Abstract
The variations and dynamics of essential and toxic metal(loid)s in patients with COVID‐19 may associate with the progression and fatal outcome of the disease, which still remains to investigate. In the present study, a retrospective analysis was performed in a cohort of 306 confirmed COVID‐19 patients admitted to Tongji hospital (Wuhan, China) from February 10 to March 15, 2020. Whole blood levels of essential and/or toxic metal(loid)s were analyzed, including magnesium, calcium, chromium, manganese, iron, copper, zinc, arsenic, cadmium, mercury, thallium, and lead according to the disease severity and outcome. Compared to the non‐severe COVID‐19 patients, severe cases showed significant higher levels of whole blood calcium, chromium, and copper, but lower levels of magnesium, manganese, iron, zinc, arsenic, thallium, and lead. These differences were further found consistently across the clinical course since the disease onset by longitudinal analysis. Among the severe patients, chromium and cadmium were higher in the deceased group compared to the recovered group, while arsenic was lower. Whole blood iron, age, and sex were determined to be independent factors associated with the disease severity, while chromium, cadmium, and the comorbidity of cardiovascular disease were determined to be independent factors associated with the mortality. These results suggest that variations of whole blood metal(loid)s may be associated with the severe illness and fatal outcome of COVID‐19, which could be persistently monitored and would be helpful in the evaluation of the dynamic changes in patients with COVID‐19.
Keywords: COVID‐19, metals, metalloids, mortality, severity, whole blood
Abbreviations
- AUC
area under curve
- CC
correlation coefficient
- COVID‐19
coronavirus disease 2019
- EQA
external quality assessment
- ICP‐MS
inductively coupled plasma mass spectrometer
- IQA
internal quality assessment
- IQR
interquartile range
- LoQ
limit of quantification
- RBC
red blood cell
- ROC
receiver operating characteristic
- RT‐PCR
real‐time polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WBC
white blood cell
1. BACKGROUND
Since the first known cases were reported in December 2019, the coronavirus disease 2019 (COVID‐19) that caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread worldwide and has been classified by the World Health Organization (WHO) as a global pandemic. 1 The clinical manifestation of COVID‐19 might be asymptomatic or moderate to severe with coughing, fever, and shortness of breath. 2 Up to 10‐20% of the patients develop a severe disease characterized by interstitial pneumonia and the rapid development of acute respiratory distress syndrome or septic shock. 3 , 4 Patients with any comorbidities yielded poorer clinical outcomes than those without. 5 The most prevalent comorbidity was hypertension (22.9%), followed by diabetes (11.5%), and cardiovascular disease (9.7%). 6
Public health practices including social distancing, handwashing, and vaccinations help reduce the spread and impact of infections. Nevertheless, the global burden of infection is high, and the need for identifying the factors to reduce the risk of SARS‐CoV‐2 infection that could be adopted by a large population at a low cost with minimal risk is a medical priority at this time of crisis. 7
Given that the important role nutrition plays in immune function is well established, abnormities of micronutrient status is supposed to be associated with increased infection risk of COVID‐19. 8 Inadequate intake and status of mineral nutrients are widespread, leading to a decrease in resistance to infections and as a consequence an increase in disease burden. 9 Poor nutrient status is associated with inflammation and oxidative stress, which in turn can impact the immune system. A wealth of mechanistic and clinical data show that essential minerals, including calcium, chromium, copper, magnesium, manganese, iron, and zinc play important and complementary roles in supporting the immune system and prevent infection. 10
Beyond that, environmental pollutes are believed to contribute to the increased prevalence of chronic diseases including diabetes and cardiovascular disease, 11 , 12 , 13 and seems to be associated with immunodeficiency observed in the contemporary pandemic, such as COVID‐19. 14 It has been reported that HIV‐infected patients and people at high risk of HIV have significantly higher body burden of heavy metals. 15 , 16 However, no previous studies have examined the body burden of environmental pollutants including heavy metals among COVID‐19 patients.
In the present study, we aim to perform a retrospective analysis of the essential minerals and heavy metals in whole blood of patients with COVID‐19, and further investigate their associations with the severity of the disease (non‐severe or severe) and outcomes (recovered or deceased), respectively.
2. MATERIALS AND METHODS
2.1. Patients
The retrospective cohort study was performed in a designated hospital for COVID‐19 treatment in Tongji Hospital of Huazhong University of Science and Technology, Wuhan, Hubei Province. All the patients with confirmed COVID‐19 admitted to the main hospital in Hankou town, Wuhan, China (excluding branches of the hospital in Hanyang and Wuchang), from February 10 to March 15, 2020, were enrolled into the study. Patients who later transferred to another hospital and without the outcome being tracked were exclude in the present study. In total, 306 patients with confirmed COVID‐19 were enrolled into the study. The diagnosis and clinical classification of COVID‐19 were carried out in accordance with the Guidelines of the Diagnosis and Treatment of New Coronavirus Pneumonia published by the National Health Commission of China. 17 Patients who had epidemiology history, clinical manifestations that mimic COVID‐19, such as fever, cough, and fatigue, were initially screened in community hospitals for fever and chest X‐ray. If the patients had fever or chest X‐ray abnormality, they would be further admitted to the designated hospitals for COVID‐19 to get SARS‐CoV‐2 real‐time polymerase chain reaction (RT‐PCR) test and chest Computed Tomography (CT) Scanning. All the patients had positive results in SARS‐CoV‐2 RT‐PCR test before hospitalization. The clinical classification was briefly described as below: (1) Mild cases: Mild symptoms without sign of pneumonia on imaging. (2) Moderate cases: Fever and respiratory symptoms with radiological findings of pneumonia. (3) Severe cases: Respiratory distress (≥30 breaths/min), or Oxygen saturation ≤93% at rest, or Arterial partial pressure of oxygen (PaO2) fraction of inspired oxygen (FiO2) ≤300 mmHg, or Lesion progression within 24‐48 hours >50% by chest imaging. (4) Critical cases: Respiratory failure and requiring mechanical ventilation, or shock, or with other organ failure that requires ICU care. In the present study, the mild and moderate cases were collectively referred to as non‐severe cases, while the severe and critical cases were collectively referred to as severe cases. All the patients had determined outcomes and the characteristics are summarized in Table 1.
TABLE 1.
