Key Points
Question
Can retinal biomarkers reflect in vivo Alzheimer disease–related brain abnormalities in cognitively normal older adults?
Findings
In this cross-sectional study including 49 participants, delayed implicit time noted on multifocal electoretinogram, as well as reduced regional thickness of macula and retinal nerve fiber layer, was associated with amyloid-β deposition, and the ganglion cell complex–inner plexiform layer thickness was correlated with Alzheimer disease–related neurodegeneration in cognitively normal individuals. A screening model based on these results showed 90% diagnostic accuracy to detect amyloid-β deposition in these cognitively normal individuals.
Meaning
This study noted that retinal biomarkers can reflect in vivo Alzheimer disease–related brain abnormalities in cognitively normal older adults, suggesting the potential of retinal biomarkers as surrogate biomarkers of early Alzheimer disease.
Abstract
Importance
Retinal biomarkers reflecting in vivo brain Alzheimer disease (AD) pathologic abnormalities could be a useful tool for screening cognitively normal (CN) individuals at the preclinical stage of AD.
Objectives
To investigate the association of both functional and structural alterations of the retina with in vivo AD pathologic abnormalities in CN older adults and model a screening tool for detection of preclinical AD.
Design, Setting, and Participants
This cross-sectional study included a total of 49 CN individuals, and all assessment was done at the Seoul National University Hospital, Seoul, South Korea. All participants underwent complete ophthalmic examination, including swept-source optical coherence tomography (SS-OCT) and multifocal electroretinogram as well as amyloid-β (Aβ) positron emission tomography and magnetic resonance imaging. Data were collected from January 1, 2016, through October 31, 2017, and analyzed from February 1, 2018, through June 30, 2020.
Main Outcomes and Measures
For structural parameters of the retina, the thickness of the macula and layer-specific thicknesses, including peripapillary retinal nerve fiber layer and ganglion cell-inner plexiform layer measured by SS-OCT, were used for analysis. For functional parameters of the retina, implicit time and amplitude of rings 1 to 6 measured by multifocal electroretinogram were used.
Results
Of the 49 participants, 25 were women (51.0%); mean (SD) age was 70.6 (9.4) years. Compared with 33 CN individuals without Aβ deposition (Aβ−CN), the 16 participants with Aβ (Aβ+CN) showed reduced inner nasal macular thickness (mean [SD], 308.9 [18.4] vs 286.1 [22.5] μm; P = .007) and retinal nerve fiber layer thickness, particularly in the inferior quadrant (133.8 [17.9] vs 103.8 [43.5] μm; P = .003). In addition, the Aβ+CN group showed prolonged implicit time compared with the Aβ−CN group, particularly in ring 5 (41.3 [4.0] vs 38.2 [1.3] milliseconds; P = .002). AD-related neurodegeneration was correlated with the thickness of the ganglion cell-inner plexiform layer only (r = 0.41, P = .005). The model to differentiate the Aβ+CN vs Aβ−CN groups derived from the results showed 90% accuracy.
Conclusions and Relevance
The findings of this study showing both functional as well as structural changes of retina measured by multifocal electroretinogram and SS-OCT in preclinical AD suggest the potential use of retinal biomarkers as a tool for early detection of in vivo AD pathologic abnormalities in CN older adults.
This cross-sectional study examines the use of structural changes in the retina as biomarkers to identify amyloid-β deposition in assessment of individuals for preclinical Alzheimer disease.
Introduction
Cerebral amyloid-β (Aβ) deposition, a hallmark neuropathologic characteristic of Alzheimer disease (AD), begins approximately 15 to 20 years before the onset of clinical manifestation.1 Advances of AD biomarkers, particularly neuroimaging biomarkers including amyloid positron emission tomography (PET), enables detection of individuals with the preclinical stage of AD who already have Aβ accumulation but are still cognitively normal (CN).1,2 Moreover, attention to early therapeutic intervention at the preclinical stage is growing as evidence supporting the risk of clinical progression accumulates over several years.3
Ophthalmologic examination of the retina, which is derived from a part of the brain during fetal neurodevelopment,4,5 has been receiving increased attention as a potential tool for direct, noninvasive evaluation of changes in the central nervous system related to AD, with lower cost and higher accessibility than neuroimaging tools.2,6,7 Previous studies reported retinal changes related to the AD process in postmortem examination of the brains of individuals with AD8,9,10 and those with clinically defined AD dementia and mild cognitive impairment.11,12,13,14,15,16,17 In the preclinical AD group, a limited number of previous studies reported retinal vascular changes,18,19 and the few studies that evaluated structural retinal parameters measured by optical coherence tomography (OCT) yielded inconsistent findings, owing to a lack of controlling for clinical confounders.20,21,22 Thus, further OCT studies with well-controlled possible confounders are required to identify structural retinal alterations in preclinical AD. Moreover, to our knowledge, whether retinal function, as measured by electroretinography, is changed in preclinical AD has not been investigated.
Meanwhile, AD-related regional neurodegeneration (AD-ND), such as reduced cortical thickness in AD-signature regions (AD-CT) including temporoparietal cortices, is another key pathophysiologic process of AD.23 Previous studies reported that even individuals with preclinical AD showed reduced AD-CT measured by magnetic resonance imaging (MRI), which linked to poorer cognitive performance and future clinical progression.24,25 However, to our knowledge, no studies have explored the association of AD-ND with retinal alterations in CN older adults.
Thus, we aimed to investigate the association of both structural and functional alterations of the retina with in vivo AD pathologic characteristics, including cerebral Aβ deposition and AD-ND in CN older adults recruited from the community. We modeled a screening tool to detect preclinical AD based on parameters identified in retinal examination.
