Abstract
The COVID‐19 confinement has dramatically altered daily routines, causing decreased sleep quality in adults. This necessitates careful observation, as sleep plays a crucial role in brain maturation and poor sleep increases the risk of psychopathology, particularly in the young population. Through an online survey with one baseline (April 2020) and two follow‐up assessments (May and June 2020), we examined the effect of confinement on sleep quality in 452 babies (0–35 months) and 412 preschool children (36–71 months) from several, mainly European, countries. An acute decrease in sleep quality was found in both groups of children. However, at follow‐up assessments, this effect rebounded to the level reported for the period before the confinement. Importantly, caregiver's stress level was identified as a substantial risk factor determining lower sleep quality in both groups of children across assessments. Protective factors conserving children's sleep quality included caregiver's engagement in mindfulness techniques or childcare, and the presence of siblings and pets. In the near future, we may repeatedly experience the circumstances of abruptly enforced confinement. Our findings reveal promising pathways of action to protect young children's sleep, with which to essentially mitigate the long‐term consequences of the pandemic on brain development and mental health.
Keywords: bedtime, circadian rhythm, lockdown, sensitive period, sleep regulation, stay‐at‐home
1. INTRODUCTION
Pandemics and subsequent disease‐confinement responses can cause families and children to experience stressful and traumatic conditions (Sprang & Silman, 2013). Masten and Obradovic state “families often infect each other before any individual is diagnosed, they also infect each other with fear’’ (Masten & Obradovic, 2008). Because confinement is abrupt and causes constraints in a multitude of ways, protecting core health needs is crucial. In response to confinement, there is a growing need for support strategies and recommendations tailored to children's health.
Good sleep is essential to children's health as it fosters neuronal functioning, cognitive performance, memory processes and decision making (Ednick et al., 2009). However, poor sleep behaviours (e.g., short or irregular sleep) are common in adults, despite being a recognized risk factor for major health problems, such as mood and cardiovascular disorders, obesity and diabetes (Chattu et al., 2018). Children in particular suffer health consequences from poor sleep, as short, fragmented or poorly consolidated sleep in childhood predicts later psychosocial and medical problems (Simola et al., 2014; Taveras et al., 2008). Furthermore, poor sleep in early childhood is a newly recognized risk for developing psychopathology (Cook et al., 2020).
The COVID‐19 outbreak has led to drastic confinement measures worldwide, including stay‐at‐home orders, school and daycare closures and working from home. The consequent decrease in adult sleep quality stands in close relationship to subjectively perceived psychological burden and stress (Blume et al., 2020; Cellini et al., 2020). Importantly, independent of the confinement context, stress in parents often co‐occurs with disturbed sleep of their children (Meltzer & Mindell, 2007). Whether this was also the case during the COVID‐19 outbreak in 2020 remains largely unclear. A recent study examined sleep within families in Israel 4 weeks after the first COVID‐19 confinement measures were implemented. Results indicated mild to high levels of maternal anxiety, whereas the majority of mothers perceived no change in the sleep quality and duration of their 6‐ to 72‐month‐old children (Zreik et al., 2021). However, 30% of mothers reported a decrease of their children's sleep quality, while a small number reported a positive change in their child's sleep. On the other hand, Dellagiulia et al. (2020) reported decreased sleep quality of 3‐ to 6‐year‐old children in the early phase of the confinement in Italy. Even though it improved again over a 4‐week period, the overall sleep quality remained nonetheless below initial levels. This observation is concerning and suggests that the extraordinary circumstances of confinement may pose a long‐term threat to young children's sleep. As Lionetti et al. (2020) emphasize in their work, there is considerable variability in available evidence with regards to children's sleep during the confinement, highlighting the need to take into account further individual and environmental factors as well as long‐term dynamics. For example, it remains unknown whether the confinement‐induced secondary effects were intertwined with children's sleep, (e.g., stress in parents that transfers to young children, thereby negatively affecting children's sleep quality).
Crucially, it is unknown whether protective factors exist that mitigate negative consequences. Previous work has shown that factors such as family income and parental education positively affect sleep behaviour during childhood (Newton et al., 2020). Unfortunately, such factors are largely unmodifiable and thus cannot provide immediate solutions to support healthy sleep of young children in challenging circumstances. However, concepts known to alleviate potential risk factors may provide new pathways to indirectly promote healthy sleep behaviour in young children. For example, parental stress can be reduced by applying stress‐relieving strategies such as yoga and physical activity (Smyth et al., 2020). Finally, sleep hygiene recommendations include consistent bedtime routines and sleep schedules, which have proven their potential to improve children's sleep (Werner et al., 2015).
To identify such associations during the COVID‐19 confinement, we longitudinally examined the dynamics of sleep behaviour in young children and the corresponding familial circumstances. In addition to children between 3 and 6 years of age, our sample includes children below 3 years of age, thus extending previous work to the youngest population. We believe that studying this population is of particular importance, as detecting risks as early as possible in development is crucial for successful implementation of protective measures. Based on the first findings in adults (Blume et al., 2020; Cellini et al., 2020) as well as children (Dellagiulia et al., 2020; Zreik et al., 2021), we hypothesized that confinement acutely decreases young children's sleep quality. We expected caregiver's stress to be significantly associated with this decrease, due to the previously recognized link between parental stress and sleep problems in children (Meltzer & Mindell, 2007). In addition, we explored further determinants of the confinement circumstances across repeated assessments throughout the confinement to identify protective factors and gain a comprehensive understanding of this extraordinary situation. Therefore, the aim of this study was to test whether the COVID‐19 confinement induced (1) acute and/or (2) persisting consequences for young children's sleep, as well as to identify environmental determinants of such changes.
2. METHODS
2.1. Study design
Data on 864 young children were collected with an online survey in German, French, Italian, Spanish and English languages (SoSci Survey; Leiner, 2020). Participants were recruited through social media, childcare institutions and medical practices. Institutional ethics committees approved the procedure. Informed consent was obtained from the person completing the survey. During the acute phase of the confinement in April 2020, 781 primary caregivers (M age = 36.2 ± 4.9 years, 738 females) of children from newborns (0 months) to 71 months (i.e., 0–5.9 years) completed the baseline survey. Of those, 175 participated again in the first follow‐up assessment in May 2020, and 149 also participated in a second follow‐up assessment in June 2020. All caregivers with at least one child of up to 6 years of age were included in the study. We only excluded caregivers who reported that their working and childcare arrangements were not affected by the confinement measures at all at the baseline assessment. Caregivers provided the data for each of their children separately by means of a loop in the survey following the same instructions for each child. Specifications of confinement conditions in relation to countries and the corresponding numbers of participants are presented in Table S1. Sixty‐five percent of caregivers were European, 0.3% Asian, 0.3% North African, 0.8% North American, 0.1% Central American, 2% South American, 0.9% Slavic and 0.3% Middle Eastern. Forty‐five percent of caregivers reported having a university degree, 13% a tertiary educational degree, 8% a secondary educational degree and 1% a basic educational degree as their highest level of education.