Basic characteristics of the patients with COVID‐19
| All patients (N = 306) | Non‐severe (N = 202) | Severe (N = 104) | ||
|---|---|---|---|---|
| Age, median (IQR), y | 63.0 (53.3‐70.0) | 58.0 (50.3‐66.0) | 69.0 (62.0‐78.0) | <0.001 |
| Sex | 0.019 | |||
| Male | 148 (48.4%) | 88 (43.6%) | 60 (57.7%) | |
| Female | 158 (51.6%) | 114 (56.4%) | 44 (42.3%) | |
| Outcome | <0.001 | |||
| Recovered | 291 (95.1%) | 202 (100%) | 89 (85.6%) | |
| Deceased | 15 (4.9%) | 0 (0%) | 15 (14.4%) | |
| Length of stay (IQR), d | 30 (19‐41) | 22 (17‐33) | 41 (35‐46) | <0.001 |
| Onset to admission (IQR), d | 21 (11‐34) | 27 (12‐38) | 15 (9‐21) | <0.001 |
| Initial symptoms | ||||
| Fever | 202 (66.0%) | 125 (61.9%) | 76 (73.1%) | 0.051 |
| Cough | 207 (67.6%) | 131 (64.9%) | 75 (72.1%) | 0.199 |
| Feebleness | 85 (27.8%) | 54 (26.7%) | 30 (28.8%) | 0.695 |
| Chest tightness | 110 (35.9%) | 68 (33.7%) | 41 (39.4%) | 0.319 |
| Shortness of breath | 72 (23.5%) | 37 (18.3%) | 35 (33.7%) | 0.003 |
| Diarrhea | 38 (12.4%) | 29 (14.4%) | 8 (7.7%) | 0.090 |
| Comorbidities | ||||
| Hypertension | 118 (38.6%) | 67 (33.2%) | 51 (49.0%) | 0.007 |
| Cardiovascular disease | 42 (13.7%) | 22 (10.9%) | 20 (19.2%) | 0.045 |
| Diabetes | 59 (19.3%) | 31 (15.3%) | 28 (26.9%) | 0.015 |
| Malignancy | 14 (4.6%) | 8 (4.0%) | 6 (5.8%) | 0.473 |
| Cerebrovascular disease | 19 (6.2%) | 10 (5.0%) | 9 (8.7%) | 0.204 |
| None | 68 (22.2%) | 47 (23.3%) | 21 (10.4%) | 0.540 |
| Blood routine indicators | ||||
| Lymphocyte, × 109/L | 1.44 (1.09‐1.86) | 1.54 (1.24‐1.95) | 1.16 (0.89‐1.54) | <0.001 |
| Monocyte, × 109/L | 0.52 (0.41‐0.64) | 0.50 (0.41‐0.6) | 0.57 (0.43‐0.84) | 0.001 |
| Neutrophil, × 109/L | 3.49 (2.55‐4.9) | 3.20 (2.52‐4.23) | 4.26 (2.77‐7.15) | <0.001 |
| Platelet, × 109/L | 213 (172‐259) | 216 (183‐256) | 207 (156‐274) | 0.404 |
| Red blood cell, × 1012/L | 3.89 (3.45‐4.36) | 4.13 (3.73‐4.48) | 3.45 (3.07‐3.86) | <0.001 |
| Hemoglobin, g/L | 120 (105.25‐133) | 125.5 (114‐136) | 107.5 (95.25‐120.75) | <0.001 |
| WBC, × 10⁹/L | 5.83 (4.75‐7.38) | 5.60 (4.72‐6.73) | 6.75 (5‐9.39) | <0.001 |
| Biochemical indicators | ||||
| Albumin, plasma, g/L | 40.05 (37.1‐42.08) | 40.6 (38.23‐42.5) | 38.1 (36.43‐39.9) | <0.001 |
| Prealbumin, plasma, mg/L | 245.5 (212‐287.75) | 249.5 (221.75‐290) | 233.5 (187.25‐271) | 0.044 |
| Calcium, plasma, mmol/L | 2.19 (2.13‐2.25) | 2.2 (2.14‐2.25) | 2.19 (2.11‐2.28) | 0.999 |
| Magnesium, plasma, mmol/L | 0.86 (0.81‐0.9) | 0.85 (0.8‐0.88) | 0.86 (0.82‐0.93) | 0.068 |
| Ferritin, serum, µg/L | 424.45 (255.73‐761.38) | 321.8 (171.6‐518.9) | 613.9 (343‐1083.3) | <0.001 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in Wuhan, China (No.:TJ‐IRB20200201). All the procedures involving human samples conformed to the principles outlined in the Declaration of Helsinki. Informed consent was obtained in all cases. All the analyses were performed on existing samples collected during standard diagnostic tests, posing no extra burden to patients.
2.2. Data collection
A medical record review was performed to collect the information on demographic characteristics (age, gender, preexisting comorbidities, and the date of symptoms onset), clinical symptoms and signs, laboratory test results (blood routine indicators and biochemical indicators) that were measured on the same day that the urine specimen for trace elemental analysis was collected. The outcomes were obtained from electronic medical records for the final analysis, which included recovered/discharged and deceased.
2.3. Sample preparation and instrumental analysis
At least 2 mL of blood was collected in 6 mL special polyethylene terephthalate vacuum vials, for trace elements, containing 10 mg of potassium ethylenediaminetetraacetic acid (BD Vacutainer Trace Element Tubes. Ref.:368381. Royal blue cap). Standard stainless steel needles were used (Precision glide, Becton Dickinson). All measurements were carried out in the Department of laboratory medicine, Tongji hospital of Tongji Medical College in Huazhong University of Science & Technology, with a quadruple inductively coupled plasma mass spectrometer (ICP‐MS) equipped with a concentric glass nebulizer and a cyclonic spray chamber ((7700x ICP‐MS system, Agilent Technologies, USA). Analyses were performed in standard mode and dynamic collision cell mode. For Cadmium (Cd), Mercury (Hg), Thallium (Tl), and Lead (Pb), the assays were run in standard mode. For Magnesium (Mg), Calcium (Ca), Copper (Cu), Zinc (Zn), Iron (Fe), Chromium (Cr), Manganese (Mn), and Arsenic (As), the assays were run in dynamic collision cell mode (collision cell gas: helium, >99.995%). The detailed operating conditions of ICP‐MS (Agilent 7700×) is listed in Table S1. The preparations of calibration standards were described in our previous report, 18 and as listed in Table S2. The Subdivision of elements analyzed in blood samples were according to the concentration ranges for the preparation of external calibration standards. Blood samples (200 µL) were diluted 1:20 (v/v) with an aqueous solution containing 0.1% (v/v) of Triton X‐100 (Sigma‐Aldrich, France) and 0.1% of nitric acid (≥69%, Merck, Germany). The samples were vortexed in a table‐top vortexer for 30 s. The diluted samples were then quantified by ICP‐MS. The limit of quantification (LoQ) was determined as 0.03 mg/L (magnesium), 0.37 mg/L (calcium), 0.50 µg/L (chromium), 0.85 µg/L (manganese), 4.30 mg/L (iron), 3.0 µg/L (copper), 0.002 mg/L (zinc), 0.23 µg/L (arsenic), 0.06 µg/L (cadmium), 1.15 µg/L (mercury), 0.05 µg/L (thallium), and 0.95 µg/L (lead), as previously reported. 18
2.4. Quality control and quality assurance
Internal quality assessment (IQA) was carried out during analysis by using the lyophilized whole blood materials from Seronorm: L‐2 (ref.:210205) and L‐3 (ref.:210305) (https://www.sero.no/products/seronorm‐trace‐elements‐whole‐blood/). The target value of all the 12 elements in the Seronorm Whole Blood materials are listed in Table S3, which were traceable to the NIST Standard Reference Materials (https://www.nist.gov/srm), as shown in Table S3. And another low concentration homemade quality controls L‐1 were prepared by dissolving the lyophilized Seronorm L‐2 in five times of solvent. During the ICP‐MS analysis in this study, internal quality controls were tested following every 20 specimens to ensure quality throughout screening. The Z‐score was calculated, and the values within the +2 to −2 range were satisfactory and indicated analytical trueness. The measured data from internal quality controls by using the Seronorm whole Blood materials are summarized in Table S4, which were all satisfactory (Z‐scores estimated based on the target value were all within the +2 to −2 range) and the precision of measurements for all the three levels were lower than 8.0%. In addition, to further estimate the method accuracy, standard addition recovery by using the pooled whole blood specimen from the patient were assessed, as shown in Table S5. Recovery of two levels of standard addition were ranged from 85% to 110%.