Methods
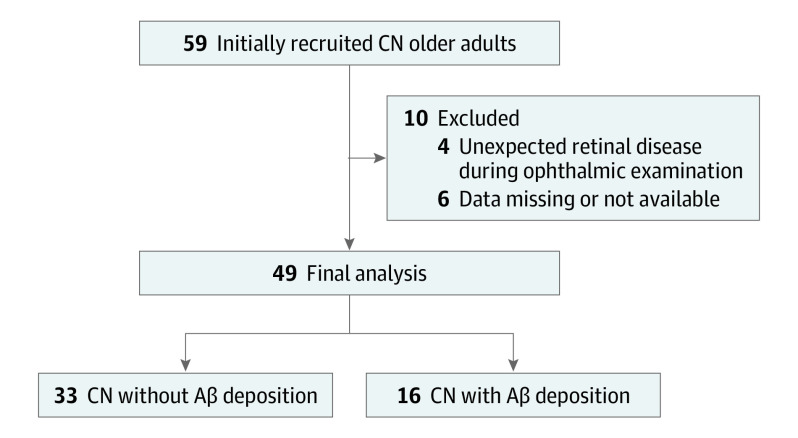
In this study, CN older adults aged 55 to 90 years, with a global Clinical Dementia Rating score of 0 (indicating no clinical impairment), and without a diagnosis of mild cognitive impairment or dementia were included. Eligible individuals were recruited from January 1, 2016, to October 31, 2017, from the cohort of the Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer Disease, an ongoing prospective cohort study (Figure 1; eMethods in the Supplement).6 Data analysis was conducted from February 1, 2018, to June 30, 2020. The study was approved by the institutional review board of Seoul National University Hospital, Seoul, Republic of Korea. Written informed consent was obtained from all participants. There was no incentive or compensation for participation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Recruitment of Participants.
Abbreviations: Aβ, amyloid-β; CN, cognitively normal.
In all participants, simultaneous 3-dimensional carbon 11–labeled Pittsburgh compound B positron emission tomography ([11C] PiB-PET) and 3-dimensional T1-weighted MRI (3.0 T Biograph mMR PET-MR scanner; Siemens) was carried out; details of acquisition and preprocessing of [11C]PiB-PET and MRI have been described previously26 and within the eMethods in the Supplement. Individuals were classified as either the Aβ-positive group (Aβ+CN) if the standardized uptake value ratio was greater than 1.4 in at least 1 of the 4 regions of interest, including the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal regions, where Aβ accumulation occurs early in AD,27 or as the Aβ-negative group (Aβ−CN) if the standardized uptake value ratio of all 4 regions of interest was less than or equal to 1.4.23,28,29 The eMethods and eFigure 1 in the Supplement provide more detailed information on the rationale regarding the definition of Aβ positivity.
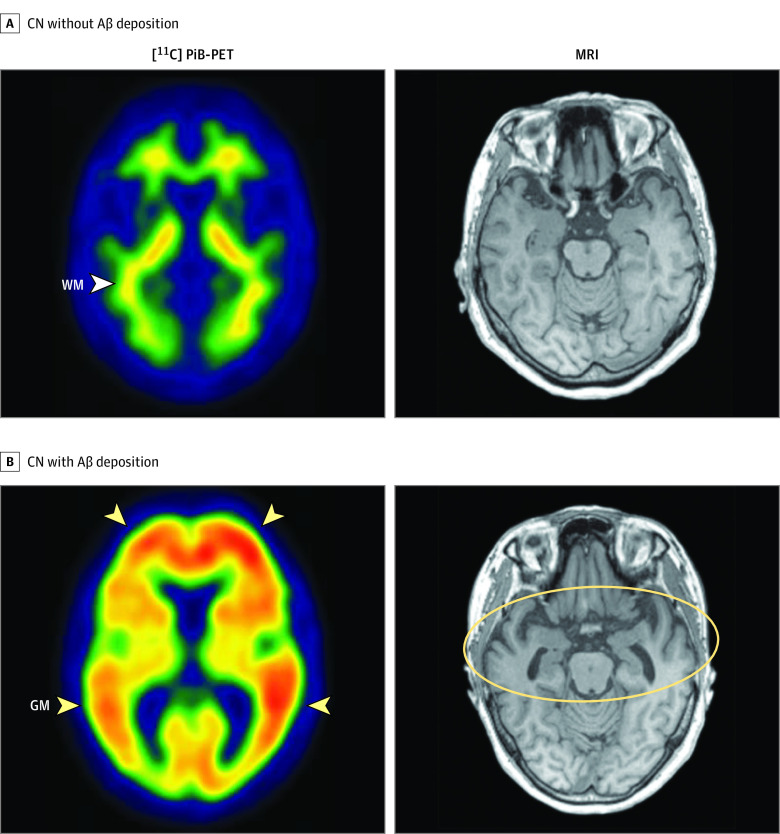
For measurement of AD-ND, all T1 MR images went through automatic segmentation using FreeSurfer, version 5.3, with manual corrections if minor segmentation errors were detected during visual inspection. Subsequently, according to the method of previous studies,25,30 AD-CT was calculated as the mean cortical thickness (millimeters) of the AD-signature regions, including the entorhinal, parahippocampal, middle temporal, angular gyrus, posterior cingulate cortex, and precuneus, using the Desikan-Killany atlas.31 Representative images of [11C] PiB-PET and MRI from individuals in the Aβ+CN and Aβ−CN groups are provided in Figure 2.