2.2. Quantification of sleep
Validated sleep instruments were used for the two age groups: babies (0–35 months) were assessed with the Brief Infant Sleep Questionnaire (BISQ) (Sadeh, 2004) and preschool children (36–71 months) were assessed with the Children's Sleep Habits Questionnaire (Owens et al., 2000). The English questionnaires were translated by the authors into German, French, Italian and Spanish. At least two native speakers reached agreement for each translation. For adequate referencing of sleep within the age norms, analyses were performed separately for the two age groups: n = 452 babies aged 0–35 months (M age = 1.5 ± 0.8 years, 233 girls) and n = 412 preschool children aged 36–71 months (M age = 4.5 ± 1 years, 178 girls). For comparability of age groups and to streamline the multi‐dimensionality of sleep patterns, we selected four primary variables: bedtime, latency of sleep, duration of sleep and sleep fragmentation.
Babies’ bedtimes were quantified with an open question to caregivers: When (clock time) does your child usually fall asleep for the night? Caregivers of preschool children were presented with the statement: My child goes to bed at the same time at night. The frequency of its occurrence within the past week was rated on a 5‐point scale: 1 = never (0 days/week), 2 = rarely (1 day/week), 3 = sometimes (2–4 days/week), 4 = usually (5–6 days/week) or 5 = always (7 days/week).
The latency of sleep in babies was assessed with the open question: How long does it take to put your child to sleep in the evening? For preschool children, the statement “My child falls asleep within 20 min after going to bed” was presented with the above‐mentioned 5‐point response scale.
The duration of sleep in babies was quantified with the question: How much time does your child spend in sleep during the NIGHT (between 7 in the evening and 7 in the morning)? In preschool children, the statement “My child sleeps about the same duration each 24‐hr‐day (night‐time sleep and naps combined)” was quantified with the above‐mentioned 5‐point response scale.
In babies, “average number of awakenings per night” was assessed to quantify sleep fragmentation. In preschool children, the statement “How often does your child wake up during the night?’’ was presented with the above‐mentioned 5‐point response scale.
2.3. Risk and protective factors
We characterized the individual extent of confinement in the participating families by examining their social‐contextual situations. The following variables assessed general circumstances, potential risk factors and potential protective factors: child's (exact) age [age], sex (boy/girl/other) [sex], presence of siblings (yes/no) [siblings] or pets (yes/no/sometimes) [pets]; change in caregiver's working arrangements (5‐point scale with 1 = not affected to 5 = affected a lot) [work]; change in caregiver's childcare arrangements (5‐point scale with 1 = not affected to 5 = affected a lot) [childcare arrangements]; time caregiver spent on childcare (in min/day) [childcare]; caregiver's current quarantine status (yes/no/don't know) [quarantine]; caregiver's fear of being infected (5‐point scale with 1 = no fear to 5 = a lot of fear) [fear of infection]; change in caregiver's adherence to isolation recommendations across time (5‐point scale with 1 = decreased a lot to 5 = increased a lot) [adherence to isolation recommendations]; change in caregiver's level of stress (5‐point scale with 1 = decreased a lot to 5 = increased a lot) [stress]; change in caregiver's social interactions (5‐point scale with 1 = decreased a lot to 5 = increased a lot) [social interaction]; and time caregiver spent on mindfulness strategies (in min/day) [mindfulness]. The variables were selected to capture the degree of confinement (i.e., work, childcare arrangements, quarantine, adherence to isolation recommendations, social interaction) and caregiver's well‐being (i.e., fear, stress). In addition, we explored potentially modifiable factors with a positive impact (i.e., siblings, pets, childcare, mindfulness). These factors were selected based on previous investigations of concepts alleviating the negative determinants of children's sleep, such as caregiver's stress (Newton et al., 2020; Smyth et al., 2020).
2.4. Statistical analysis
2.4.1. Part 1: Acute dynamics at baseline assessment
Although this study was not launched until the confinement, we quantified the dynamics of children's sleep at the baseline assessment by addressing both the current situation “since the confinement”, as well as the time “before the confinement” (retrospectively). We will refer to these as “during” and “before” throughout the report. To quantify the acute effect of confinement on sleep, the difference in responses “before”–“during” was calculated for the baseline assessment. In babies, this yielded the change in minutes for bedtimes, latency and duration of sleep and the average change in the number of nocturnal awakenings for sleep fragmentation. In preschool children, this approach revealed positive (becoming more frequent) or negative (becoming less frequent) effects of confinement.
Outliers were excluded whenever exceeding 1.5 interquartile ranges above the upper quartile or below the lower quartile. Wilcoxon signed‐rank tests revealed changes from “before” to “during” the confinement in four sleep variables (bedtime, sleep latency, sleep duration and number of awakenings) for babies and preschool children separately. To test factors affecting changes in sleep variables (“before”–“during”), we applied linear mixed models with fixed factors age, sex, siblings, pets, work, childcare arrangements, childcare, quarantine, fear of infection, adherence to isolation recommendations, stress, social interaction and mindfulness. Participants’ identification number was included as a random effect to account for inter‐individual differences. Missing values (on average 7%) were imputed by means of predictive mean matching (Buuren, 2018) with seven imputations and 20 iterations each as previously recommended for longitudinal questionnaire data (Nooraee et al., 2018). For each sleep variable and for the two age groups, the best fitting model was determined separately, through backward selection based on the Akaike information criterion (AIC). We used the software R and the packages nlme, MASS and mice. All p‐values were corrected for multiple testing (i.e., four sleep variables) by means of the false discovery rate (Benjamini & Hochberg, 1995).
2.4.2. Part 2: Persistent dynamics across follow‐up assessments
The same statistical procedures were applied to longitudinal data across assessments, including baseline, and first and second follow‐up. In follow‐up surveys, only the prevailing situation was assessed, with referrals to the time period “since completing the last survey”. The longitudinal analysis thus included absolute values without the quantification of a temporal change. Time (1 to 3 for the three assessments) was included as an additional factor to examine evolution across the three time‐points of assessment.
3. RESULTS
The two goals of this study were to test whether the COVID‐19 confinement induced (1) acute and/or (2) persisting consequences for young children's sleep. Moreover, we aimed to identify environmental determinants of potential changes in children's sleep behaviour. In the following paragraphs, we present the results for the two analyses examining acute and persistent dynamics separately. Acute dynamics (Part 1) represent the change from before (assessed retrospectively) to during the confinement (baseline assessment). Persisting dynamics (Part 2) reflect longitudinal analyses (across assessments, including baseline, and first and second follow‐up). Furthermore, we grouped the resulting determinants of children's sleep into risk and protective factors, depending on whether they relate to children's sleep negatively or positively. Finally, for simplification, we only provide details regarding the evolution of the examined determinants averaged across all caregivers (i.e., caregivers of babies and caregivers of preschool children pooled together), because the reports on these variables were similar for the two groups.
