked with 5% milk powder dissolved in 0.1% tris‐buffered saline solution (TBS, Bio‐Rad). 15 Western blots (WBs) were performed using human sera (5 patients with SARS‐CoV‐2 infection, confirmed by nucleic acid detection and 5 healthy controls) and with specific anti‐2019‐nCoV‐Spike (S1 subunit) and anti‐CoV‐nucleoprotein antibodies (recombinant monoclonal antibodies (MABs) manufactured by ProteoGenix, Schiltigheim, France). The membrane was washed with TBS‐Tween‐20 (Sigma‐Aldrich) and incubated with alkaline phosphatase‐conjugated mouse anti‐Human IgG (A‐9544, Sigma‐Aldrich), anti‐human IgA (A3063, Sigma‐Aldrich), and anti‐human IgM (A3437, Sigma‐Aldrich), for 1 h at RT. Membrane analysis was subsequently performed by alkaline phosphatase substrate (NBT/BCIP Ready‐to‐Use Tablets, Merck, Darmstadt, Germany) according to the manufacturer's instructions.
2.7. Enzyme‐linked immunosorbent assay (ELISA)
Ninety‐six‐well ELISA (Biomat, Los Angeles, CA, US) plates were coated overnight at RT with inactivated purified whole SARS‐CoV‐2 virus. Non‐specific binding sites were blocked with PBS solution supplemented with 2.5% BSA. Serum samples were added to wells at a 1:100 dilution to sample buffer (Sample Diluent, Diesse Diagnostica Senese) and incubated for 1 h at RT. For IgM detection, sera were first mixed with anti‐Human IgG sorbent. Concomitantly, recombinant anti‐2019‐nCoV‐(S1)‐Spike Subunit and anti‐CoV‐Nucleoprotein antibodies (ProteoGenix) were diluted in sample buffer. After washing, horseradish peroxidase‐conjugated mouse monoclonal anti‐human IgG, IgA, and IgM (Diesse Diagnostica Senese SpA, Italy) were diluted and added to the wells. Following 1 h at RT, the plates were washed, and the signal was developed with tetramethylbenzidine substrate (Diesse Diagnostica Senese SpA). The reaction was stopped with a sulfuric acid‐containing solution, and measurements were performed by a Multiskan® EX microplate spectrophotometer reader (Thermo Fisher Scientific) at a wavelength of 450 nm.
3. RESULTS
Each batch of viral antigen produced was quantified before SDS‐PAGE and WB analysis. SDS‐PAGE was used to analyze two different batches of viral antigen to visualize the structural proteins present in the SARS‐CoV‐2 virus, with and without fetal serum 5%. The batch containing fetal serum was 0.671 g/L concentrated, versus the serum‐free batch at 0.178 g/L. SDS‐PAGE results showed that the composition of the structural proteins were similar in both production batches, as depicted in Figure 1, and contained the four structural proteins (S, N, M, and E), as expected.
FIGURE 1.
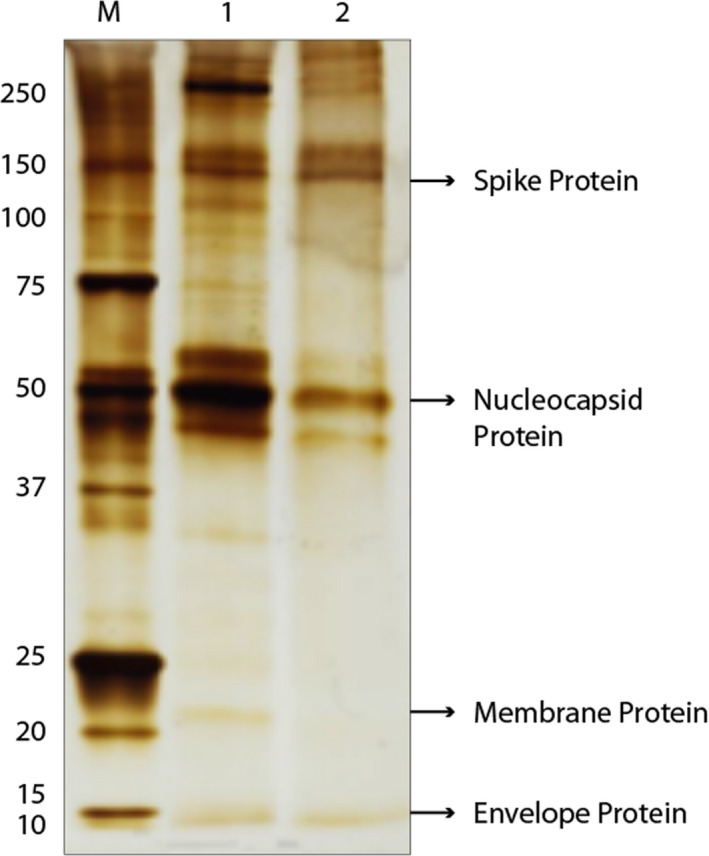
Viral antigen quantification. SDS‐PAGE in non‐reducing conditions of the two SARS‐CoV‐2 batches, with (line 1) and without (line 2) fetal serum. The arrows indicate SARS‐CoV‐2 M protein, molecular markers, expressed in kD
Western blots and ELISA investigations were carried out with sera of convalescent patients (after a confirmed SARS‐CoV‐2 infection) and sera from healthy donors, collected prior to COVID‐19 pandemic (before June 2019). Both analyses were concurrently performed with recombinant MABs to verify the findings.