Figure 2. Representative Images of Carbon 11–Labeled Pittsburgh Compound B Positron Emission Tomography ([11C] PiB-PET) and Magnetic Resonance Imaging (MRI) .
A, A cognitively normal (CN) older adult in the group with no amyloid-β (Aβ) deposition with a typical negative amyloid scan of [11C] PiB-PET (left), showing only nonspecific uptake in the white matter (WM) tract (arrowhead) and no PiB uptake in cerebral gray matter (GM), as well as no significant bilateral temporal atrophy in brain MRI (right). B, A CN older adult in the group with Aβ deposition with a typical positive amyloid scan of [11C] PiB-PET, showing PiB retention in cerebral GM (left, arrowheads) and bilateral temporal atrophy in MRI (right, yellow circle).
In all participants, complete ophthalmic evaluation was conducted, including the best-corrected visual acuity, intraocular pressure, slitlamp biomicroscopy, and fundus examination. Eyes with a history of retinal surgery, evidence of the epiretinal membrane, glaucoma, or branch retinal vein occlusion were excluded. One eye per individual was randomly selected and included for the study. Best-corrected visual acuity was recorded in Snellen equivalents and subsequently transformed to logMAR values. Among 59 CN older adults initially recruited, 10 individuals were excluded after ophthalmic examination owing to unexpected retinal disease (n = 6) noted during ophthalmic examination and missing or unavailable data (n = 4). Thus, a total of 49 individuals were included in the final analysis (Figure 1). The mean interval between PET-MRI scan and ophthalmic examination was 77 days (range, 12-178 days).
To investigate the structural changes in the retina related to in vivo AD pathologic characteristics, including cerebral Aβ deposition and AD-ND, the following factors were assessed: (1) the association between the thickness of the macula and both AD biomarkers (Aβ positivity and AD-CT), (2) the association between changes in the specific retinal layer (in particular, thickness of the ganglion cell complex–inner plexiform layer [GCIPL] and peripapillary retinal nerve fiber layer [RNFL]),32,33 and (3) whether the retinal structural parameters were associated with AD biomarkers. Subregional analyses were conducted to identify region-specific associations.
Macular thickness was measured using swept-source optical coherence tomography (SS-OCT) (DRI-OCT-1 Atlantis; Topcon) as described in the eMethods in the Supplement. Retinal thickness map was overlapped with a 6 × 6-mm grid of the Early Treatment Diabetic Retinopathy Study (ETDRS) to assess the different mean values of each sector: central fovea, 4 parafoveal sectors (nasal, inferior, temporal, and superior), and 4 perifoveal sectors (nasal, inferior, temporal, and superior). After obtaining the macular thickness at each ETDRS area, the average macular thickness in the central fovea, inner ring (parafovea), and outer ring (perifovea) was calculated and used for analysis.
In addition, the GCIPL thickness (GCIPL-T) and RNFL thickness (RNFL-T) were measured using the same SS-OCT and procedure as that used for macular thickness (eMethods in the Supplement). The mean GCIPL-T was calculated based on regional GCIPL-Ts in 6 sectors (superior, superotemporal, temporal, inferotemporal, inferior, inferonasal, and superonasal), and the mean RNFL-T was calculated as the mean RNFL-T measured in 4 sectors (superior, temporal, inferior, and nasal).
The mfERG (RETI-scan; Roland Consult, Stasche & Finger GmbH) was recorded using ERG-Jet electrodes, according to the International Society for Clinical Electrophysiology of Vision guideline for clinical multifocal electroretinography.34 Details on the methods are provided in the eMethods in the Supplement. For data analysis, the hexagonal stimulus fields were grouped by eccentricity and, subsequently, the P1 amplitude and implicit time of rings 1 to 6, the standard measurement for amplitude and timing of mfERG, were analyzed.
Statistical Analysis
Comparisons of demographic and clinical variables between the Aβ+CN and Aβ−CN groups were performed using an independent t test for continuous variables and χ2 test for categorical variables. With regard to Aβ deposition, analysis of covariance was performed to compare retinal structural parameters between these 2 groups after controlling for covariates including age, sex, apolipoprotein E e4 (APOE4) positivity, and best-corrected visual acuity. All P values were 2-sided and were not corrected for multiple analyses. Analysis of covariance was also performed to compare differences in the retinal functional parameters of rings 1 to 6. In terms of AD-ND, partial correlation analyses between retinal parameters and AD-CT were performed after adjusting the covariates, with subsequent subregional analyses. In addition, multivariate logistic regression analyses were performed to model a screening tool for detection of preclinical AD based on significant findings from the aforementioned analyses. The outcome variable was Aβ positivity, as defined above, indicating whether CN older adults have preclinical AD. Three variables (ie, regional macular thickness, regional GCIPL-T or RNFL-T, and functional parameters measured by mfERG) that showed the most significant P value in each analysis between the Aβ+CN and Aβ−CN groups were selected and entered into the logistic regression analyses, with the abovementioned covariates as fixed variables. The final model was selected using the backward likelihood ratio method. Details for the receiver operating characteristic curve analyses are provided in the eMethods in the Supplement. All statistical analyses were performed using SPSS, version 25 (IBM Corp) and MedCalc for Windows, version 19.2.0 (MedCalc Software).