External quality assessment (EQA) was carried out recently by participation in the NCCL (National Center for Clinical Laboratories) external quality assessment (EQA) scheme (2020) (https://www.nccl.org.cn/ptCn), and the College of American Pathologists (CAP) proficiency program (2020) (https://www.cap.org/laboratory‐ improvement/proficiency‐testing), as the Department of laboratory medicine of Tongji hospital is a ISO15189 accredited laboratory, and certified by College of American Pathologist (CAP). For NCCL EQA scheme, whole blood element panel (magnesium, calcium, iron, copper, and zinc) (scheme code: NCCL‐C‐29, 2020) and whole blood lead (scheme code: NCCL‐C‐26, 2020) were assessed, and were all acceptable, as listed in Table S6. For CAP proficiency testing, whole blood trace element panel (chromium, manganese, copper, zinc, arsenic, mercury, and lead) (scheme code: TMWB‐A 2020) and whole blood cadmium (scheme code: CD‐B 2020) (CD‐A were canceled due to the pandemic) were assessed, as shown in Table S7.
2.5. Data analysis
Descriptive statistics were performed with continuous variables estimated as median and interquartile range (IQR), and categorical variables summarized as frequencies and proportions. The reference range of each trace elements in Wuhan residents were described as we recently reported. 18 Considering the distributions of the elements tested by the Kolmogorov‐Smirnov test were mostly not normal, the nonparametric tests were used in our data analysis. The Mann‐Whitney U test was used to compare the results between different disease severities and different outcomes, respectively. The Spearman correlation test and its statistical significance were used to assess the correlation between the different elements. The trace elements were tested for their discriminative power in determining the predictive criteria for disease progression and fatal outcome by calculating the area under the receiver operating characteristic (ROC) curve. Sensitivity and specificity were calculated according to ROC curves for each parameter. Multivariate logistic regression was performed to adjust the effect from whole blood elements, age and sex, and the comorbidities of hypertension, diabetes, and cardiovascular disease. For data analysis, individual results below the limit of quantification (LoQ) were replaced by the (LoQ/2) value. All analyses were performed using SPSS version 22.0 (SPSS, Chicago, USA) and P < .05 was considered statistically significant.
3. RESULTS
The present study included a total of 306 hospitalized patients with confirmed COVID‐19. The median age was 63.0 years old (IQR: 53.3‐70.0). Among them, 158 (51.6%) were female and all were of Wuhan residents. All the patients were classified on admission according to the severity of illness, as severe cases (N = 104 (34.0%)) or non‐severe cases (N = 202 (66.0%)) (Table 1). All the patients had determined outcomes. Of which, 202 (100.0%) non‐severe cases and 89 (85.6%) severe cases recovered, and the remained 15 (14.4%) severe cases deceased (Table 1). In comparison with non‐severe patients, the severe cases were older (Median: 69 vs 58 years), more males (57.7% vs 43.6%), had shorter intervals between symptoms onset and admission to hospital (15 vs 27 days), and longer hospital stay (41 vs 22 days). The severe cases also showed a higher frequency of shortness of breath (33.7% vs 18.3%) and an underlying disorder, including hypertension (49.0% vs 33.2%), cardiovascular disease (19.2% vs 10.9%), and Diabetes (26.9% vs 15.3%), had a higher frequency of fatal outcome (14.4% vs 0%) (Table 1). Laboratory parameters including albumin, prealbumin, calcium, magnesium, ferritin, and blood cell counts were obtained (Table 1). Compared with non‐severe patients, the severe cases showed higher levels of ferritin (Median: 613.9 vs 321.8 µg/L), and monocyte (0.57 vs 0.50, × 109/L), neutrophil (4.26 vs 3.20, × 109/L), and WBC (6.75 vs 5.60, × 109/L), and showed lower levels of albumin (3.81 vs 4.06, g/L), prealbumin (233.5 vs 249.5, mg/L), lymphocyte (1.16 vs 1.54, × 109/L), red blood cell (3.45 vs 4.13, × 1012/L), and hemoglobin (107.5 vs 125.5, g/L).
We determined the levels of 12 elements in whole blood from all the included COVID‐19 patients by using a validated ICP‐MS‐based method and with quality assurance. Of which, nine elements including copper, zinc, calcium, iron, magnesium, lead, manganese, arsenic, and cadmium had all measure values above the LoQ, while the other three elements including chromium, mercury, and thallium showed relatively low levels in whole blood, as 1.62% (5/306) of chromium, 24.18% (74/306) of mercury, and 54.90% (168/306) of thallium had measured values below the LoQ. Among these measured values below the LoQ, all the chromium measurements (5/5, 100.0%), and more than half of the mercury (42/74, 56.8%) and thallium (90/168, 53.6%) measurements were found from the non‐severe group.
For all these patients, the median of the whole blood metal(loid)s were 39.13 mg/L (magnesium), 64.54 mg/L (calcium), 0.85 µg/L (chromium), 12.05 µg/L (manganese), 419.87 mg/L (iron), 865.02 µg/L (copper), 6.44 mg/L (zinc), 1.43 µg/L (arsenic), 0.70 µg/L (cadmium), 1.56 µg/L (mercury), <0.05 µg/L (thallium), and 11.56 µg/L (lead), respectively, as listed in Table 2. Among them, most were within the reference range, except that the median of whole blood chromium was above the upper limit.