WB of the convalescent serum samples showed a varying immune response between patients (Figure 2) and reducing and non‐reducing conditions appeared to have a considerable effect on the response toward N and S proteins. Reducing conditions displayed only a band which corresponded to the N protein (Figure 2A), supporting the hypothesis that the immunodominant epitopes of the S protein are mainly conformational rather than linear. Only one serum among those analyzed appeared to recognize the M protein (Figure 2B). Presence of IgA (Figure 2C) and IgM (Figure 2D) were also detected, in non‐reducing conditions.
FIGURE 2.
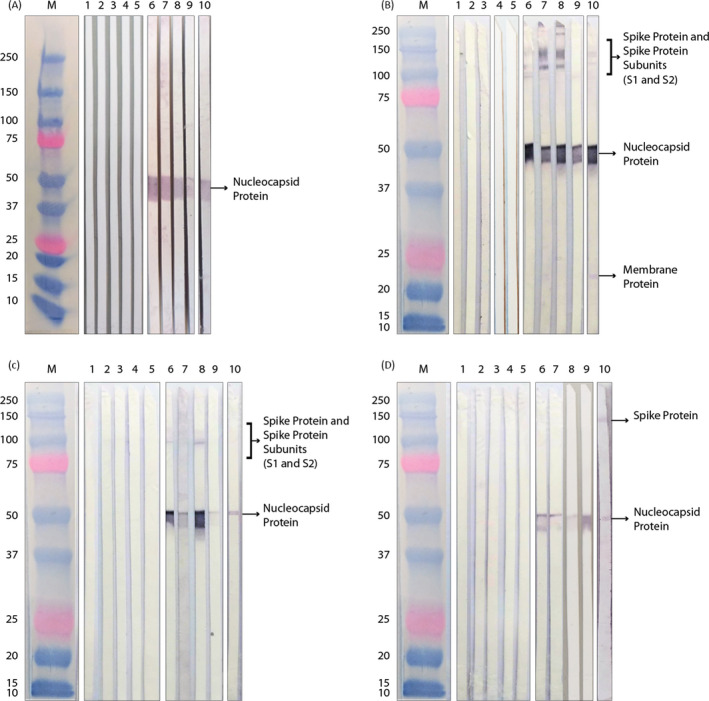
Analysis of structural proteins of SARS‐CoV‐2. Wester blotting of healthy donors (samples 1–5) and patients (samples 6–10) sera, in reducing (A), and non‐reducing condition (B), IgG detection. Presence of IgA (C) and IgM (D) was also detected. The arrows indicate SARS‐CoV‐2 M protein, molecular markers, expressed in kD
Similarly, the analysis with commercial MAB specifically targeting Subunit 1 of the S and the N proteins demonstrated the same differences between reducing and non‐reducing electrophoresis conditions as those observed with the serum samples, as shown in Figure 3A,B, respectively.
FIGURE 3.