Results
Of total 49 participants included in the study, 16 individuals were Aβ+CN and 33 individuals were Aβ−CN (Table). The population comprised 25 women (51.0%) and 24 men (49.0%); mean (SD) age was 70.6 (9.4) years. In the comparison of macular thickness, macular thickness in the inner ring was reduced in the Aβ+CN compared with the Aβ−CN cohort, after controlling for covariates (adjusted mean difference [ADM], 11.76 μm; 95% CI, 1.82-21.69 μm; P = .02) (eTable 1 in the Supplement); macular thickness in the central subfield and outer ring was similar between the 2 groups. Subsequent subregional analyses showed that macular thickness of the Aβ+CN cohort was reduced, particularly in the inner nasal subfield of macula, compared with that of the Aβ−CN individuals (mean [SD], Aβ+CN, 286.1 [22.5] vs Aβ−CN, 308.9 [18.4] μm; ADM, 15.88 μm; 95% CI, 4.59-27.16 μm; P = .007) (Figure 3A; eTable 2 in the Supplement).
Table. Demographic and Clinical Characteristics of Participants.
| Characteristic | No. (%) | |
|---|---|---|
| Aβ−CN (n = 33) | Aβ+CN (n = 16) | |
| Age, mean (SD), y | 68.4 (9.7) | 75.2 (7.1) |
| Sex | ||
| Women | 15 (45.5) | 10 (62.5) |
| Men | 18 (54.5) | 6 (37.5) |
| Educational level, mean (SD), y | 12.4 (5.1) | 10.8 (4.1) |
| APOE4 carrier status | 9 (27.3) | 7 (43.8) |
| Hypertension | 11 (33.3) | 7 (43.8) |
| Diabetes | 2 (6.1) | 3 (18.8) |
| Coronary heart disease | 2 (6.1) | 1 (6.3) |
| Hyperlipidemia | 11 (33.3) | 3 (18.8) |
| Best-corrected visual acuity, mean (SD), logMAR | −0.01 (0.09) | 0.07 (0.12) |
| Cerebral Aβ deposition, mean (SD), SUVR | 1.11 (0.05) | 1.89 (0.36) |
| AD-signature cortical thickness, mean (SD), mm | 2.49 (0.16) | 2.47 (0.14) |
Abbreviations: Aβ, amyloid-β; AD, Alzheimer disease; APOE4, apolipoprotein E e4 allele; CN, cognitively normal; SUVR, standardized uptake value ratio.
Figure 3. Comparison of Structural and Functional Parameters of Retina Measured by Swept-Source Optical Coherence Tomography and Multifocal Electroretinography Between Cognitively Normal (CN) Individuals With and Without Amyloid-β Deposition.
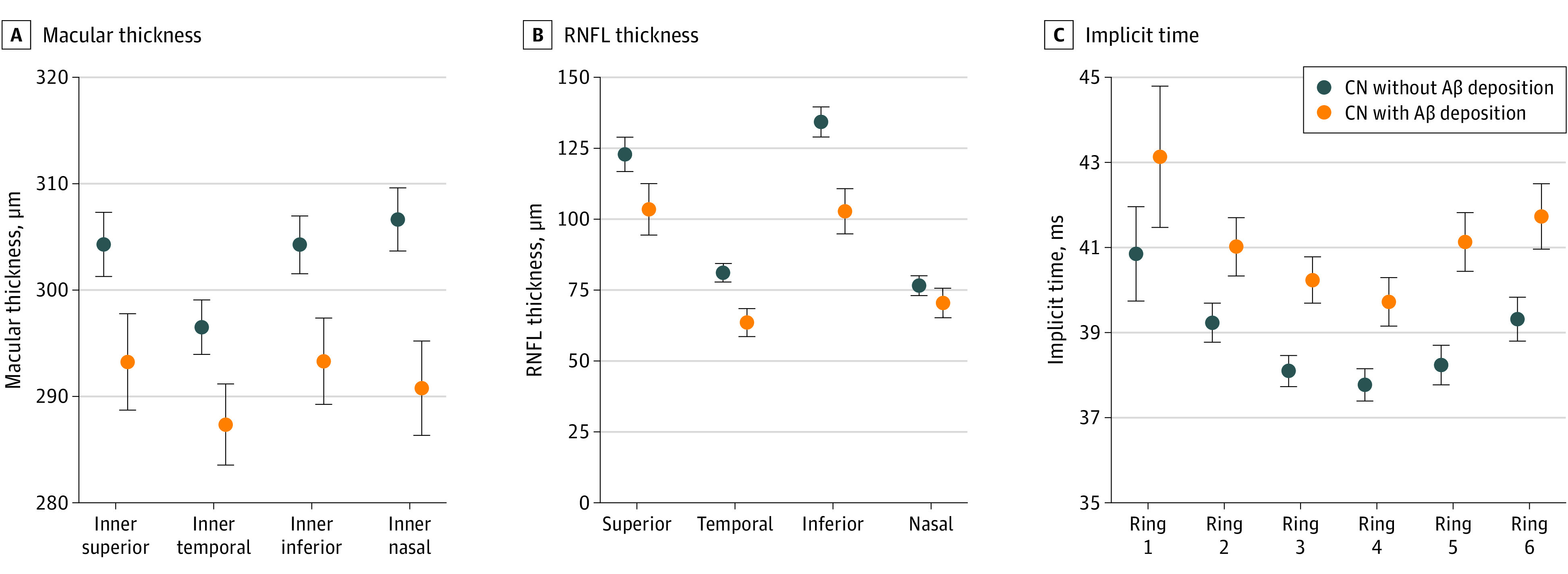
A, Macular thickness of the area in the inner ring of macula. B, Subregional comparison of the thickness of the retinal nerve fiber layer (RNFL) quadrant. C, Implicit time from ring 1 to 6. Dot and error bar indicates adjusted mean and SEM obtained from analysis of covariance after adjusting covariates (age, sex, APOE4, and best-corrected visual acuity).