TABLE 2.
Levels of whole blood metal(loid)s in non‐severe and severe patients with COVID‐19
| Elements (reference range), IQR | Disease status, median (IQR) | |||
|---|---|---|---|---|
| All | Non‐severe | Severe | P | |
| Mg (31.9‐48.0), mg/L | 39.13 (36.63‐41.94) | 39.46 (37.07‐42.31) | 38.33 (35.18‐40.4) | .002 |
| Ca (46.9‐66.8), mg/L | 64.54 (60.61‐69.12) | 63.55 (60.02‐67.47) | 68.20 (61.85‐71.93) | <.001 |
| Cr (<0.50‐0.76), µg/L | 0.85 (0.72‐1.08) | 0.81 (0.69‐0.97) | 1.03 (0.8‐1.3) | <.001 |
| Mn (8.1‐18.5), µg/L | 12.05 (9.74‐14.77) | 12.96 (10.63‐15.43) | 10.56 (8.63‐13.13) | <.001 |
| Fe (406.5‐576.7), mg/L | 419.87 (363.42‐465.34) | 436.8 (393.26‐479.73) | 370.24 (330.04‐428.21) | <.001 |
| Cu (634.1‐999.4), µg/L | 865.02 (779.75‐987.13) | 838.55 (770.47‐950.13) | 929.73 (828.52‐1080.02) | <.001 |
| Zn (4.3‐7.8), mg/L | 6.44 (5.84‐7.09) | 6.61 (5.91‐7.25) | 6.18 (5.67‐6.79) | .001 |
| As (0.93‐8.14), µg/L | 1.43 (1.05‐1.84) | 1.59 (1.25‐1.99) | 1.09 (0.77‐1.45) | <.001 |
| Cd (0.22‐6.44), µg/L | 0.70 (0.49‐0.99) | 0.71 (0.48‐1.01) | 0.7 (0.5‐0.96) | .654 |
| Hg (<1.15‐5.97), µg/L | 1.56 (1.15‐2.11) | 1.68 (1.2‐2.1) | 1.45 (<1.15‐2.12) | .119 |
| Tl (<0.05‐0.09), µg/L | <0.05 (<0.05‐0.06) | 0.05 (<0.05‐0.06) | <0.05 (<0.05‐0.05) | <.001 |
| Pb (8.7‐48.1), µg/L | 11.56 (9.06‐15.73) | 12.37 (9.51‐16.32) | 10.57 (8.78‐14.51) | .012 |
Abbreviations: As, arsenic; Ca, calcium; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Hg, mercury; Mg, magnesium; Mn, manganese; Pb, lead; Tl, thallium; Zn, Zinc.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Between severe and non‐severe group, except for cadmium and mercury, the other 10 metal(loid)s including magnesium, calcium, chromium, manganese, iron, copper, zinc, arsenic, and thallium displayed significant differences (Table 2, Figure S1). Among them, whole blood calcium, chromium, and copper were higher in the severe patients, while magnesium, manganese, iron, zinc, arsenic, thallium, and lead were lower (P < .05). For the severe patients, chromium and cadmium were found to be higher in the deceased group compared to the recovered group, while Arsenic were found to be lower (P < .05) (Table 3, Figure S2). The longitudinal changes of these metal(loid)s were further analyzed according to the disease severity. As shown in Figure 1, the higher levels of whole blood calcium, chromium, and copper and lower levels of magnesium, manganese, iron, zinc, arsenic, thallium, and lead in the severe group than the non‐severe group seemed to be consistently existed across the clinical course since the disease onset.
TABLE 3.
Levels of whole blood metal(loid)s in recovered and deceased patients with severe COVID‐19
| Elements | Outcomes, median (IQR) | P | |
|---|---|---|---|
| Recovered | Deceased | ||
| Age, median (IQR), y | 69 (62‐77.5) | 69 (67‐81) | .311 |
| Sex | .185 | ||
| Male | 49 (55.1%) | 11 (73.3%) | |
| Female | 40 (44.9%) | 4 (26.7%) | |
| Whole blood elements (reference range), IQR | |||
| µMg (31.9‐48.0), mg/L | 38.29 (35‐40.49) | 38.39 (36.18‐40.19) | .681 |
| µCa (46.9‐66.8), mg/L | 68.72 (62.39‐71.92) | 64.53 (60.51‐72.57) | .491 |
| Cr ( <0.50‐0.76), µg/L | 0.96 (0.78‐1.19) | 1.5 (1.05‐1.87) | .001 |
| Mn (8.1‐18.5), µg/L | 10.63 (8.74‐13.25) | 10.38 (8.13‐13.01) | .687 |
| Fe (406.5‐576.7), mg/L | 371.33 (331.57‐430.26) | 342.58 (316.07‐413.32) | .424 |
| Cu (634.1‐999.4), µg/L | 925.22 (828.7‐1076.39) | 999.29 (820.21‐1114.67) | .392 |
| Zn (4.3‐7.8), mg/L | 6.15 (5.65‐6.79) | 6.22 (5.81‐6.99) | .508 |
| As (0.93‐8.14), µg/L | 1.14 (0.84‐1.52) | 0.85 (0.52‐0.96) | .002 |
| Cd (0.22‐6.44), µg/L | 0.63 (0.45‐0.9) | 0.8 (0.77‐1.78) | .002 |
| Hg (<1.15‐5.97), µg/L | 1.45 (<1.15‐2) | 1.82 (<1.15‐3.29) | .187 |
| Tl (<0.05‐0.09), µg/L | <0.05 (<0.05‐0.05) | <0.05 (<0.05‐< 0.05) | .622 |
| Pb (8.7‐48.1), µg/L | 10.55 (8.92‐14.31) | 10.64 (6.42‐16.9) | .647 |
Abbreviations: As, arsenic; Ca, calcium; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Hg, mercury; Mg, magnesium; Mn, manganese; Pb, lead; Tl, thallium; Zn, Zinc.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
FIGURE 1.