3.1. Part 1: Acute Dynamics at Baseline Assessment (April 2020)
3.1.1. Children's sleep during the acute phase of the confinement
We observed an acute worsening of sleep in both age groups (Figure 1). Specifically, babies experienced a prolongation of sleep latency (by 8 ± 21 min), a delay of bedtime (by 21 ± 42 min) and a shortening of sleep duration (by 6 ± 53 min) during the confinement in comparison to the time before. Furthermore, we found an increasing trend in the number of nocturnal awakenings (by 0.13 ± 1.25; p =.05). Similarly, preschool children experienced a reduction in consistency of several sleep variables in the acute phase of the confinement: less regular bedtimes (by 0.40 ± 0.85 points), less frequently falling asleep within 20 min (by 0.31 ± 0.87 points), increased day‐to‐day variability of sleep duration (by 0.16 ± 0.69 points) and increased sleep fragmentation (by 0.13 ± 0.72 points).
FIGURE 1.
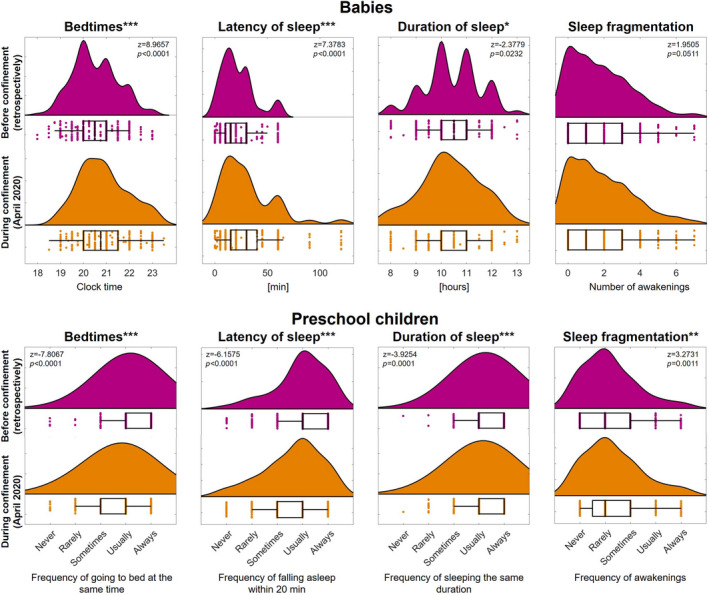
Acute decrease in sleep quality in babies (0–35 months) and preschool children (36–71 months) as a consequence of the COVID‐19 confinement in April 2020. Raincloud plots indicate the distribution of core sleep quality measures (bedtimes, latency of sleep, duration of sleep and sleep fragmentation) before and during the confinement (452 babies and 412 preschool children). Dots represent individual subjects and box plots indicate the first and third quartiles (whisker length 1.5 * interquartile range; vertical lines represent the median). Statistics (z, p) are based on Wilcoxon signed‐rank tests (before versus during the confinement). Data were collected with age‐specific assessments: Brief Infant Sleep Questionnaire (BISQ) for babies and Children's Sleep Habits Questionnaire for preschool children. Consequently, we note the quantification of absolute values for babies and reports of frequency of occurrence for preschool children. The significance levels are indicated as *p < 0.05, **p < 0.01 and ***p < 0.001 after correcting for multiple testing by means of the false discovery rate. This figure was based on Blume et al. (2020) for the purpose of comparability
3.1.2. Risk factors during the acute phase of the confinement
Several dimensions of sleep behaviour in young children were associated with the change in stress level of the primary caregiver. The caregivers reported an increase in stress of 3.55 ± 1.13 points in April (as compared to before the confinement). This increase was associated with later bedtimes, longer sleep latency, shorter sleep duration and increased sleep fragmentation of their babies (Table 1). Similarly, caregiver's increased stress was associated with less regular bedtimes in preschool children. Also, caregivers who were in quarantine (36%) reported longer sleep latency for their babies as compared to caregivers who were not in quarantine. In contrast, no significant acute association of quarantine status was observed with any of the sleep variables in preschool children.
TABLE 1.
Factors acutely associated with sleep of babies (0–35 months) and preschool children (36–71 months) during the COVID‐19 confinement in April 2020
| Babies | Preschool Children | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedtimes (min) | Latency of sleep (min) | Duration of sleep (min) | Number of awakenings | Frequency of going to bed at the same time | Frequency of falling asleep within 20 min | Frequency of sleeping the same duration | Frequency of awakenings | |||||||||
| Factor | b | p | b | p | b | p | b | p | b | p | b | p | b | p | b | p |
| Stress | 4.15 | 0.026 | 3.11 | 0.002 | −4.94 | 0.026 | 0.18 | 0.002 | −0.10 | 0.018 | −0.07 | 0.093 | −0.04 | 0.165 | – | – |
| Quarantine | – | – | 4.97 | 0.037 | −7.32 | 0.192 | 0.17 | 0.192 | – | – | – | – | – | – | – | – |
| Mindfulness | – | – | 0.15 | 0.255 | – | – | – | – | 0.02 | <0.001 | 0.02 | <0.001 | – | – | – | – |
| Siblings | −6.51 | 0.170 | −4.44 | 0.076 | – | – | −0.17 | 0.170 | 0.23 | 0.021 | 0.16 | 0.072 | – | – | 0.13 | 0.072 |
| Childcare | 0.01 | 0.196 | −0.01 | 0.044 | – | – | −0.00 | 0.044 | – | – | 0.00 | 0.017 | – | – | −0.00 | 0.292 |
| Pets | – | – | – | – | – | – | −0.32 | 0.029 | – | – | – | – | 0.28 | <0.001 | – | – |
| Work | −4.20 | 0.026 | – | – | 5.49 | 0.015 | – | – | – | – | – | – | – | – | – | – |
| Age | 13.78 | <0.001 | 3.13 | 0.017 | 7.50 | 0.017 | 0.16 | 0.027 | −0.12 | 0.011 | −0.11 | 0.011 | – | – | – | – |
| Sex | – | – | – | – | 8.22 | 0.339 | – | – | 0.14 | 0.273 | – | – | – | – | 0.10 | 0.273 |
Unstandardized beta coefficients (b) and corrected p‐values (p) from the linear mixed model are shown for the following factors: caregiver's stress, quarantine status, time caregiver spent on mindfulness practices, presence of siblings, time caregiver spent on childcare, presence of pets, change in caregiver's working arrangements, child's age and sex. Missing values indicate that the factor did not survive statistical backward selection. Significant associations (p < 0.05 after correcting for multiple testing by means of the false discovery rate) are presented in bold. Results are based on the baseline assessment and considering the change in sleep variables from before (assessed retrospectively) to during the confinement (April 2020). We note the difference between sleep variables for the two groups of children, with babies’ sleep assessed in absolute terms and preschool children's sleep assessed in terms of frequency according to a 5‐point scale.