In the comparison of retinal layer thickness between the 2 groups (eTable 3 in the Supplement), the mean RNFL-T was decreased in the Aβ+CN group compared with that in the Aβ−CN group (ADM, 20 μm; 95% CI, 5.96-34.05 μm; P = .006), while the mean GCIPL-T was similar between the 2 groups. In the subregional analyses of RNFL-T (Figure 3B; eTable 4 in the Supplement), the Aβ+CN group showed lower RNFL-T in the superior, temporal, and inferior quadrants compared with the Aβ−CN group. The most prominent difference was observed in the RNFL-T of the inferior quadrant (mean [SD], Aβ+CN, 103.8 [43.5] vs Aβ−CN, 133.8 [17.9] μm; ADM = 31.49 μm; 95% CI, 11.18-51.8 μm; P = .003).
With regard to the functional parameters of the retina, the amplitude of rings 1 to 6 was similar between the 2 groups (eTable 5 in the Supplement), whereas the Aβ+CN participants had higher implicit time in rings 2 to 6 compared with the Aβ−CN participants. The group difference for ring 5 showed the smallest P value (mean [SD], Aβ+CN, 41.3 [4.0] vs Aβ−CN, 38.2 [1.3] milliseconds; ADM = 2.89 milliseconds; 95% CI, 1.13-4.66 milliseconds; P = .002) (Figure 3C).
In partial correlation analyses between structural retinal parameters and AD-CT with adjustment for covariates, the macular thickness in the central subfield, inner, and outer rings was not correlated with AD-CT (eTable 6 in the Supplement). The mean GCIPL-T was correlated with AD-CT (r = 0.41; P = .005), whereas the mean RNFL-T was not correlated with AD-CT (r = −0.02; P = .89) (Figure 4). In subregional analyses, correlation between AD-CT and GCIPL-T for the superior (r = 0.40; P = .006) and superonasal (r = 0.41; P = .005) subfields were relatively more prominent compared with other subfields (eFigure 2 in the Supplement). This pattern of associations did not change after added adjustment for cerebral Aβ deposition. In contrast to the association between implicit time and Aβ deposition, no such association between implicit time and AD-CT was observed in partial correlation analyses after adjustment of covariates (eTable 7 in the Supplement).
Figure 4. Association of Retinal Structural Parameters by Swept-Source Optical Coherence Tomography With Alzheimer Disease (AD) Signature Cortical Thickness (AD-CT).
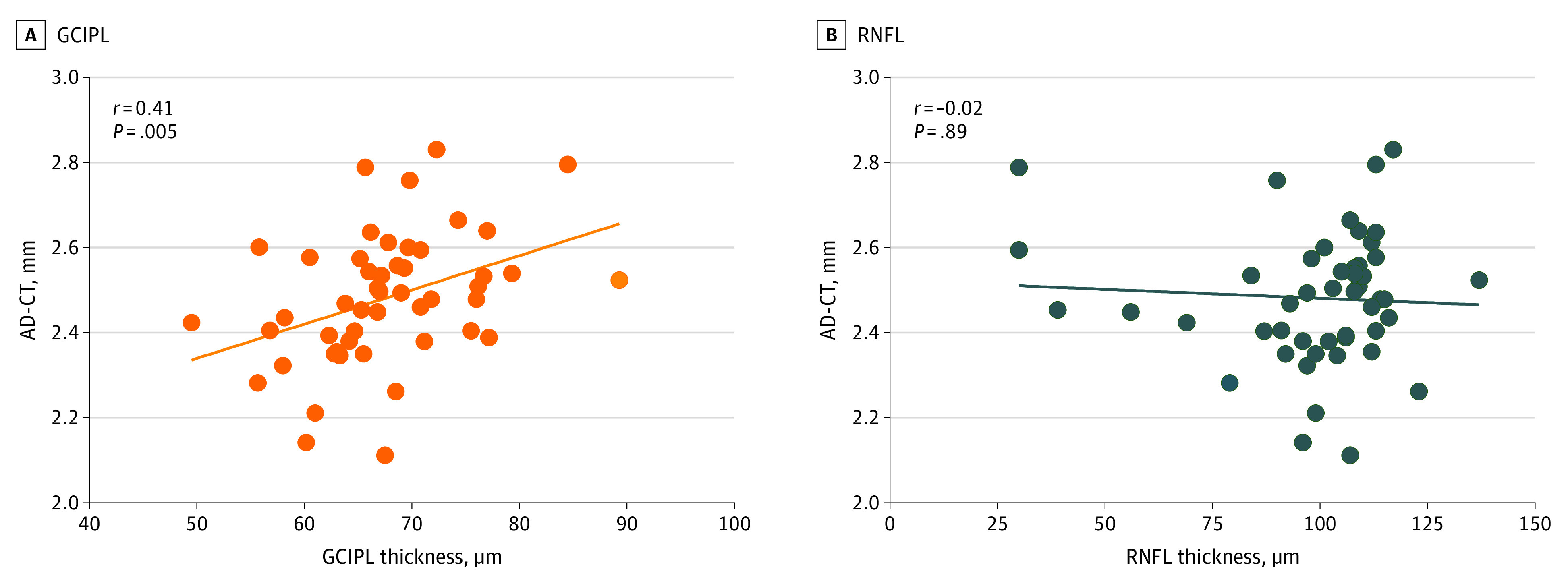
The mean ganglion cell-inner plexiform layer (GCIPL) thickness was associated with AD-CT (A), but the mean retinal nerve fiber layer (RNFL) thickness was not associated with AD-CT (B).