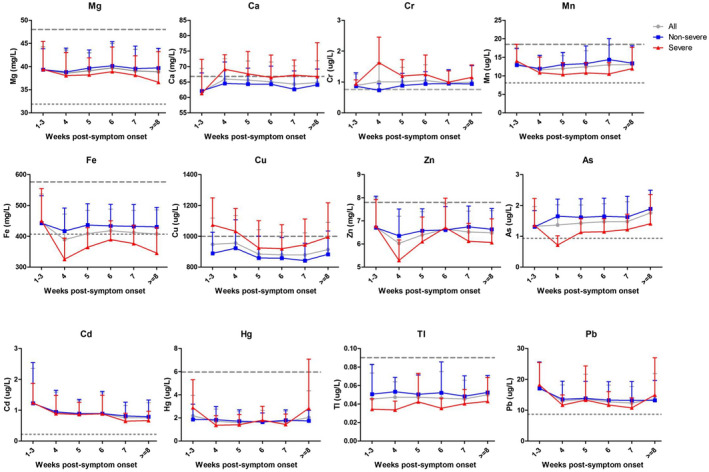
Longitudinal dynamic profiles of the 12 metal(loid)s in whole blood of 306 COVID‐19 patients according to the disease severity (non‐severe, severe, and total cases). The mean values were delineated on weeks of disease onset. Data are shown as mean ± SD. As, arsenic; Ca, calcium; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Hg, mercury; Mg, magnesium; Mn, manganese; Pb, lead; Tl, thallium; Zn, zinc
The correlation matrix was further established to check the inter‐metal(loid) correlations, as summarized in Figure 2. The most positive correlations were found among the essential metals magnesium, manganese, iron, and zinc, with the highest correlation coefficient (CC) of 0.64 for iron‐zinc, followed by 0.55 for iron‐magnesium, and 0.51 for iron‐manganese. Among the heavy metals, arsenic‐thallium showed the highest coefficient of 0.43. The most negative correlations were found as iron‐calcium (CC = −0.59), zinc‐calcium (CC = −0.52), and iron‐chromium (CC = −0.35).
FIGURE 2.
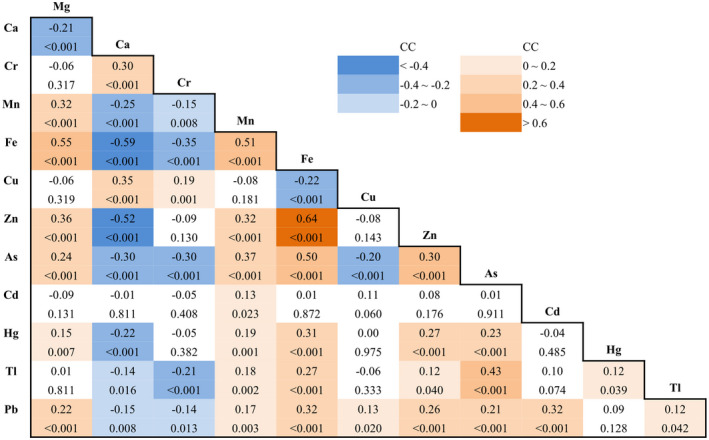
Correlation matrix among the whole blood elements. Spearman correlation coefficients (CC, above) and P values (below, italic) for the spearman correlation between each two of the 12 elements. Green and blue color represent positively and negatively correlations, respectively. Correlation coefficients (CC, above) and P values (below). As, arsenic; Ca, calcium; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Hg, mercury; Mg, magnesium; Mn, manganese; Pb, lead; Tl, thallium; Zn, Zinc
Given associations of inflammatory processes with COVID‐19 evolution, we further assessed the correlations of the 12 whole blood elements with other blood parameters including albumin, prealbumin, calcium, magnesium, ferritin, and blood cell counts (lymphocyte, monocyte, neutrophil, RBC, and WBC) and hemoglobin (Figure S3), respectively. The highest correlations were found between whole blood iron and hemoglobin (CC = 0.75), iron and RBC (CC = 0.70). The hemoglobin and RBC were also found to be positively correlated with lead (CC = 0.36 and 0.33, respectively), arsenic (CC = 0.35 and 0.47), zinc (CC = 0.33 and 0.31), manganese (CC = 0.30 and 0.48), and magnesium (CC = 0.37 and 0.33), but negatively correlated with calcium (CC = −0.53 and −0.50) and chromium (CC = −0.33 and −0.35) (Figure S3).
For those elements displayed a statistically significant difference at univariate analysis between different disease severities or outcomes, we calculated the optimum diagnostic cutoff points of severe vs non‐severe group and recovered vs deceased group, respectively, by using the ROC curve method. The specified cutoff points and the sensitivity, specificity, and the area underneath the ROC curve belonging to those cutoff points are shown in Table S8.
For the disease severity, two indicators had AUC > 0.70, including iron and arsenic (Table S8). Considering the correlations existed between iron and arsenic (CC = 0.50, P < .001), only iron was used by applying binary logistic regression to the predetermined cutoff values. Age, sex, and whole blood iron were determined to be independently predictive of severe illness (P < .05). The risk of a severe illness increased 4.19, 1.97, and 3.49 times in the presence of age above 65 years old, male, and iron <376.67 mg/L, respectively (Table 4). These indicators collectively could discriminate 73.2% of the patients that might progress to severe illness (data not shown).
TABLE 4.
The risk factors for severe illness and fatal outcome in COVID‐19 patients by using cutoff points
| Severe illness | ||||
|---|---|---|---|---|
| Non‐severe | Severe | OR (95% CI) | P | |
| Age, years | ||||
| ≥65 | 61 | 73 | 4.19 (2.41‐7.29) | <.001 |
| <65 | 141 | 31 | ||
| Sex | ||||
| Male | 88 | 60 | 1.97 (1.15‐3.39) | .014 |
| Female | 114 | 44 | ||
| Fe, mg/L | ||||
| <376.67 | 39 | 57 | 3.49 (1.99‐6.14) | <.001 |
| ≥376.67 | 163 | 47 | ||
| Fatal outcome | ||||
| Recovered | Deceased | OR (95% CI) | P | |
| Cr, µg/L | ||||
| ≥1.32 | 15 | 10 | 9.60 (2.14‐42.96) | .0031 |
| <1.32 | 74 | 5 | ||
| Cd, µg/L | ||||
| ≥0.68 | 40 | 14 | 12.73 (1.36‐119.01) | .026 |
| <0.68 | 49 | 1 | ||
| Cardiovascular diseases | ||||
| Y | 15 | 5 | 6.91 (1.29‐37.01) | .024 |
| N | 74 | 10 | ||
Abbreviations: Cd, cadmium; Cr, chromium; Fe, iron.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
For the disease outcome, three indicators had AUC > 0.70, including chromium, arsenic, and cadmium (Table S8), and used for the multivariate logistic regression. Finally, whole blood chromium, cadmium, and the comorbidity of cardiovascular diseases were determined to be independently predictive of fatal outcome (P < .05). The risk for a fatal outcome increased 9.595, 12.734, and 6.91 times in the presence of chromium ≥ 1.32 µg/L, cadmium ≥ 0.68 µg/L, and cardiovascular diseases, respectively (Table 4). These indicators collectively could discriminate 87.5% of the patients that might progress to severe illness (data not shown).