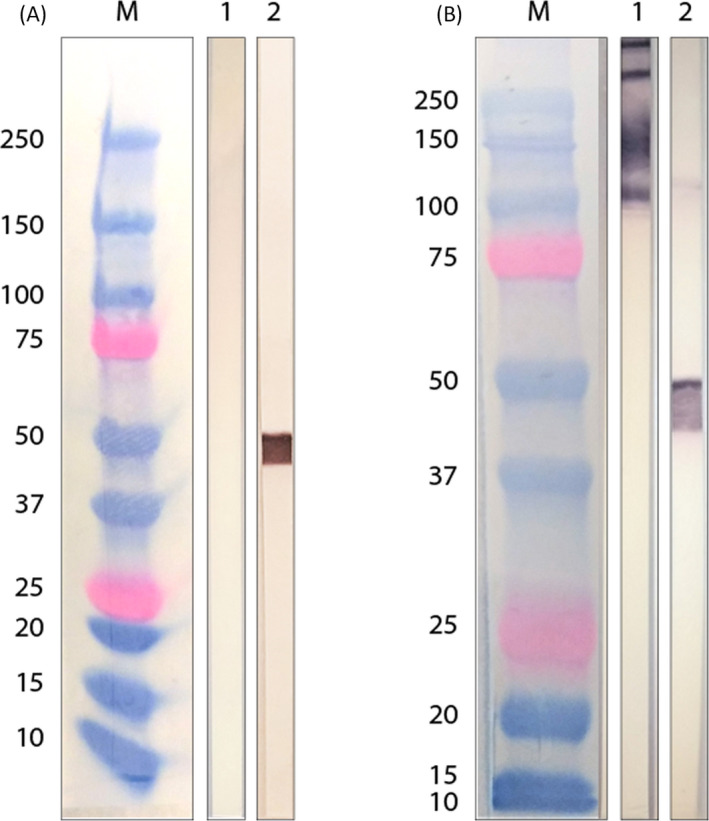
Analysis of structural proteins of SARS‐CoV‐2 with recombinant monoclonal antibodies. Western blotting of commercial MABs, in (A) reducing and (B) non‐reducing condition
Similarly, the sera (Table 1) and commercial MABs (Table 2) were tested with ELISA and were found to have the same results as those obtained by WB, and successfully identified all of the confirmed SARS‐CoV‐2‐positive individuals.
TABLE 1.
ELISA results of SARS‐CoV‐2‐negative and SARS‐CoV‐2‐positive human sera
| Human IgG | Human IgA | Human IgM | ||||
|---|---|---|---|---|---|---|
| Abs | Index | Abs | Index | Abs | Index | |
| Neg 1 | 0.125 | 0.5 | 0.089 | 0.2 | 0.241 | 0.6 |
| Neg 2 | 0.098 | 0.2 | 0.263 | 0.6 | 0.120 | 0.3 |
| Neg 3 | 0.076 | 0.2 | 0.258 | 0.6 | 0.218 | 0.5 |
| Neg 4 | 0.069 | 0.4 | 0.076 | 0.2 | 0.089 | 0.2 |
| Neg 5 | 0.103 | 0.6 | 0.154 | 0.4 | 0.247 | 0.6 |
| Pos 1 | 2.558 | 7.8 | 2.524 | 5.8 | 1.363 | 3.4 |
| Pos 2 | 2.807 | 8.5 | 1.744 | 4.0 | 0.751 | 1.9 |
| Pos 3 | 2.166 | 6.6 | 1.827 | 4.2 | 0.504 | 1.3 |
| Pos 4 | 0.812 | 2.5 | 0.561 | 1.3 | 1.109 | 2.8 |
| Pos 5 | 2.416 | 7.3 | 0.716 | 1.6 | 0.695 | 1.7 |
Values are expressed in absorbance (λ = 450 nm) and index. Index is determined as a cutoff calculated from an arbitrary value, of the average of the negative seras plus 3 standard deviations. Positive values in bold.
TABLE 2.
ELISA results of commercial recombinant monoclonal antibodies, anti‐S1 spike subunit, and anti‐nucleocapsid
| Anti‐S1 spike | Abs | Anti‐nucleocapsid | Abs |
|---|---|---|---|
| 2 × 10−2 g/L | 2.582 | 2 × 10−2 g/L | 2.759 |
| 5 × 10−3 g/L | 2.378 | 5 × 10−3 g/L | 2.839 |
| 12 × 10−4 g/L | 1.775 | 12 × 10−4 g/L | 2.834 |
| 3 × 10−4 g/L | 1.012 | 3 × 10−4 g/L | 2.806 |
| 75 × 10−6 g/L | 0.436 | 75 × 10−6 g/L | 2.606 |
| 19 × 10−6 g/L | 0.235 | 19 × 10−6 g/L | 0.921 |
| 1 × 10−5 g/L | 0.186 | 1 × 10−5 g/L | 0.683 |
| 5 × 10−6 g/L | 0.095 | 5 × 10−6 g/L | 0.275 |
Values are expressed in absorbance (λ = 450 nm).