Based on the abovementioned results, 3 variables with the smallest P value in each analysis between the Aβ+CN and Aβ−CN groups—inner nasal subfield of macula, inferior quadrant RNFL-T, and the implicit time of ring 5—were entered into the logistic regression analysis, and covariates were entered as fixed variables. A final model including inner nasal subfield of macula and inferior quadrant RNFL-T was selected (χ2 = 26.636; P < .001; −2 log-likelihood = 35.28). In receiver operating characteristic curve analyses (eFigure 3 in the Supplement), compared with the reference model derived from the logistic regression analysis with only fixed variables (area under the curve, 0.77; 95% CI, 0.63-0.92; sensitivity 75.00%, specificity 72.73%), a final model showed higher diagnostic accuracy (area under the curve, 0.90; 95% CI, 0.81-0.99), with sensitivity 93.75% and specificity 78.79% at cutoff based on the Youden index (difference between areas, 0.13; 95% CI, 0.02-0.24; P = .03).
Discussion
Our cross-sectional study revealed characteristic change of the structure and function of the retina in the Aβ+CN. In addition, the findings showed patterns of association between each AD pathologic characteristic and measurement values of the retina in CN older adults, indicating that cerebral Aβ deposition and AD-ND may have differential association with specific changes of the retina.
We observed a decrease in macular thickness at the inner ring of the ETDRS area, particularly in the inner nasal part of the ETDRS area, in the Aβ+CN cohort compared with in the Aβ−CN cohort. A previous study reported reduction of macular thickness particularly at the inner segments, which plays an important role in central visual function, in patients with AD dementia compared with controls.35 The collective results of that study and the current study highlight that Aβ pathologic factors may preferentially affect the inner ring of macula from the preclinical stage of AD.
Our study revealed that RNFL-T, but not GCIPL-T, was reduced in the Aβ+CN compared with the Aβ−CN participants and the reduction of RNFL-T of the inferior quadrant was particularly prominent compared with other quadrants. Previous OCT studies in patients with clinically defined AD dementia reported reduced RNFL-T in all quadrants,12,13,14,16,17 while a previous study in individuals with mild cognitive impairment reported reduced RNFL-T, particularly in the inferior quadrant, compared with controls.15 The collective findings from this previous study in a mild cognitive impairment group15 and ours in a CN group suggests that the RNFL-T inferior quadrant might be more vulnerable to Aβ deposition and noted changes in early-stage AD compared with other quadrants. Moreover, our study is in agreement with a recent epidemiologic study that reported selective association of the baseline RNFL-T with risk of AD dementia.36 The RNFL is composed of the axons from the retinal ganglion cell bodies that are densely localized in the GCIPL layer, and the axons of the RNFL connect to the postsynaptic neurons in the brain.37 Previous studies have reported that vulnerability of the white matter to the AD process, which begins before onset of cognitive decline,38,39 is associated with brain Aβ deposition.40 Given retinal Aβ deposition in patients with AD dementia and in an AD mouse model,8,9 thinning of the RNFL may be related to early retinal Aβ deposition.
With regard to AD-ND, GCIPL-T was associated with AD-CT, even after controlling for Aβ; however, RNFL-T, macular thickness, and functional retinal parameters were not associated with AD-CT, indicating an Aβ-independent association of changes in GCIPL-T with AD-CT. Given that GCIPL, which is the layer where retinal ganglion cell bodies exist, corresponds to the neuronal cell bodies in the brain, we can postulate that neuronal cell bodies in the retina, as well as those in the gray matter of AD-signature cortices, might be commonly vulnerable to non–Aβ-related neuropathologic changes that can occur in CN older adults.23,41 In addition, considering that GCIPL thinning in the inferior and inferotemporal subfields is a potential early indicator of glaucoma,42,43 positive association of AD-CT with GCIPL-T only at the superior and superonasal subfields suggests that a different pathogenic mechanism underlies AD and glaucoma, although further studies are required to elucidate underlying mechanism.
In this study, retinal electrophysiologic parameters measured by mfERG, particularly the implicit time, but not the amplitude, were different in the Aβ+CN vs the Aβ−CN participants, suggesting that not only retinal structure, but also retinal function, undergo changes from the asymptomatic stage of AD. Implicit time, but not amplitude, is known to be a more sensitive parameter to evaluate injury in degenerative diseases of the receptors44 and is more susceptible than amplitude in various diseases, including retinitis pigmentosa, macular dystrophy, and diabetes.45,46,47,48 Thus, our finding suggests that delayed implicit time is a potential sensitive indicator of early retinal neuronal change related to cerebral Aβ deposition.
Limitations
To our knowledge, this study is the first to investigate the association of in vivo AD pathologic abnormalities with functional changes of the retina as measured by electroretinography, as well as structural changes using SS-OCT in CN older adults. Nevertheless, our study has limitations. First, it is not possible to determine the temporal consequences and causal association between retinal alterations and in vivo AD pathologic abnormalities using a cross-sectional design and relatively small sample size. Second, because of rather cumbersome aspects of mfERG, wide use of mfERG in clinical practice might be limited. Third, given the use of the backward likelihood ratio method and the relatively small sample size, as well as the fact that only individuals without any eye disease were included in our study, our findings cannot be applied to the general population.
