Abstract
Objective
We conducted this study to investigate whether the COVID‐19 pandemic impacted the rate of DKA and previously identified risk factors in children presenting with T1D.
Methods
We performed an extension of a retrospective analysis of all paediatric patients (age ≤ 18) newly diagnosed with T1D within a tertiary care referral centre between 01/01/2017 and 09/14/2020. Demographics, insurance coverage and clinical documents 30 days before their T1D diagnosis were abstracted to assess for symptoms at diagnosis, laboratory values (blood glucose, HbA1c, venous pH and bicarbonate) and any healthcare encounters within 30 days of their diagnosis of T1D.
Results
412 patients with T1D [171 F:241 M; 370 pre‐COVID era:42 post‐COVID era] were included. The percentages of DKA diagnoses at admission were very similar between the pre‐COVID and post‐COVID groups (47% vs. 48%), as were the severity (13% vs. 14% mild DKA; 33% vs. 31% moderate or severe DKA).
Conclusion
There were no fluctuations in the rate of DKA among paediatric patients newly diagnosed with T1D throughout the coronavirus pandemic in central Pennsylvania.
Keywords: type 1 diabetes, diabetic ketoacidosis, COVID‐19
We conducted this study to investigate whether the COVID‐19 pandemic impacted the rate of DKA and previously identified risk factors in children presenting with T1D. We performed an extension of a retrospective analysis of all paediatric patients (age ≤ 18) newly diagnosed with type 1 diabetes within a tertiary care referral centre between 01/01/2017 and 09/14/2020. There were no fluctuations in the rate of DKA among paediatric patients newly diagnosed with T1D throughout the coronavirus pandemic in central Pennsylvania.

Abbreviations
- T1D
type 1 diabetes
- DKA
diabetic ketoacidosis
1. INTRODUCTION
The majority of patients with Type 1 Diabetes Mellitus (T1D) present with similar symptoms including polyuria, polydipsia, weight loss and fatigue. Unfortunately, some patients progress to diabetic ketoacidosis (DKA) at the time of diagnosis of T1D 1 . Children diagnosed in DKA have increased risk of morbidity, mortality, poor glycaemic control, higher medical costs and healthcare resource utilization including ICU level care 1 , 2 . Previous research has identified the predictors for children to present in DKA, which include younger children (under 5 years of age), Hispanic or African American race, low socioeconomic status, misdiagnosis at an initial clinical encounter and lack of private health insurance 3 . Thus far, there are limited data on the impact of the COVID‐19 pandemic on the rate and severity of DKA in children at initial diagnosis of T1D. Interestingly, some geographic locations noted an increase in DKA frequency during the COVID‐19 pandemic, while others reported no change 4 , 5 . We conducted this study to investigate whether the COVID‐19 pandemic impacted the rate of DKA and previously identified risk factors in children presenting with T1D in a single tertiary care referral centre in central Pennsylvania.
2. METHODS
We performed an extension of a retrospective analysis of all paediatric patients (age ≤ 18) newly diagnosed with T1D within a tertiary care referral centre between 01/01/2017 and 09/14/2020. Demographics, insurance coverage, and all clinical documents 30 days before their T1D diagnosis were abstracted to assess for symptoms at diagnosis (polyuria, polydipsia, nocturia, weight loss, nausea, vomiting, altered mental status, infection, vision changes and autism spectrum disorder), laboratory values (blood glucose, HbA1c, venous pH and bicarbonate) and any healthcare encounters within 30 days of their diagnosis of T1D. We performed descriptive statistics and univariate analyses [evaluating children diagnosed with T1D during the pre‐COVID‐19 era (diagnosed between 1/1/2017 and 2/28/2020) and post‐COVID‐19 era (diagnosed between 03/01/2020‐ and 09/14/2020) associated with the incidence of DKA], followed by logistic regression analysis (incorporating key clinical factors previously associated with DKA and the pre‐ or post‐ COVID‐19 era classification). The pH at diagnosis was used to classify DKA as mild DKA (7.25‐<7.30) or moderate/severe DKA (<7.25).