3.1.3. Protective factors during the acute phase of the confinement
Several protective factors for young children's sleep were identified. Time caregivers spent on mindfulness strategies had beneficial effects on young children's sleep. Caregivers reported a slight increase in time spent on mindfulness strategies (by 1.76 ± 10.04 min) in April, but also the number of caregivers who performed mindfulness strategies increased from 10% before the confinement to 12% in April. Although there was no significant acute effect of mindfulness time on babies’ sleep, the acute effects of increased caregivers’ mindfulness time on preschool children's sleep were broad and included more regular bedtimes and more frequently reported short sleep latency (Table 1).
The presence of siblings had a positive effect on the quality of sleep. Forty‐three percent of caregivers reported having more than one child. Although several positive effects of siblings on babies’ sleep were found, these did not reach significance. In contrast, preschool children with siblings showed significantly more regular bedtimes (Table 1).
Another relevant factor was the time caregivers spent on childcare activity. Surprisingly, caregivers reported an acute decrease in childcare time (by 21.46 ± 481.54 min) in April. We note that the participants often reported having difficulties in estimating the time spent on different activities during the early phase of the confinement due to unstructured and overlapping activities experienced in this period. Nevertheless, more childcare time was associated with shorter sleep latency and less sleep fragmentation in babies (Table 1). In preschool children, we observed more frequent short sleep latency. These findings indicate notably higher sleep quality in children of caregivers who spent more time with their children during the confinement.
Furthermore, the presence of pets showed a positive effect on sleep quality. Twenty‐six percent of caregivers reported having pets in their household. The presence of pets was associated with lower sleep fragmentation in babies and lower day‐to‐day variability of sleep duration in preschool children (Table 1).
A final determining factor was the context of work arrangements. Caregivers whose work arrangements were strongly affected by the confinement (on average 3.84 ± 1.27 points in April) reported earlier bedtimes and longer sleep duration in their babies, whereas no such effects were observed in preschool children (Table 1). We note that the details of changes in work arrangements were unknown to us and acknowledge that these might have included severe events such as loss of employment. Nevertheless, we based the definition of this factor as protective on its overall effect on children's sleep, which was positive.
3.1.4. Role of age and sex during the acute phase of the confinement
Sleep behaviour changes during childhood maturation (Iglowstein et al., 2003) and varies depending on sex (Plancoulaine et al., 2015). For babies, older age at baseline was associated with acute consequences of confinement, including later bedtimes, longer sleep latency, longer sleep duration and increased sleep fragmentation (Table 1). Similarly, older preschool children showed less regular bedtimes and less frequent short sleep latency as compared to younger children in the same group (i.e., preschool children).
No significant sex differences were found with regards to the acute reaction to the confinement.
Our models revealed no additional acute effects in relation to young children's sleep quality for the remaining factors (i.e., childcare arrangements, fear of infection, adherence to isolation recommendations and social interaction).
3.2. Part 2: Persistent dynamics across follow‐up assessments (April, May, June 2020)
3.2.1. Children's sleep throughout the confinement
Interestingly, divergent trajectories were found for the investigated sleep behaviours throughout the confinement (Figure 2). Already at the first follow‐up in May 2020, sleep latency in babies returned to initial values, that is, the retrospective reports for the period before the confinement (April = 30 ± 24 min, May = 21 ± 12 min, June = 22 ± 11 min as compared to 21 ± 15 min before the confinement). In contrast, sleep duration further decreased over the follow‐up period (April = 10:18 ± 01:10 h, May = 10:10 ± 01:03 h, June = 10:04 ± 01:22 h as compared to 10:33 ± 00:58 h before the confinement). Babies’ bedtimes remained unchanged across follow‐up assessments (clock times: April = 20:55 ± 01:06, May = 20:54 ± 01:04, June = 20:52 ± 01:01 as compared to 20:26 ± 00:59 before the confinement). Similarly, the number of awakenings demonstrated no significant changes across assessments (April = 1.86 ± 1.70, May = 1.49 ± 1.39, June = 1.58 ± 1.49 as compared to 1.62 ± 1.49 before the confinement).
FIGURE 2.
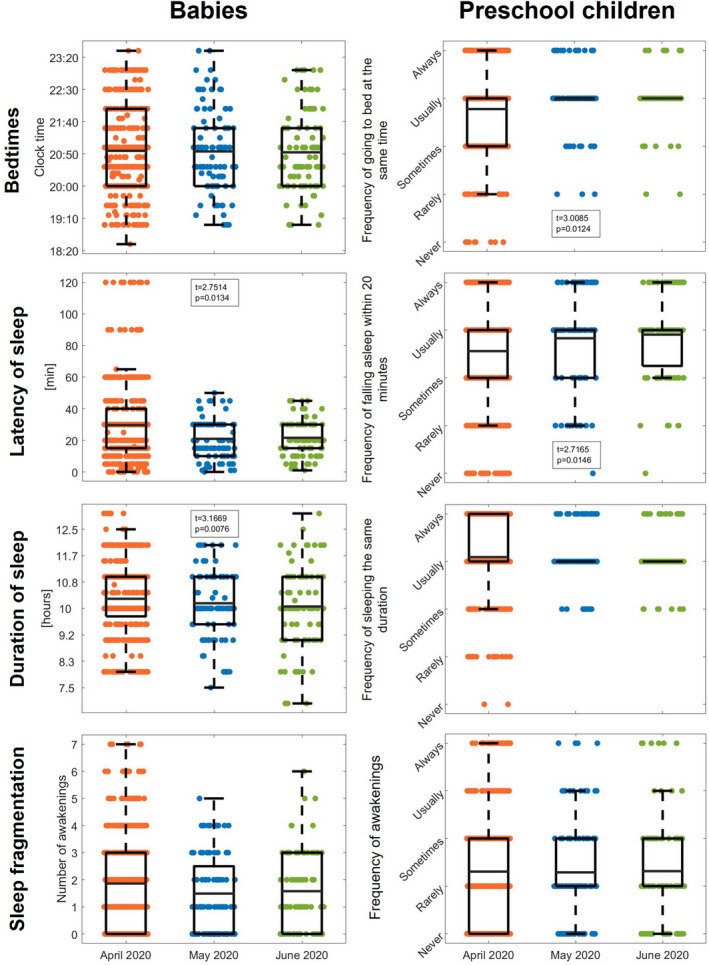
Evolution of sleep variables across the COVID‐19 confinement period from April to June 2020 in babies (0–35 months) and preschool children (36–71 months). Box plots, indicating the first and third quartiles as well as the mean (whisker length 1.5 * interquartile range), and scattered dots display core sleep measures (bedtimes, latency of sleep, duration of sleep and sleep fragmentation) for baseline assessment (April 2020; in orange; 452 babies and 412 preschool children), first follow‐up (May 2020; in blue; 96 babies and 100 preschool children) and second follow‐up (June 2020; in green; 90 babies and 95 preschool children). t‐ and p‐values depict significant time effects from our linear mixed model after correcting for multiple comparisons by means of the false discovery rate. Due to age‐specific assessment tools, absolute values are presented for babies and reports of frequency of occurrence for preschool children
In preschool children, bedtimes became more regular throughout the follow‐up period (April = 3.78 ± 0.89 points, May = 3.98 ± 0.60 points, June = 4.00 ± 0.50 points as compared to 4.15 ± 0.70 points before the confinement). Furthermore, short sleep latency (i.e., below 20 min) was more frequently reported (April = 3.56 ± 1.13 points, May = 3.83 ± 0.95 points, June = 3.90 ± 0.85 points as compared to 3.90 ± 1.05 points before the confinement). In contrast, the regularity of sleep duration remained stable across follow‐up assessments (April = 4.09 ± 0.74 points, May = 4.16 ± 0.54 points, June = 4.10 ± 0.49 points as compared to 4.25 ± 0.62 points before the confinement). Similarly, sleep fragmentation showed no changes across assessments (April = 2.31 ± 1.11 points, May = 2.29 ± 1.08 points, June = 2.32 ± 1.06 points as compared to 2.24 ± 1.12 points before the confinement).