Conclusions
This cross-sectional study found that CN older adults with cerebral Aβ deposition had not only structural but also functional changes of the retina. The specific patterns of retinal changes may indicate the presence of different types of AD pathologic abnormalities: in vivo cerebral Aβ deposition and AD-ND. Our findings suggest that retinal biomarkers may be used as a screening tool for early detection of AD, although further evidence to validate the findings are needed.
eMethods. Detailed Methods
eTable 1. Comparison of Macular Thickness of Retina Between the Aβ–CN and Aβ+CN Groups
eTable 2. Subregional Comparison of the Thickness of the Inner Ring of the Macula Between the Aβ–CN and Aβ+CN Groups
eTable 3. Comparison of Retinal Layer Thickness Between the Aβ–CN and Aβ+CN Groups
eTable 4. Subregional Comparison of the Thickness of the RNFL Quadrants Between the Aβ–CN and Aβ+CN Groups
eTable 5. Comparison of Functional Parameters of Retina Measured by mfERG Between the Aβ–CN and Aβ+CN Groups
eTable 6. Partial Correlation Analysis Between Regional Macular Thickness and AD-CT in CN Individuals
eTable 7. Partial Correlation Analysis Between Functional Parameters of Retina Measured by mfERG and AD-CT in CN Individuals
eFigure 1. Histogram of Global [11C] PiB Retention (SUVR), a Continuous Variable, Indicating Global Aβ Deposition in All CN Older Adults in This Study
eFigure 2. Subregional Analyses of the Correlations Between Alzheimer Disease-Signature Cortical Thickness (AD-CT) and Ganglion Cell-Inner Plexiform Layer Thickness (GCIPL-T)
eFigure 3. Receiver Operating Characteristic (ROC) Curve Analysis of the Model to Detect CN Older Adults With Preclinical AD Using Retinal Imaging Biomarkers
eReferences
References
- 1.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parnetti L, Chipi E, Salvadori N, D’Andrea K, Eusebi P. Prevalence and risk of progression of preclinical Alzheimer’s disease stages: a systematic review and meta-analysis. Alzheimers Res Ther. 2019;11(1):7. doi: 10.1186/s13195-018-0459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikram MK, Cheung CY, Wong TY, Chen CP. Retinal pathology as biomarker for cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2012;83(9):917-922. doi: 10.1136/jnnp-2011-301628 [DOI] [PubMed] [Google Scholar]
- 5.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44-53. doi: 10.1038/nrneurol.2012.227 [DOI] [PubMed] [Google Scholar]
- 6.Byun MS, Yi D, Lee JH, et al. ; KBASE Research Group . Korean Brain Aging Study for the Early Diagnosis And Prediction of Alzheimer’s Disease: methodology and baseline sample characteristics. Psychiatry Investig. 2017;14(6):851-863. doi: 10.4306/pi.2017.14.6.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao H, Zhu Z, Peng Y. Potential utility of retinal imaging for Alzheimer’s disease: a review. Front Aging Neurosci. 2018;10:188. doi: 10.3389/fnagi.2018.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2(16):93621. doi: 10.1172/jci.insight.93621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(suppl 1):S204-S217. doi: 10.1016/j.neuroimage.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501(2):364-372. doi: 10.1016/0006-8993(89)90653-7 [DOI] [PubMed] [Google Scholar]
- 11.Chan VTT, Sun Z, Tang S, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126(4):497-510. doi: 10.1016/j.ophtha.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppola G, Di Renzo A, Ziccardi L, et al. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS One. 2015;10(8):e0134750. doi: 10.1371/journal.pone.0134750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285-2289. doi: 10.1167/iovs.06-1029 [DOI] [PubMed] [Google Scholar]
- 14.Iseri PK, Altinaş O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26(1):18-24. doi: 10.1097/01.wno.0000204645.56873.26 [DOI] [PubMed] [Google Scholar]
- 15.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113(7):523-526. doi: 10.1016/j.clineuro.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 16.He XF, Liu YT, Peng C, Zhang F, Zhuang S, Zhang JS. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer’s disease: a meta-analysis. Int J Ophthalmol. 2012;5(3):401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112(10):1860-1867. doi: 10.1016/S1388-2457(01)00620-4 [DOI] [PubMed] [Google Scholar]
- 18.Frost S, Kanagasingam Y, Sohrabi H, et al. ; AIBL Research Group . Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. 2013;3:e233. doi: 10.1038/tp.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 2018;136(11):1242-1248. doi: 10.1001/jamaophthalmol.2018.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement (Amst). 2018;10:196-209. doi: 10.1016/j.dadm.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder PJ, Johnson LN, Lim YY, et al. Nonvascular retinal imaging markers of preclinical Alzheimer’s disease. Alzheimers Dement (Amst). 2016;4:169-178. doi: 10.1016/j.dadm.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golzan SM, Goozee K, Georgevsky D, et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):13. doi: 10.1186/s13195-017-0239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997-1005. doi: 10.1016/S1474-4422(14)70194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racine AM, Brickhouse M, Wolk DA, Dickerson BC; Alzheimer’s Disease Neuroimaging Initiative . The personalized Alzheimer’s disease cortical thickness index predicts likely pathology and clinical progression in mild cognitive impairment. Alzheimers Dement (Amst). 2018;10:301-310. doi: 10.1016/j.dadm.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth M, Villeneuve S, Haase CM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70(12):1512-1519. doi: 10.1001/jamaneurol.2013.4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byun MS, Kim HJ, Yi D, et al. ; KBASE Research Group . Differential effects of blood insulin and HbA1c on cerebral amyloid burden and neurodegeneration in nondiabetic cognitively normal older adults. Neurobiol Aging. 2017;59:15-21. doi: 10.1016/j.neurobiolaging.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Fantoni E, Collij L, Lopes Alves I, Buckley C, Farrar G; AMYPAD consortium . The spatial-temporal ordering of amyloid pathology and opportunities for PET imaging. J Nucl Med. 2020;61(2):166-171. doi: 10.2967/jnumed.119.235879 [DOI] [PubMed] [Google Scholar]