3. RESULTS
The analysis included 412 paediatric patients with T1D [171 F:241 M; 370 pre‐COVID‐19 era:42 post‐COVID‐19 era] (TABLE 1). In children diagnosed with T1D in the post‐COVID era, the peak occurrences of T1D were in ages 5‐13 (Interquartile Range [25%‐75%]), males (23, 54.8%), white race (33, 78.6%) and children with Medicaid (16, 38.1%) or military/government (13, 31%) insurance. 8 (19%) children had private insurance and 5 (11.9%) were uninsured (TABLE 1). The rate of DKA was similar between the pre‐COVID‐19 and post‐COVID‐19 groups (47% vs 48%), as was DKA severity (13% vs. 14% mild DKA; 33% vs. 31% moderate or severe DKA). There were no temporal associations with the rate of DKA in respect to COVID‐19 (FIGURE 1); however, age (0‐3 and 9‐13 years), misdiagnosis during a preceding healthcare encounter, presenting to the emergency department directly, elevated HbA1c (>10.0%/13.4mmol/L), and altered mental status (a known symptom of DKA) were associated with increased risk of DKA on multivariable analysis (TABLE 2).
TABLE 1.
Data summary of the 412 patients [370 Pre‐COVID and 42 Post‐COVID] included in this study
| Pre‐COVID (N = 370) | Post‐COVID (N = 42) | |
|---|---|---|
| Age | ||
| Mean (Standard deviation) | 10.0 (4.29) | 9.2 (4.55) |
| Median | 11.0 | 9.0 |
| Interquartile range (25th, 75th percentile) | 7.0, 13.0 | 5.0, 13.0 |
| Range | (0.0‐18.0) | (1.5‐17.0) |
| Sex | ||
| Female | 152 (41.1%) | 19 (45.2%) |
| Male | 218 (58.9%) | 23 (54.8%) |
| Race | ||
| White | 261 (70.5%) | 33 (78.6%) |
| Hispanic or Latino | 41 (11.1%) | 4 (9.5%) |
| Black or African American | 31 (8.4%) | 2 (4.8%) |
| Two or more races | 16 (4.3%) | 0 |
| Asian | 3 (0.8%) | 0 |
| Other | 11 (3.0%) | 3 (7.1%) |
| Missing | 7 | 0 |
| BMI Percentile | ||
| Mean (standard deviation) | 48.9 (34.64) | 54.9 (28.94) |
| Median | 48.2 | 60.8 |
| Interquartile range (25th, 75th percentile) | 15.4, 85.3 | 34.5, 77.1 |
| Range | (0.0‐99.9) | (0.0‐99.9) |
| Insurance | ||
| Medicaid | 137 (37.0%) | 16 (38.1%) |
| Private | 206 (55.7%) | 8 (19.0%) |
| Self‐pay | 21 (5.7%) | 5 (11.9%) |
| Military | 4 (1.1%) | 13 (31.0%) |