3.2.2. Risk factors throughout the confinement
We observed a persistent decrease in caregivers’ stress to 2.82 ± 1.10 points in May (since the last assessment, i.e., April) and to 2.72 ± 1.15 points in June (since the last assessment, i.e., May). Nevertheless, we observed a trend towards an association of greater levels of caregiver's stress with longer sleep latency and increased sleep fragmentation in babies across assessments (p =.07). Furthermore, greater caregiver's stress was significantly associated with less regular bedtimes and less frequent short sleep latency in preschool children (Table 2).
TABLE 2.
Factors persistently associated with sleep of babies (0–35 months) and preschool children (36–71 months) across the COVID‐19 confinement period from April to June 2020
| Babies | Preschool children | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedtimes (min) | Latency of sleep (min) | Duration of sleep (min) | Number of awakenings | Frequency of going to bed at the same time | Frequency of falling asleep within 20 min | Frequency of sleeping the same duration | Frequency of awakenings | |||||||||
| Factor | b | p | b | p | b | p | b | p | b | p | b | p | b | p | b | p |
| Stress | – | – | 1.80 | 0.074 | −4.05 | 0.109 | 0.09 | 0.076 | −0.06 | 0.031 | −0.10 | 0.023 | −0.05 | 0.051 | – | – |
| Quarantine | 25.93 | <0.001 | 3.12 | 0.099 | −18.03 | 0.003 | 0.39 | 0.002 | – | – | – | – | 0.11 | 0.236 | −0.15 | 0.236 |
| Mindfulness | 0.36 | 0.103 | 0.15 | 0.095 | 0.52 | 0.089 | 0.02 | <0.001 | 0.01 | 0.023 | 0.01 | 0.007 | 0.01 | 0.038 | 0.01 | 0.009 |
| Siblings | – | – | −3.28 | 0.102 | −13.31 | 0.064 | 0.32 | 0.064 | 0.40 | <0.001 | – | – | 0.16 | 0.023 | −0.20 | 0.051 |
| Childcare | −0.01 | 0.171 | −0.01 | <0.001 | – | – | – | – | – | – | – | – | 0.00 | 0.050 | −0.00 | 0.025 |
| Pets | −11.03 | 0.202 | – | – | – | – | – | – | – | – | – | – | 0.11 | 0.227 | – | – |
| Work | – | – | – | – | – | – | – | – | – | – | 0.05 | 0.479 | – | – | – | – |
| Age | 7.12 | 0.050 | 3.26 | 0.009 | – | – | −0.33 | <0.001 | – | – | – | – | 0.07 | 0.039 | −0.24 | <0.001 |
| Sex | – | – | – | – | – | – | – | – | – | – | −0.28 | 0.011 | −0.14 | 0.044 | – | – |
Unstandardized beta coefficients (b) and corrected p‐values (p) from the linear mixed model are shown for the following factors: caregiver's stress, quarantine status, time caregiver spent on mindfulness practices, presence of siblings, time caregiver spent on childcare, presence of pets, change in caregiver's working arrangements, child's age and sex. Missing values indicate that the factor did not survive statistical backward selection. Significant associations (p < 0.05 after correcting for multiple testing by means of the false discovery rate) are presented in bold. Results were derived from the longitudinal analysis across assessments (baseline, and first and second follow‐up), including absolute values of sleep variables (i.e., without the quantification of a temporal change). We note the difference between sleep variables for the two groups of children, with babies’ sleep assessed in absolute terms and preschool children's sleep assessed in terms of frequency according to a 5‐point scale.
Caregivers who were in quarantine (36% in April, 17% in May, 4% in June) reported later bedtimes, shorter sleep duration and increased sleep fragmentation for their babies across assessments as compared to caregivers who were not in quarantine (Table 2). In contrast to the broad effect on babies’ sleep, no significant persistent effect of quarantine status was observed for any of the sleep variables in preschool children.
3.2.3. Protective factors throughout the confinement
Interestingly, caregivers temporarily reduced their time spent on mindfulness strategies from April to May (by 4.88 ± 10.84 min), with only 3% of caregivers engaging in such activities. Afterwards, it increased again (by 1.50 ± 5.25 min) in June, with 10% of caregivers engaging in mindfulness practices, corresponding to the level before the confinement. Across assessments, more mindfulness time was associated with increased sleep fragmentation in babies as well as preschool children. Furthermore, more mindfulness time was associated with more regular bedtimes, more frequently reported short sleep latency and lower day‐to‐day variability of sleep duration in preschool children (Table 2).
The presence of siblings showed no significant persistent effect on babies’ sleep. However, preschool children with siblings showed more regular bedtimes and a more regular sleep duration compared to preschool children without siblings across assessments (Table 2).
The time caregivers invested in childcare increased from April to May (by 6.46 ± 454.09 min) and from May to June (by 21.33 ± 430.62 min). A persistent effect of childcare time across assessments was found for babies in decreased sleep latency. Moreover, more childcare time was associated with less frequent awakenings in preschool children across assessments (Table 2).
In contrast to the acute effects at baseline assessment (Part 1), we found no significant persistent effect of pets on young children's sleep across the confinement period, suggesting that pets have a positive impact only in acute phases.
Although the caregivers’ working arrangements remained affected throughout the confinement (3.81 ± 1.31 points in May, 3.06 ± 1.41 points in June), there were no significant persistent effects of this variable on children's sleep.
3.2.4. Role of age and sex throughout the confinement
For babies, older age was associated with longer sleep latency and reduced sleep fragmentation across assessments. Similarly, older preschool children's sleep demonstrated a reduced day‐to‐day variability of sleep duration and less frequent awakenings (Table 2).
Although no effects of sex were found in the baby group, preschool girls showed poorer sleep quality compared to boys in the same group, including less frequent short sleep latency and higher day‐to‐day variability of sleep duration (Table 2).
We found no persistent effects of childcare arrangements, fear of infection, adherence to isolation recommendations and social interaction on children's sleep across assessments.