4. DISCUSSION
To our knowledge, this is the first study focusing on the associations of the whole blood metals/metalloids with the severity and outcome of COVID‐19. We performed a retrospective study of 306 patients with COVID‐19 for their whole blood levels of essential minerals including magnesium, calcium, chromium, manganese, iron, copper, zinc, and toxic metal(loid)s including arsenic, cadmium, mercury, thallium, and lead according to the disease severity (severe or non‐severe) and outcomes (recovered or deceased), respectively. Our data revealed that the whole blood magnesium, cadmium, chromium, manganese, iron, copper, zinc, arsenic, and thallium displayed significant differences between severe and non‐severe group (P < .05), and the whole blood chromium, cadmium, and arsenic displayed differences between the deceased group and recovered group (P < .05). These data suggested the whole blood levels of essential minerals and toxic metal(loid)s may be associated with the disease progression and fatal outcome, and could be potential risk factors correlated with the severe illness and mortality of COVID‐19.
For the essential minerals, as the world awaits an effective vaccine, nutrition may play an important and safe role in helping mitigate patient morbidity and mortality. Optimal nutritional status of relevant nutrients is important for a well‐functioning immune system during the COVID‐19 crisis. 8 , 9 Among the many essential nutrients, magnesium is the second most abundant intracellular cation after potassium, and involved in >600 enzymatic reactions in the body, including those contributing to the exaggerated immune and inflammatory responses exhibited by COVID‐19 patients. 19 Magnesium deficiency primes phagocytes, enhances granulocyte oxidative burst, activates endothelial cells, and increases the levels of cytokines, thus, promoting inflammation. 20 Several aspects of the COVID‐19 mimic the metabolic events shown to occur during latent subclinical magnesium deficiency, such as a drop of T cells, increased plasma concentration of inflammatory cytokines, and endothelial dysfunction. 19 , 21 Our data suggested the significant lower levels of magnesium in severe patients with COVID‐19 than in the non‐severe patients, which is consistent with the hypothesis by Iotti et al. that a low Mg status might foment the transition from mild to severe clinical manifestations of the COVID‐19. 21 Constant monitoring of magnesium status was thought as an possible strategy to influence disease contraction and progression. 19
Calcium was reported as a key role in viral fusion for many enveloped viruses such as SARS‐CoV, MERS‐CoV, and Ebola virus. 22 Low levels of serum total and ionized calcium were reported in COVID‐19 patients. 23 Hypocalcemia had already shown to be common in patients with SARS and in patients with Ebola virus disease, and may occur also in COVID‐19. 22 However, a mild increase of whole blood total calcium in severe patients compared with the non‐severe cases (68.20 vs 63.55 mg/L) were observed in the present study. Considering that the vast majority of blood calcium were in plasma rather than within blood cells, meanwhile the blood cell counts in severe COVID‐19 patients were changed significantly, therefore, it seems to be the variations of the blood cells, especially for the red blood cell counts (RBCs) which deceased to a much lower levels in severe patients than the non‐severe cases (3.15 vs 4.13, ×1012/L), that inversely lead to the relatively increase of calcium concentrations in whole blood. This result needs to be further confirmed in future studies.
Chromium is a naturally occurring heavy metal found commonly in the environment, and also is an essential micronutrient involved in carbohydrate, lipid, and protein metabolism primarily by increasing insulin efficiency. 24 , 25 Previous study has confirmed the drastic increase of urinary chromium in patients with diabetes. 24 Chromium is of significant importance in altering the immune response by immunostimulatory or immunosuppressive processes as shown by its effects on T and B lymphocytes, macrophages, cytokine production, and the immune response that may induce hypersensitivity reactions. 26 As the lymphocyte abnormities and cytokine dysfunctions has been commonly reported in severe COVID‐19 patients, 3 the higher levels of chromium in severe and deceased COVID‐19 patients, observed in our study, may be associated with the immune dysfunction in COVID‐19, and the mechanisms need further clarification in the future.
Transition metals such as manganese, iron, copper, and zinc are essential for all forms of life, as 30% of enzymes require a metal cofactor. 27 Clinical deficiency of manganese, iron, or zinc in the host increases the incidence of infectious disease and mortality, 28 , 29 , 30 which is consistent, to some extent, with our findings that the whole blood manganese, iron, or zinc decreased in the severe COVID‐19 patients than non‐severe patients. Among them, manganese has been reported of playing an important role in innate immune activation and host antiviral defense, as it released from organelles into the cytosol upon virus infection and facilitates the activation of cGAS and STING signaling. 28 For iron, systemic dysregulation resulted from COVID‐19 hyperinflammation has been recently reviewed. 31 Serum iron level was reported as a potential predictor of COVID‐19 severity and mortality. 32 Decreased serum iron level could predict the transition of COVID‐19 from mild to severe and critical illness, 32 , 33 which was in good agreement with our findings. Zinc is one of the micronutrients that could be consumed to reduce the intensity of SARS‐CoV‐2 infection and perhaps lessen the respiratory tract infection through the antiviral actions 7 . Zinc deficiency can contribute to defective cell‐mediated immunity and to increased susceptibility to various infections, including pneumonia. 34 , 35 Several lines of evidence suggest a link between zinc and COVID‐19, including the observation that chloroquine, a drug being repurposed for COVID‐19, is a known zinc ionophore. 36 SARS‐CoV and SARS‐CoV‐2 use the host zinc metalloenzyme, ACE2, as an entry point to cells. 37 Consistent with these overall findings, our data suggested the lower level of whole blood zinc together with the transition metal manganese and iron were associated with the severe illness of COVID‐19. Our findings revealed that the essential minerals including manganese, iron, zinc, and magnesium positively correlated with each other with high coefficients, which implied a possible synergistic effect of these metals on the disease.
In contrast to the transition metals above, copper change showed a different pattern: it increased in the severe patients compared with the non‐severe cases. This difference may be associated with the two sides of copper: it is an essential micronutrient for both pathogens and the animal hosts they infect, and also is toxic in cells due to its redox properties and ability to disrupt active sites of metalloproteins. 38 Animal host can thwart pathogen growth by limiting their copper nutrients, similar to the well‐documented nutritional immunity effects for starving microbes of essential zinc, manganese, and iron nutrients. 38 Meanwhile, a common hallmark of infection irrespective of the agent (viral, bacterial, and fungal) is a marked and progressive rise in serum copper, including the lung infections. 39 , 40 , 41 In addition, the increased serum copper to zinc ratio has been reported to be associated with the inflammation conditions, 42 similar to the findings in this study.