4. DISCUSSION
Since the early phase of the COVID‐19 outbreak, nucleic acid tests (NATs) from nasopharyngeal swabs have played a fundamental role in the identification of SARS‐CoV‐2 infection, mainly in the acute stages of the disease. 16 However, the need for reliable serological assays is rapidly growing. It is well acknowledged that serological assays play a fundamental role in providing epidemiological analysis, and enhance the understanding of immunity profiles from recovered patients, which allows for development and implementation of serologic therapies (e.g. convalescent plasma) and vaccines. 8 Furthermore, several studies support the role of serological testing for more reliable detection of a positive infection, 17 , 18 however, NATs currently remain the gold standard for early infection diagnosis.
Antibody kinetics, following infection with nCoV, are characterized by similarities in IgM and IgG titers. 19 In addition, IgA antibodies have recently been reported to be associated with severe forms of COVID‐19. 20 For these reasons, Diesse Diagnostica Senese SpA in cooperation with Spallanzani Institute, developed serological diagnostic kits to rapidly detect all three of these specific antibodies (IgM, IgG, IgA), by using an indirect ELISA. Compared to other commercial serological tests which are based on specific recombinant viral antigens (such as N or S proteins), these assays are based on crude extracts from SARS‐CoV‐2 whole virus which increases the possibility of specific antibody detection. Diesse Diagnostica started by directly culturing the virus in mammalian cells and observed a substantial growth of SARS‐CoV‐2 virus in these Vero E6 cells. BPL treatment was confirmed to efficiently inactivate the virus without a negative impact on immunogenicity and has recently been used to generate an inactivated vaccine preparation for SARS‐CoV‐2. 21
The SDS‐PAGE and WB analyses showed that the culturing, inactivation, and concentration methods used are adequate for whole virus production, as all of the structural protein (S, M, N, and E protein) were present, with their native conformations preserved. Our promising results have confirmed the innovation underlying our approaches of viral culturing and inactivation (developed in order to obtain a native antigen) and a patent application on these methods has been filed by our company.
Convalescent sera from patients with SARS‐CoV‐2 infection provided confirmation of which viral proteins induced reactivity. As the N and S proteins were the most immunogenic, as previously reported in literature, 22 , 23 they were identified as the primary diagnostic candidate for antibody tests. Results from the WB analysis identified that sera from infected patient's contained both conformational and linear antibodies for the N protein, but only conformational epitopes for the S protein. As shown by the results of this study, this native antigen can therefore be successfully used for the development of serological ELISA tests, to identify the subclasses of specific antibodies against SARS‐CoV‐2. Extensive experimentation has been conducted following this first analysis to confirm these findings and three diagnostic kits for IgG, IgM, and IgA have been approved for commercial use: Enzy‐well SARS‐CoV‐2 IgG (91400), Enzy‐well SARS‐CoV‐2 IgM (91401), and Enzy‐well SARS‐CoV‐2 IgA (91402). Analytical performance (including sensitivity and specificity) of our serological kits has met the criteria required by the European authority for marketing authorization. CE marking was obtained on April 23rd, 2020. In the following months, 4 new kits were developed by our company using a disposable device applied on Diesse Chorus and Chorus TRIO instruments. Tests are based on the same immunoenzymatic method for the qualitative determination of IgG‐M‐A class and total antibodies to SARS‐CoV‐2 virus in human serum [Chorus SARS‐CoV‐2 IgG (81400); Chorus SARS‐CoV‐2 IgM (81401); Chorus SARS‐CoV‐2 IgA (81402); Chorus SARS‐CoV‐2 Screen Serum (81406)].
The ELISA results clearly correlate with the immunological status of the individuals analyzed, allowing identification of positive and negative subjects. This confirms that the antigen, obtained by our advanced and innovative method, is suitable for the development of highly sensitive and specific serological assays. A second wave COVID‐19 pandemic is emerging in Europe and worldwide, yet we are still far from having serological evidence of SARS‐CoV‐2. Specific serological assays are urgent and this ELISA test may represent an efficient strategy for qualitative and differential detection of the specific anti‐SARS‐CoV‐2 antibodies (IgG, IgM, and IgA).
CONFLICT OF INTEREST
All the authors declare to be Diesse Diagnostica Senese SpA employees. A patent application on a method reported in the research (entitled “Method for inactivating SARS‐CoV‐2 and uses thereof,” numbered EP20175656) has been filed by Diesse Diagnostica Senese SpA. AB, VR, and SV are the inventors named on patent application.