4. DISCUSSION
This investigation examined the influence of the COVID‐19 confinement on young children's sleep as reported by their primary caregivers in an internationally distributed online survey. A fundamental acute change in response to the confinement in April 2020 was significantly decreased sleep quality in babies and preschool children. Throughout the two subsequent months, this effect largely disappeared and sleep quality normalized. Most importantly, caregiver's stress due to the confinement was identified as the dominant negative determinant of children's sleep. Stress not only governed the strong acute change at baseline, but it furthermore persisted to determine children's sleep throughout the confinement period. Fortunately, the factors positively influencing children's sleep included caregiver's mindfulness practices, time spent on childcare and the presence of siblings in the household, demonstrating both acute as well as persistent associations.
4.1. Are young children more severely affected than adults?
We demonstrate negative secondary consequences of the confinement that are notable in several domains of sleep quality in babies and preschool children. Our results agree with findings among 3‐ to 6‐year‐old children in Italy (Dellagiulia et al., 2020), as well as with adults assessed in Italy, Switzerland, Germany and Austria (Blume et al., 2020; Cellini et al., 2020). The observation that adults in the USA and Italy notably delayed their bedtime during the acute confinement period (Cellini et al., 2020; Wright et al., 2020) agrees with our observations in young children.
Interestingly, although adults prolonged their sleep during the early confinement (Cellini et al., 2020; Wright et al., 2020), sleep duration continuously decreased throughout the confinement period in our group of babies. The cohort of 3‐ to 6‐year‐old children in Italy showed a similar initial decrease in sleep duration during the early phase of the confinement (Dellagiulia et al., 2020). However, this group of children was older than our baby group and, importantly, recovered across a 4‐week period. Unfortunately, the structure of the questionnaire we applied for the assessment of sleep behaviour in preschool children did not allow examination of sleep duration. In this age group, only the variability of sleep duration was assessed, a measure not directly comparable to the approach of Dellagiulia et al. (2020). On the other hand, Zreik et al. (2021) reported no change in maternal perception of children's sleep duration in 40% of the study population (6‐ to 72‐month‐old children from Israel) 4 weeks after the first confinement measures were implemented. Nevertheless, 35% of mothers indicated a decrease in sleep duration of their babies. In alignment with the association of caregivers’ stress with children's sleep in our study, the authors reported that maternal anxiety was negatively linked to children's sleep duration. Thus, the findings overlap to a large extent, and the large variability might have been influenced by cultural and methodological differences. Nevertheless, the continuous shortening of nocturnal sleep we observed in the group younger than 3 years is alarming, as this age range constitutes a highly vulnerable developmental period encompassing the most rapid brain growth across the lifespan (Dekaban & Sadowsky, 1978). It is quite remarkable that nocturnal sleep duration in this group decreased by 29 min from the period before the confinement until our last assessment, given that normative data show that nocturnal sleep duration increases up to 3 years of age (Scholle et al., 2011). A chronic loss of half an hour of sleep is sizable, as the National Sleep Foundation (USA) (Hirshkowitz et al., 2015) recommends sleep durations of 14–17 h at age 0 to 3 months, 12–15 h at age 4 to 11 months, and 11–14 h between 1 and 2 years.
An additional contrast to adults was found in the regularity of sleep duration. During the early confinement period, adults experienced more similarity between work days and free days regarding their sleep duration as compared to before the confinement (Blume et al., 2020), whereas the preschool children in our study showed acutely decreased day‐to‐day similarity in sleep duration. We conclude that young children's sleep is more severely harmed by the implementation of confinement in comparison to the effect confinement had on adults’ sleep. This observation may be explained by children's increased sensitivity towards environmental influences due to the rapid and dramatic changes associated with development (Rice & Barone, 2000).
4.2. Unique evolution of young children's sleep behaviours across confinement
Throughout the assessments, young children's sleep mostly recovered to the same levels reported for the period before the confinement. This confirms that the retrospective assessments at baseline were not significantly affected by recall bias. However, several sleep variables underwent a unique evolution across assessments. For example, bedtimes and sleep duration demonstrated no improvement across the confinement period. One possible explanation for this result is that the multi‐dimensionality of sleep (Buysse, 2014) reflects a variety of different mechanisms underlying each investigated sleep variable, some of which are in the midst of their development in the cohort investigated here. For example, bedtimes may reflect the child's or even caregiver's chronotype (i.e., morningness/eveningness) and thus somewhat link to their circadian rhythm, whereas sleep duration is determined by prior time spent awake, thus reflecting sleep need accumulated throughout wakefulness (Skeldon et al., 2016). Furthermore, it is plausible to assume that further contextual factors such as physical exercise and exposure to daylight (Altena et al., 2020), including the seasonal increase in daylight hours, are involved, which were not examined in this work. Additionally, regular food intake aligns the metabolic processes that facilitate the maintenance of a daily rhythm of body physiology (Depner et al., 2018), representing another potential factor influencing sleep beyond the scope of our investigation.
In contrast to the negative impact on bedtimes and sleep duration, we observed an improvement in other sleep variables across the confinement period, which may reflect families’ adapting to the new circumstances. Alternatively, this improvement may also relate to the decrease of caregiver's stress, which was detected as the main risk factor influencing young children's sleep.
4.3. Psychological consequences of confinement situations
Stress‐related sleep problems are common in adults, but this frustrating relationship is rather bidirectional (Altena et al., 2016). The decrease of adults’ sleep quality during the confinement highly depended on the increase in perceived burden (Blume et al., 2020), symptomatology of stress, depression and anxiety (Cellini et al., 2020). Our findings extend this state of knowledge by demonstrating that caregivers’ stress not only worsened their own sleep, but also affected the sleep of their offspring.
We must consider that confinement measures trigger secondary – psychological – consequences that go beyond the direct effect on physiological health. In 2003, Canada implemented quarantine as an extreme type of confinement in response to the outbreak of SARS, which caused considerable psychological distress in a large fraction of the population. Up to 30% showed symptoms of post‐traumatic stress disorder and depression (Hawryluck et al., 2004). Shortly after the outbreak of COVID‐19, the prevalence of acute post‐traumatic stress symptoms in China was 4.6%. Notably, poor sleep quality was identified as a risk factor (Sun et al., 2020). These recent insights illustrate the need to identify interventions that improve sleep quality as a global matter of urgent action.
4.4. Young children's sleep can be protected during confinement
Fortunately, several dimensions of sleep are modifiable and can be targeted with sleep interventions. One example is sleep hygiene, including regular sleep schedules and calming bedtime routines, which effectively improves children's sleep and leads to fewer behavioural problems (Werner et al., 2015). A healthy sleep intervention comprising similar aspects was shown to improve not only infant sleep, but also elevate maternal well‐being (Symon & Crichton, 2017), which further supports a bidirectional relation between young children's sleep and caregivers’ well‐being.