- 28.Choe YM, Sohn BK, Choi HJ, et al. Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol Aging. 2014;35(7):1519-1525. doi: 10.1016/j.neurobiolaging.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 29.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(16):6820-6825. doi: 10.1073/pnas.0900345106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not β-amyloid in cognitively normal older individuals. J Neurosci. 2013;33(13):5553-5563. doi: 10.1523/JNEUROSCI.4409-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 32.Lee EK, Yu HG. Ganglion cell-inner plexiform layer thickness after epiretinal membrane surgery: a spectral-domain optical coherence tomography study. Ophthalmology. 2014;121(8):1579-1587. doi: 10.1016/j.ophtha.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 33.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010;51(1):217-222. doi: 10.1167/iovs.09-3468 [DOI] [PubMed] [Google Scholar]
- 34.Hood DC, Bach M, Brigell M, et al. ISCEV guidelines for clinical multifocal electroretinography (2007 edition). Doc Ophthalmol. 2008;116(1):1-11. doi: 10.1007/s10633-007-9089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha LP, Lopes LC, Costa-Cunha LV, et al. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS One. 2016;11(4):e0153830. doi: 10.1371/journal.pone.0153830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutlu U, Colijn JM, Ikram MA, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 2018;75(10):1256-1263. doi: 10.1001/jamaneurol.2018.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko F, Muthy ZA, Gallacher J, et al. ; UK Biobank Eye & Vision Consortium . Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol. 2018;75(10):1198-1205. doi: 10.1001/jamaneurol.2018.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araque Caballero MA, Suárez-Calvet M, Duering M, et al. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease. Brain. 2018;141(10):3065-3080. doi: 10.1093/brain/awy229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer’s disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822(3):416-422. doi: 10.1016/j.bbadis.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao LL, Decarli C, Kriger S, et al. Associations between white matter hyperintensities and β amyloid on integrity of projection, association, and limbic fiber tracts measured with diffusion tensor MRI. PLoS One. 2013;8(6):e65175. doi: 10.1371/journal.pone.0065175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dani M, Brooks DJ, Edison P. Suspected non-Alzheimer’s pathology—is it non-Alzheimer’s or non-amyloid? Ageing Res Rev. 2017;36:20-31. doi: 10.1016/j.arr.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 42.Choi JA, Shin HY, Park HL, Park CK. The pattern of retinal nerve fiber layer and macular ganglion cell-inner plexiform layer thickness changes in glaucoma. J Ophthalmol. 2017;2017:6078365. doi: 10.1155/2017/6078365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong ZM, Wollstein G, Schuman JS. Clinical utility of optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):OCT556-OCT567. doi: 10.1167/iovs.16-19933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19(5):607-646. doi: 10.1016/S1350-9462(00)00013-6 [DOI] [PubMed] [Google Scholar]
- 45.Park JY, Kim SH, Park TK, Ohn YH. Multifocal electroretinogram findings after intravitreal bevacizumab injection in choroidal neovascularization of age-related macular degeneration. Korean J Ophthalmol. 2011;25(3):161-165. doi: 10.3341/kjo.2011.25.3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneck ME, Bearse MA Jr, Han Y, Barez S, Jacobsen C, Adams AJ. Comparison of mfERG waveform components and implicit time measurement techniques for detecting functional change in early diabetic eye disease. Doc Ophthalmol. 2004;108(3):223-230. doi: 10.1007/s10633-004-8745-z [DOI] [PubMed] [Google Scholar]
- 47.Seeliger M, Kretschmann U, Apfelstedt-Sylla E, Rüther K, Zrenner E. Multifocal electroretinography in retinitis pigmentosa. Am J Ophthalmol. 1998;125(2):214-226. doi: 10.1016/S0002-9394(99)80094-4 [DOI] [PubMed] [Google Scholar]
- 48.Miyake Y, Horiguchi M, Tomita N, et al. Occult macular dystrophy. Am J Ophthalmol. 1996;122(5):644-653. doi: 10.1016/S0002-9394(14)70482-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eTable 1. Comparison of Macular Thickness of Retina Between the Aβ–CN and Aβ+CN Groups
eTable 2. Subregional Comparison of the Thickness of the Inner Ring of the Macula Between the Aβ–CN and Aβ+CN Groups
eTable 3. Comparison of Retinal Layer Thickness Between the Aβ–CN and Aβ+CN Groups
eTable 4. Subregional Comparison of the Thickness of the RNFL Quadrants Between the Aβ–CN and Aβ+CN Groups
eTable 5. Comparison of Functional Parameters of Retina Measured by mfERG Between the Aβ–CN and Aβ+CN Groups
eTable 6. Partial Correlation Analysis Between Regional Macular Thickness and AD-CT in CN Individuals
eTable 7. Partial Correlation Analysis Between Functional Parameters of Retina Measured by mfERG and AD-CT in CN Individuals
eFigure 1. Histogram of Global [11C] PiB Retention (SUVR), a Continuous Variable, Indicating Global Aβ Deposition in All CN Older Adults in This Study
eFigure 2. Subregional Analyses of the Correlations Between Alzheimer Disease-Signature Cortical Thickness (AD-CT) and Ganglion Cell-Inner Plexiform Layer Thickness (GCIPL-T)
eFigure 3. Receiver Operating Characteristic (ROC) Curve Analysis of the Model to Detect CN Older Adults With Preclinical AD Using Retinal Imaging Biomarkers
eReferences