| Missing | 2 | 0 |
| Type of Initial Healthcare Encounter | ||
| Primary care provider | 266 (71.9%) | 27 (64.3%) |
| Emergency department | 104 (28.1%) | 15 (35.7%) |
| Outcome of Primary Care Provider Appointment | ||
| Referred to ED for concern of T1D | 216 (58.4%) | 24 (57.1%) |
| Misdiagnosis and discharged without concern of T1D | 50 (13.5%) | 3 (7.1%) |
| No PCP visit (went to ED directly) | 104 (28.1%) | 15 (35.7%) |
| Language | ||
| English | 349 (94.3%) | 39 (92.9%) |
| Other primary language | 20 (5.4%) | 3 (7.1%) |
| Missing | 1 | 0 |
| Family History of Type 1 Diabetes | ||
| First degree relative | 42 (11.4%) | 4 (9.5%) |
| Second degree relative | 73 (19.7%) | 9 (21.4%) |
| No family history | 229 (61.9%) | 27 (64.3%) |
| Missing | 26 | 2 |
| Blood Glucose | ||
| Mean (standard deviation) | 503.6 (198.41) | 486.8 (217.68) |
| Median | 470.0 | 455.0 |
| Interquartile range (25th, 75th percentile) | 378.0, 596.0 | 358.0, 581.5 |
| Range | (176.0‐1500.0) | (86.0‐1172.0) |
| Initial A1c | ||
| Mean (standard deviation) | 12.0 (2.38) | 12.2 (2.47) |
| Median | 11.7 | 12.7 |
| Interquartile range (25th, 75th percentile) | 10.3, 13.9 | 11.0, 14.0 |
| Range | (6.2‐18.6) | (5.8‐16.5) |
| Altered Mental Status | ||
| No | 349 (94.3%) | 40 (95.2%) |
| Yes | 20 (5.4%) | 2 (4.8%) |
| Missing | 1 | 0 |
| Autism | ||
| No | 343 (92.7%) | 39 (92.9%) |
| Yes | 26 (7.0%) | 3 (7.1%) |
| Missing | 1 | 0 |
| DKA at Diagnosis of T1D | ||
| No | 198 (53.5%) | 22 (52.4%) |
| Yes | 172 (46.5%) | 20 (47.6%) |
| Severity of DKA at Diagnosis of T1D | ||
| No DKA | 198 (53.5%) | 22 (52.4%) |
| Mild DKA | 49 (13.2%) | 6 (14.3%) |
| Moderate or severe DKA | 123 (33.2%) | 13 (31.0%) |
| Missing | 0 | 1 |
Abbreviations: ED, emergency department; PCP, primary care provider.
FIGURE 1.
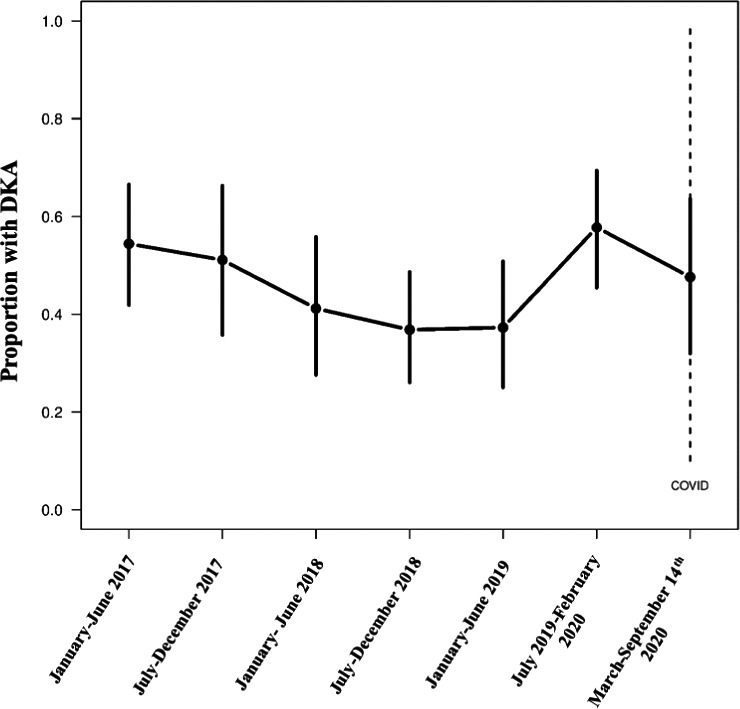
Time series of the proportion of patients with a DKA diagnosis at the time of admission by approximately 6‐month periods from 1 January 2017 to 14 September 2020. The vertical lines for each period represent the 95% confidence interval for the proportion
TABLE 2.