Our study revealed several factors that can protect young children's sleep. For example, the practice of mindfulness strategies by caregivers was associated with better quality of their children's sleep. Specifically, preschool children of caregivers who spent more time applying mindfulness techniques showed more regular bedtimes and sleep duration, and more frequent short sleep latency. Interestingly, caregivers applying mindfulness techniques also reported more frequent awakenings in their babies and preschool children, which possibly reflects an effect of increased sensory awareness, as can be achieved through yoga practice (Rivest‐Gadbois & Boudrias, 2019). Previous reports indeed confirm that positive parent personality, as reflected by optimism, empathy and mindfulness, can moderate the parents’ stress and enhance the quality of their children's sleep (Miadich et al., 2019). Our findings extend this concept by showing that caregivers’ active engagement in mindfulness techniques effectively protects their young children's sleep during the extreme condition of a pandemic confinement.
Further protective factors for young children's sleep were identified in caregivers’ working arrangements (i.e., working from home during the confinement) and in the time caregivers spent on childcare. Both most likely arise from increased time caregivers spent at home during the confinement and thus with their children. The increased time family members spent together was recently proposed as a possible positive consequence of the confinement (Altena et al., 2020). Furthermore, the presence of siblings and pets in a household showed a positive effect on young children's sleep. It is possible that these partially compensate for the limited social interactions under confinement conditions.
4.5. Will there be consequences for young children's development?
Sleep is intertwined with developmental processes and crucial for the maturation of central brain functions in animals (Jha et al., 2005). Sleep also plays a mediating role in human health (Garrison, 2015). Particularly the first years of life demand adequate sleep: poor sleep in infancy and early childhood increases the risk of later psychosocial and medical problems (Simola et al., 2014; Taveras et al., 2008). Accordingly, we are relieved to report that many dimensions of young children's sleep normalized after the first month of confinement. Nevertheless, it is crucial to track behavioural consequences that may emerge in this population in the following years. Moreover, the sleep behaviours that are easily modifiable should be targeted to promote healthy developmental outcomes.
Practical recommendations to limit the effect of stress on sleep should be tailored to adults and include elements of cognitive behavioural therapy (Altena et al., 2020). Our investigation shows that balancing stress will not only benefit adults, but also their young children. Given the severe acute changes in response to confinement we observed for young children, it is of utmost importance to raise public awareness and provide timely psychosocial interventions in comparable situations. To this end, public health officials, infectious diseases physicians, psychiatrists and psychologists need to provide sleep‐specific guidance to support those families at risk of adverse psychological, social and developmental consequences of confinement.
4.6. Limitations
The sudden global implementation of the COVID‐19 confinement provided a unique insight into family dynamics under extreme conditions. However, the social‐distancing regulations allowed only for subjective methodology in our study, as they prevented a physiological objective assessment of sleep (e.g., actimetry, sleep electroencephalogram). Consequently, our analyses are based on caregivers’ subjective reports. We thus cannot rule out the possibility that the observed changes in young children's sleep behaviour were subject to caregivers’ misperception (e.g., due to elevated stress during the confinement). Furthermore, the examined sleep variables were not directly comparable between the two age groups (i.e., babies and preschool children) due to different assessment instruments. To account for this, we analysed and presented the data for the two groups separately. Although the terms “infants” and “toddlers” would be more suitable to precisely describe the younger group, we decided to refer to them as “babies” for simplification. It is important to consider that the baby group also included newborns of only up to 3 months of age. With regards to sleep behaviour, this is a highly dynamic age encompassing rapid changes and large inter‐individual variability. However, the number of babies of up to 3 months of age was low (n = 15 accounting for 3% of the baby group) and they showed changes in sleep variables from the period before the confinement that were similar to those of the older babies in the sample. The only exception was that the number of awakenings in the newborn subgroup decreased from before to during the confinement by 0.6, whereas the older baby subgroup (3 months and older) increased by 0.15 (Wilcoxon rank sum test: z=−2.14, p =.03). We thus strongly believe that the overall dynamics are not driven by this subgroup of subjects.
Another limiting factor is the large number of participants who dropped out of the study after the first assessment. However, we note that across follow‐up assessments similar numbers of participants could be maintained. A further limitation of the study is the lack of momentary assessments before the confinement, as baseline data were based on retrospective assessments. This was impossible to address due to the abruptness of the confinement situation. However, at follow‐up assessments, we observed a rebound to levels of retrospective assessments for several sleep variables, suggesting that our findings were not subject to significant recall bias. Nevertheless, we cannot rule out the possibility that referring to the unspecific period of time in the past (“before the confinement”) has affected our results. However, we evaluated that referencing a specific time‐point before the confinement may be difficult for caregivers of the youngest children in the study population, in which changes in sleep behaviour can occur within very short time‐scales. Finally, the widespread geographic distribution of our sample raises concerns with regards to possible cultural differences but also differences in confinement regulations. Although we were not able to control for cultural differences due to an already complex statistical model, we attempted to account for differences in confinement regulations by including multiple determinants of the confinement situation as factors in our analyses.
We note that our distinction between risk and protective factors depending on whether they affect children's sleep in a negative or a positive manner was based on adults’ sleep. We acknowledge that our current understanding of “good” and “poor” sleep in early life is still limited. For example, we define increased sleep fragmentation as an indicator of poor sleep in young children. However, it has been suggested that fragmented sleep in the first months of life may serve a central role in preventing sudden infant death (Goto et al., 1999; Mosko et al., 1997). It is thus possible that some of the observed effects described as negative would rather show positive consequences for development. However, this issue is beyond the scope of our study and remains an open question for future research.
4.7. Conclusions
Our findings demonstrate that primarily in the acute period of the COVID‐19 confinement, the perceived stress of primary caregivers was associated with drastically decreased sleep quality in their young children. However, several protective factors have promising potential to counteract such negative impacts. It is of immediate concern to protect children's sleep, especially during periods of confinement, as chronically disturbed sleep in vulnerable periods of early life can negatively impact brain development (Cook et al., 2020). Notably, the pandemic is already causing a rise in childhood mental health issues that are expected to far outlast the crisis (Holmes et al., 2020). Consequently, beyond monitoring success in disease containment, we strongly recommend tracking the well‐being of adults as well as young children.
CONFLICTS OF INTEREST
No conflicts of interest declared.
AUTHOR CONTRIBUTIONS
SK, AM, CM and MB designed and conducted the study. AM performed the analyses. SK, AM and VC wrote the manuscript. All authors discussed the results and contributed to the final manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank Océane Minot, Debora Castiglioni, Valentine Horii and Dr. Maria Berrozpe for their help with data collection, Dr. Sarah Schoch for scientific advice and all the participants for taking part in the study. We acknowledge funding from the Swiss National Science Foundation (PCEFP1‐181279 to SK) and the University of Zurich (Medical Faculty; Forschungskredit FK‐18‐047; Stiftung für wissenschaftliche Forschung STWF‐17‐008 to SK).
Markovic A, Mühlematter C, Beaugrand M, Camos V, Kurth S. Severe effects of the COVID‐19 confinement on young children’s sleep: A longitudinal study identifying risk and protective factors. J Sleep Res. 2021;30:e13314. 10.1111/jsr.13314
DATA AVAILABILITY STATEMENT
The datasets are available from the corresponding author on reasonable request and pending ethics approval.