AUTHOR CONTRIBUTIONS
Helena Cerutti: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, and Writing ‐ original draft and review & editing. Giulia Tesi, Claudia Soldatini, and Marinunzia Castria: Investigation and Methodology. Veronica Ricci, Marco Natale Vaccaro, Stefania Tornesi, and Simona Toppi: Methodology. Silvana Verdiani and Alessandra Brogi: Supervision, Validation, and Project administration.
Supporting information
App S1
ACKNOWLEDGMENTS
The authors thank “Laboratory of Virology at National Institute for Infectious Diseases ‘L. Spallanzani’ IRCCS” who have provided the viral strain used in our research. Editorial support was provided by Content Ed Net, with the helpful contribution of Viola Guardigni, MD, in medical revising of the article and Mr. Bilal Bham in the final reading of the article for English language improvement.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [HC], upon reasonable request. Data regarding commercial kits are available at the website https://www.diesse4covid19.it.
REFERENCES
- 1. World Health Organization . Laboratory testing of human suspected cases of novel coronavirus (nCoV) infection [published online ahead of print January 21, 2020]. https://apps.who.int/iris/bitstream/handle/10665/330374/WHO‐2019‐nCoV‐laboratory‐2020.1‐eng.pdf. Accessed November 2020.
- 2. WHO . Weekly operational update on COVID‐19 ‐ 30 October 2020.
- 3. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perlman S, Netland J. Coronaviruses post‐SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439‐450. 10.1038/nrmicro2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis [published correction appears in J Med Virol. 2020 Aug 2]. J Med Virol. 2020;92(4):418‐423. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Preprint. bioRxiv. 2020. 10.1101/2020.02.11.944462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. 10.1002/jmv.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirkcaldy RD, King BA, Brooks JT. COVID‐19 and Postinfection Immunity: Limited Evidence, Many Remaining Questions [published online ahead of print, 2020 May 11]. JAMA. 2020;323(22):2245–2246. 10.1001/jama.2020.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budowsky EI, Friedman EA, Zheleznova NV, Noskov FS. Principles of selective inactivation of viral genome. VI. Inactivation of the infectivity of the influenza virus by the action of beta‐propiolactone. Vaccine. 1991;9(6):398‐402. 10.1016/0264-410x(91)90125-p [DOI] [PubMed] [Google Scholar]
- 10. Budowsky EI, Smirnov YUA, Shenderovich SF. Principles of selective inactivation of viral genome. VIII. The influence of beta‐propiolactone on immunogenic and protective activities of influenza virus. Vaccine. 1993;11(3):343‐348. 10.1016/0264-410x(93)90197-6 [DOI] [PubMed] [Google Scholar]
- 11. Chowdhury P, Topno R, Khan SA, Mahanta J. Comparison of β‐propiolactone and formalin inactivation on antigenicity and immune response of West Nile virus. Adv Virol. 2015;2015:616898. 10.1155/2015/616898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logrippo GA, Hartman FW. Antigenicity of beta‐propiolactone‐inactivated virus vaccines. J Immunol. 1955;75(2):123‐128.PMID: 13242811. [PubMed] [Google Scholar]
- 13. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 1976;72:248‐254. 10.1006/abio.1976.9999. PMID: 942051. [DOI] [PubMed] [Google Scholar]
- 14. Laemmli U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680‐685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 15. Burnette WN. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112(2):195‐203. 10.1016/0003-2697(81)90281-5 [DOI] [PubMed] [Google Scholar]
- 16. Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS‐CoV‐2 [published online ahead of print, 2020 May 6]. JAMA. 2020;323(22):2249. 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019‐n‐Cov infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383(18):1724‐1734. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database Syst Rev. 2020;6(6):CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali OH, Bomze D, Risch L, et al. Severe COVID‐19 is associated with elevated serum IgA and antiphospholipid IgA‐antibodies. Clin Infect Dis. 2020;ciaa1496. 10.1093/cid/ciaa1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. 10.1126/science.abc1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID‐19: current issues and challenges. Journal of Clinical Microbiology. 2020;58(6):e00512‐20. Published 2020 May 26. 10.1128/JCM.00512-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontou PI, Braliou GG, Dimou NL, Nikolopoulos G, Bagos PG. Antibody tests in detecting SARS‐CoV‐2 infection: a meta‐analysis. Diagnostics (Basel). 2020;10(5):319. 10.3390/diagnostics10050319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [HC], upon reasonable request. Data regarding commercial kits are available at the website https://www.diesse4covid19.it.