Besides the minerals discussed above, environmental pollutants, including the heavy metals or metalloids, are believed to be associated with the viral epidemic/pandemic events and prevalence. 14 In the present study, the heavy metals in whole blood were investigated, among which, cadmium was found to be higher in the deceased cases. Cadmium has cumulative toxicity to many organs due to its long biological half‐life. It was reported that exposure to cadmium and lead could cause adverse effect on human health on the respiratory system with lung function impairment. 43
However, the arsenic, lead, and thallium showed lower levels in whole blood of the severe cases than the non‐severe cases, and the arsenic also showed a lower level in deceased cases than the recovered cases. These results were unexpected, as exposure to heavy metals was commonly reported to induce respiratory dysfunction and positively correlated with the occurrence of respiratory diseases. 44 In blood, heavy metals were distributed unequally in plasma and blood cells. For arsenic and lead, the content of the two trace elements in red blood cells is much higher than in plasma. Since the red blood cell counts (RBCs) showed a much lower levels in severe patients with COVID‐19 than those non‐severe cases (3.15 vs 4.13, × 1012/L) (Table 1), the decrease of the circulatory arsenic and lead in severe patients with COVID‐19 may be due to the abnormalities of RBCs.
In fact, abnormalities of peripheral blood system in patients with COVID‐19 include lower counts of RBCs, lymphocytes, platelets, and hemoglobin, and higher levels of neutrophils. Considering that the heavy metals in whole blood were at relatively low levels in all the COVID‐19 patients (all within the normal range or mildly lower than the lower limit), we suspected the variations of the heavy metals like arsenic, lead, thallium, and cadmium in severe or decrease COVID‐19 patients may be attributed to the abnormalities of peripheral blood system. 45 The positive correlations among arsenic, thallium, and lead revealed by our results implied a similar mechanism existed for these elements. These results need to be verified and clarified in the future studies. Furthermore, future studies focused on the investigation of the elemental levels not in whole blood but distinguishing the cellular part from the liquid one may contribute to a further understanding of the abnormalities.
There are some limitations in the present study. The study was based on only 306 severe (N = 104) or non‐severe (N = 202) patients with COVID‐19 in Wuhan of China, among which 291 patients recovered and discharged while 15 cases deceased. Considering the relatively small sample size, future multicenter studies on a larger cohort will be needed to verify the findings. In addition, the present study mainly focused on the associations of disease severity and mortality with whole blood metal(loid)s, but did not exclude the effect of comorbidities, which should also be noticed in future studies. Moreover, the baseline levels of chromium, mercury, and thallium in whole blood were very close to or lower than the LoQ of our method of analysis, thus, the results should be carefully interpreted. The data in the present study might provide a reference for future ecological investigations that evaluate the presence of metals and metalloids in environmental matrices such as soils, 46 , 47 , 48 waters, 49 air, 50 investigating their correlation with epidemiological data and blood concentrations (serum / plasma / cells) of COVID‐19 patients.
In conclusion, we provided a comprehensive analysis of the abnormalities of 12 metal(loid)s for the COVID‐19 disease. Whole blood calcium, chromium, and copper were higher, while magnesium, manganese, iron, zinc, arsenic, thallium, and lead were lower in the severe patients. Among the severe patients, chromium and cadmium were higher, while arsenic was lower in the deceased group. Our study determined that the iron, arsenic, age, and sex were independent factors associated with the disease severity, while chromium, cadmium, and the comorbidities of cardiovascular disease were independent factors associated with the mortality. These results suggest variations of whole blood metal(loid)s as associated factors correlated with the disease progression and fatal outcome, which could be persistently monitored, and would be helpful in the evaluation of the dynamic changes in patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
H‐L. Zeng and L. Cheng conceived the idea of the study; H‐L. Zeng, Q. Yang, and P. Yuan analyzed the data; H‐L. Zeng, Q. Yang, and X. Wang interpreted the results; H‐L. Zeng and L. Cheng wrote the paper; all authors discussed the results and revised the manuscript.
ETHICS
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in Wuhan, China (No.:TJ‐IRB20200201). All the procedures involving human samples conformed to the principles outlined in the Declaration of Helsinki. Informed consent was obtained in all cases. All the analysis were performed on existing samples collected during standard diagnostic tests, posing no extra burden to patients.
Supporting information
Fig S1‐S3
Table S1‐S8
ACKNOWLEDGMENTS
The authors thank all the medical care workers who participated in the sample collection.
Zeng H‐L, Yang Q, Yuan P, Wang X, Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID‐19. The FASEB Journal. 2021;35:e21392. 10.1096/fj.202002346RR
Funding information
The work was supported by National Natural Science Foundation of China (31600666).
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W‐J, Ni Z‐y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarzi‐Puttini P, Giorgi V, Sirotti S, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337‐342. [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population‐level observational study. Lancet Digit Health. 2020;2(4):e201‐e208. 10.1016/s2589-7500(20)30026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh AK, Gillies CL, Singh R, et al. Prevalence of co‐morbidities and their association with mortality in patients with COVID‐19: a systematic review and meta‐analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. 10.1111/dom.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Razzaque MS. COVID‐19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J Exp Med. 2020;251(3):175‐181. 10.1620/tjem.251.175 [DOI] [PubMed] [Google Scholar]
- 8. Iddir M, Brito A, Dingeo G, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID‐19 crisis. Nutrients. 2020;12(6): 1562. 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well‐functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. 10.3390/nu12041181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system‐working in harmony to reduce the risk of infection. Nutrients. 2020;12(1): 236. 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rhee SY, Hwang YC, Woo JT, et al. Blood lead is significantly associated with metabolic syndrome in Korean adults: an analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc Diabetol. 2013;12:9. 10.1186/1475-2840-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009‐2010. Diabet Med: J Brit Diabet Association. 2013;30(4):e143‐e148. 10.1111/dme.12103 [DOI] [PubMed] [Google Scholar]
- 13. Tellez‐Plaza M, Guallar E, Howard BV, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421‐429. 10.1097/EDE.0b013e31828b0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsatsakis A, Petrakis D, Nikolouzakis TK, et al. COVID‐19, an opportunity to reevaluate the correlation between long‐term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem Toxicol. 2020;141:111418. 10.1016/j.fct.2020.111418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X, Hu H, Hong YA. Body burden of heavy metals among HIV high risk population in USA. Environ Pollut. 2017;220(Pt B):1121‐1126. 10.1016/j.envpol.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 16. Xu X, Hu H, Dailey AB, Kearney G, Talbott EO, Cook RL. Potential health impacts of heavy metals on HIV‐infected population in USA. PLoS One. 2013;8(9):e74288. 10.1371/journal.pone.0074288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Health Commission of the People’s Republic of China. Diagnosis and Treatment Scheme of New Coronavirus Infected Pneumonia. 2020. http://www.gov.cn/zhengce/zhengceku/2020‐08/19/5535757/files/da89edf7cc9244fbb34ecf6c61df40bf.pdf.