Multivariable logistic regression model of 385 patients in respect to DKA at time of diagnosis of T1D
| Parameter | OR (95% CI) | p‐value |
|---|---|---|
| Age | ||
| 0‐3 | 4.16 (1.64‐10.6) | 0.003 |
| 4‐8 | 1.26 (0.64‐2.48) | 0.50 |
| 9‐13 | 2.21 (1.22‐4.00) | 0.009 |
| 14‐18 (ref) | 1 | |
| Sex | ||
| Male | 1.26 (0.78‐2.01) | 0.34 |
| Female (ref) | 1 | |
| Race | ||
| White | 1.70 (0.97‐2.99) | 0.07 |
| Other (ref) | 1 | |
| BMI percentile | ||
| <15th percentile | 1.11 (0.57‐2.17) | 0.75 |
| 15th to 85th percentile | 0.69 (0.39‐1.23) | 0.21 |
| >85th percentile (ref) | 1 | |
| Insurance | ||
| Private (ref) | 1 | |
| Medicaid | 1.21 (0.70‐2.07) | 0.50 |
| Other | 1.00 (0.45‐2.25) | 0.99 |
| Outcome of PCP healthcare encounter | ||
| Referred to ED for concern of T1D (ref) | 1 | |
| Misdiagnosis and discharged without concern of T1D | 3.73 (1.72‐8.11) | 0.001 |
| No PCP Visit (went to ED directly) | 1.76 (1.04‐2.96) | 0.034 |
| A1c | ||
| ≤10% (13.4mmol/L) (ref) | 1 | |
| >10% (13.4mmol/L) | 5.81 (2.96‐11.4) | <0.001 |
| Altered mental status | ||
| Yes | 9.73 (1.21‐78.0) | 0.032 |
| No (ref) | 1 | |
| Autism | ||
| Yes | 0.94 (0.3‐2.34) | 0.90 |
| No (ref) | 1 | |
| COVID era | ||
| Pre‐COVID (ref) | 1 | |
| Post‐COVID | 0.94 (0.40‐2.21) | 0.89 |
A total of 27 patients (6.6%) had a missing value for at least one of the variables included in the model. These patients were excluded when fitting the model. Odds ratios (ORs) and corresponding 95% CIs for parameters in the model are shown. Bold font signifies p‐value ≤ 0.05.
Abbreviations: ED, emergency department; PCP, primary care provider.
4. DISCUSSION
Our study was the first assessment of the rate of DKA at diagnosis of T1D within central Pennsylvania throughout the COVID‐19 pandemic. We found similar overall DKA rates and severity throughout the COVID‐19 pandemic to an earlier assessment of newly diagnosed T1D in the paediatric population in the same region 3 . Alternatively, there are reports of increased frequency 5 or severity 4 of DKA throughout the COVID‐19 pandemic in other geographic locations. Regardless, our findings suggest previously described predictors of DKA in the paediatric population persist, even in the setting of the COVID‐19 pandemic 3 . A limitation to this study was the small cohort size in the post‐COVID era. Further investigation of the rate of DKA in other regions of the US during the COVID‐19 pandemic would be of interest, particularly to elucidate any regional differences and their potential causes. The findings of this investigation offer clinicians a simple and cost‐effective means to rapidly risk‐stratify children for development of DKA throughout the COVID‐19 pandemic.
CONFLICT OF INTEREST
No authors report a conflict of interest.
AUTHOR CONTRIBUTIONS
K. T. B., V. U. and K. B. K. performed the research and designed the research study. E. S. analysed the data. K. T. B., V. U. and K. B. K. wrote and edited the final manuscript.
ETHICAL APPROVAL
The study has institutional IRB approval.
Acknowledgements
The authors would like to thank the dedicated staff of the Penn State Hershey Pediatric Diabetes Clinic.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long‐term glycemic control. Diabetes Care. 2017;40(9):1249–1255. [DOI] [PubMed] [Google Scholar]
- 2. Saydah SH, Shrestha SS, Zhang P, Zhou X, Imperatore G. Medical costs among youth younger than 20 years of age with and without diabetic ketoacidosis at the time of diabetes diagnosis. Diabetes Care. 2019;42(12):2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bogale KT, Hale DE, Schaefer E, Bangalore KK. Prevalence and factors associated with diabetic ketoacidosis at diagnosis of type 1 diabetes: A report from a tertiary medical center in Central Pennsylvania. Endocrinol Diabetes Metab. 2020. 10.1002/edm2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Has COVID‐19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870–2872. [DOI] [PubMed] [Google Scholar]
- 5. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324(8):801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