- 18. Zeng HL, Li H, Lu J, Guan Q, Cheng L. Assessment of 12 metals and metalloids in blood of general populations living in Wuhan of China by ICP‐MS. Biol Trace Elem Res. 2019;189(2):344‐353. 10.1007/s12011-018-1486-8 [DOI] [PubMed] [Google Scholar]
- 19. Wallace TC. Combating COVID‐19 and building immune resilience: a potential role for magnesium nutrition? J Am College Nutr. 2020;1‐9. 10.1080/07315724.2020.1785971 [DOI] [PubMed] [Google Scholar]
- 20. Maier JA, Castiglioni S, Locatelli L, Zocchi M, Mazur A. Magnesium and inflammation: advances and perspectives. Semin Cell Dev Biol. 2020;S1084‐9521(20):30171–30173. 10.1016/j.semcdb.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 21. Iotti S, Wolf F, Mazur A, Maier JA. The COVID‐19 pandemic: is there a role for magnesium? hypotheses and perspectives. Magnes Res. 2020; 33(2):21–27. 10.1684/mrh.2020.0465 [DOI] [PubMed] [Google Scholar]
- 22. Marazuela M, Giustina A, Puig‐Domingo M. Endocrine and metabolic aspects of the COVID‐19 pandemic. Rev Endocr Metab Disord. 2020;21(4):495–507. 10.1007/s11154-020-09569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cappellini F, Brivio R, Casati M, Cavallero A, Contro E, Brambilla P. Low levels of total and ionized calcium in blood of COVID‐19 patients. Clin Chem Lab Med. 2020;58(9):e171‐e173. 10.1515/cclm-2020-0611 [DOI] [PubMed] [Google Scholar]
- 24. Zhou Q, Guo W, Jia Y, Xu J. Comparison of chromium and iron distribution in serum and urine among healthy people and prediabetes and diabetes patients. Biomed Res Int. 2019;2019:1‐8. 10.1155/2019/3801639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewicki S, Zdanowski R, Krzyzowska M, et al. The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann Agric Environ Med: AAEM. 2014;21(2):331‐335. 10.5604/1232-1966.1108599 [DOI] [PubMed] [Google Scholar]
- 26. Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC. Effects of chromium on the immune system. FEMS Immunol Med Microbiol. 2002;34(1):1‐7. 10.1111/j.1574-695X.2002.tb00596.x [DOI] [PubMed] [Google Scholar]
- 27. Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen‐host interface. Nat Rev Microbiol. 2012;10(8):525‐537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang C, Guan Y, Lv M, et al. Manganese increases the sensitivity of the cGAS‐STING pathway for double‐stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48(4):675–687.e677. 10.1016/j.immuni.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 29. Cannas D, Loi E, Serra M, Firinu D, Valera P, Zavattari P. Relevance of essential trace elements in nutrition and drinking water for human health and autoimmune disease risk. Nutrients. 2020;12(7):2074. 10.3390/nu12072074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12):1286. 10.3390/nu9121286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID‐19 pathogenesis? Int J Infect Dis: IJID. 2020;97:303‐305. 10.1016/j.ijid.2020.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7):ofaa250. 10.1093/ofid/ofaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolondi G, Russo E, Gamberini E, et al. Iron metabolism and lymphocyte characterisation during Covid‐19 infection in ICU patients: an observational cohort study. World J Emerg Surg: WJES. 2020;15(1):41. 10.1186/s13017-020-00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman MT, Idid SZ. Can Zn Be a critical element in COVID‐19 treatment? Biol Trace Elem Res. 2020;199(2):550–558. 10.1007/s12011-020-02194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanna A, Firinu D, Zavattari P, Valera P. Zinc status and autoimmunity: a systematic review and meta‐analysis. Nutrients. 2018;10(1):68. 10.3390/nu10010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doboszewska U, Wlaz P, Nowak G, Mlyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br J Pharmacol. 2020; 177(21):4887–4898. 10.1111/bph.15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21(2):137‐144. 10.1007/s00775-016-1335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ilback NG, Frisk P, Tallkvist J, Gadhasson IL, Blomberg J, Friman G. Gastrointestinal uptake of trace elements are changed during the course of a common human viral (Coxsackievirus B3) infection in mice. J Trace Elem Med Biol. 2008;22(2):120‐130. 10.1016/j.jtemb.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 40. Cernat RI, Mihaescu T, Vornicu M, Vione D, Olariu RI, Arsene C. Serum trace metal and ceruloplasmin variability in individuals treated for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011;15(9):1239‐1245. 10.5588/ijtld.10.0445. [DOI] [PubMed] [Google Scholar]
- 41. Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(38):E5336‐E5342. 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malavolta M, Piacenza F, Basso A, Giacconi R, Costarelli L, Mocchegiani E. Serum copper to zinc ratio: relationship with aging and health status. Mech Ageing Dev. 2015;151:93‐100. 10.1016/j.mad.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 43. Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. 2018;119(1):157‐184. 10.1002/jcb.26234 [DOI] [PubMed] [Google Scholar]
- 44. Bortey‐Sam N, Ikenaka Y, Akoto O, et al. Association between human exposure to heavy metals/metalloid and occurrences of respiratory diseases, lipid peroxidation and DNA damage in Kumasi, Ghana. Environ Pollut. 2018;235:163‐170. 10.1016/j.envpol.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 45. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China. Clinica Chimica Acta. 2020;507:174‐180. 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valera P, Zavattari P, Albanese S, et al. A correlation study between multiple sclerosis and type 1 diabetes incidences and geochemical data in Europe. Environ Geochem Health. 2014;36(1):79‐98. 10.1007/s10653-013-9520-4 [DOI] [PubMed] [Google Scholar]
- 47. Valera P, Zavattari P, Sanna A, et al. Zinc and other metals deficiencies and risk of type 1 diabetes: an ecological study in the high risk Sardinia Island. PLoS ONE. 2015;10(11):e0141262. 10.1371/journal.pone.0141262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monti MC, Guido D, Montomoli C, et al. Is geo‐environmental exposure a risk factor for multiple sclerosis? a population‐based cross‐sectional study in South‐Western Sardinia. PLoS ONE. 2016;11(9):e0163313. 10.1371/journal.pone.0163313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flem B, Reimann C, Birke M, Banks D, Filzmoser P, Frengstad B. Inorganic chemical quality of European tap‐water: 2. Geographical distribution. Appl Geochem. 2015;59:211‐224. 10.1016/j.apgeochem.2015.01.016 [DOI] [Google Scholar]
- 50. Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):e04691. 10.1016/j.heliyon.2020.e04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Table S1‐S8
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