REFERENCES
- Altena, E. , Baglioni, C. , Espie, C. A. , Ellis, J. , Gavriloff, D. , Holzinger, B. , Schlarb, A. , Frase, L. , Jernelöv, S. , & Riemann, D. (2020). Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. Journal of Sleep Research, 29, e13052. 10.1111/jsr.13052 [DOI] [PubMed] [Google Scholar]
- Altena, E. , Micoulaud‐Franchi, J.‐A. , Geoffroy, P.‐A. , Sanz‐Arigita, E. , Bioulac, S. , & Philip, P. (2016). The bidirectional relation between emotional reactivity and sleep: From disruption to recovery. Behavioral Neuroscience, 130, 336–350. 10.1037/bne0000128 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Blume, C. , Schmidt, M. H. , & Cajochen, C. (2020). Effects of the COVID‐19 lockdown on human sleep and rest‐activity rhythms. Current Biology, 30, R795–R797. 10.1016/j.cub.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D. J. (2014). Sleep health: Can we define it? Does it matter? Sleep, 37, 9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini, N. , Canale, N. , Mioni, G. , & Costa, S. (2020). Changes in sleep pattern, sense of time and digital media use during COVID‐19 lockdown in Italy. Journal of Sleep Research, 29, e13074., 10.1111/jsr.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattu, V. K. , Manzar, M. D. , Kumary, S. , Burman, D. , Spence, D. W. , & Pandi‐Perumal, S. R. (2018). The global problem of insufficient sleep and its serious public health implications. Healthcare, 7(1), 1. 10.3390/healthcare7010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, F. , Conway, L. J. , Giallo, R. , Gartland, D. , Sciberras, E. , & Brown, S. (2020). Infant sleep and child mental health: A longitudinal investigation. Archives of Disease in Childhood, 105, 655–660. 10.1136/archdischild-2019-318014 [DOI] [PubMed] [Google Scholar]
- Dekaban, A. S. , & Sadowsky, D. (1978). Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology, 4, 345–356. 10.1002/ana.410040410 [DOI] [PubMed] [Google Scholar]
- Dellagiulia, A. , Lionetti, F. , Fasolo, M. , Verderame, C. , Sperati, A. , & Alessandri, G. (2020). Early impact of COVID‐19 lockdown on children’s sleep: A 4‐week longitudinal study. Journal of Clinical Sleep Medicine, 16, 1639–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner, C. M. , Melanson, E. L. , McHill, A. W. , & Wright, K. P. (2018). Mistimed food intake and sleep alters 24‐hour time‐of‐day patterns of the human plasma proteome. Proceedings of the National Academy of Sciences of the United States of America, 115, E5390–E5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ednick, M. , Cohen, A. P. , McPhail, G. L. , Beebe, D. , Simakajornboon, N. , & Amin, R. S. (2009). A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep, 32, 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison, M. M. (2015). The feedback whirlpool of early childhood sleep and behavior problems. JAMA Pediatrics, 169, 525–526. [DOI] [PubMed] [Google Scholar]
- Goto, K. , Mirmiran, M. , Adams, M. M. et al (1999). More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics, 103, 603–609. [DOI] [PubMed] [Google Scholar]
- Hawryluck, L. , Gold, W. L. , Robinson, S. , Pogorski, S. , Galea, S. , & Styra, R. (2004). SARS control and psychological effects of quarantine, Toronto, Canada. Emerging Infectious Diseases, 10, 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz, M. , Whiton, K. , Albert, S. M. et al (2015). National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health, 1, 40–43. [DOI] [PubMed] [Google Scholar]
- Holmes, E. A. , O’Connor, R. C. , Perry, V. H. et al (2020). Multidisciplinary research priorities for the COVID‐19 pandemic: A call for action for mental health science. Lancet Psychiatry, 7, 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglowstein, I. , Jenni, O. G. , Molinari, L. , & Largo, R. H. (2003). Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics, 111, 302–307. [DOI] [PubMed] [Google Scholar]
- Jha, S. K. , Jones, B. E. , Coleman, T. , Steinmetz, N. , Law, C.‐T. , Griffin, G. , Hawk, J. , Dabbish, N. , Kalatsky, V. A. , & Frank, M. G. (2005). Sleep‐dependent plasticity requires cortical activity. Journal of Neuroscience, 25, 9266–9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner, D. J. (2020). SoSci Survey. (Version 3.2.10) [Computer software]. Available at https://www.soscisurvey.de [Google Scholar]
- Lionetti, F. , Fasolo, M. , & Dellagiulia, A. (2020). On the role of moderators on children’s sleep health in response to COVID‐19. Journal of Clinical Sleep Medicine, 17(2), 353–354. 10.5664/jcsm.8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten, A. S. , & Obradovic, J. (2008). Disaster preparation and recovery: Lessons from research on resilience in human development. Ecology and Society, 13, 9. 10.5751/ES-02282-130109 [DOI] [Google Scholar]
- Meltzer, L. J. , & Mindell, J. A. (2007). Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology, 21, 67–73. 10.1037/0893-3200.21.1.67 [DOI] [PubMed] [Google Scholar]
- Miadich, S. A. , Doane, L. D. , Davis, M. C. , & Lemery‐Chalfant, K. (2019). Early parental positive personality and stress: Longitudinal associations with children’s sleep. The British Journal of Health Psychology, 24, 629–650. 10.1111/bjhp.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosko, S. , Richard, C. , & McKenna, J. (1997). Infant arousals during mother‐infant bed sharing: Implications for infant sleep and sudden infant death syndrome research. Pediatrics, 100, 841–849. 10.1542/peds.100.5.841 [DOI] [PubMed] [Google Scholar]
- Newton, A. T. , Honaker, S. M. , & Reid, G. J. (2020). Risk and protective factors and processes for behavioral sleep problems among preschool and early school‐aged children: A systematic review. Sleep Medicine Reviews, 52, 101303. 10.1016/j.smrv.2020.101303 [DOI] [PubMed] [Google Scholar]
- Nooraee, N. , Molenberghs, G. , Ormel, J. , & den Heuvel, E. R. V. (2018). Strategies for handling missing data in longitudinal studies with questionnaires. Journal of Statistical Computation and Simulation, 88, 3415–3436. 10.1080/00949655.2018.1520854 [DOI] [Google Scholar]
- Owens, J. A. , Spirito, A. , & McGuinn, M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school‐aged children. Sleep, 23, 1043–1051. 10.1093/sleep/23.8.1d [DOI] [PubMed] [Google Scholar]
- Plancoulaine, S. , Lioret, S. , Regnault, N. , Heude, B. , & Charles, M.‐A. (2015). Gender‐specific factors associated with shorter sleep duration at age 3 years. Journal of Sleep Research, 24, 610–620. [DOI] [PubMed] [Google Scholar]
- Rice, D. , & Barone, S. (2000). Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives, 108, 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest‐Gadbois, E. , & Boudrias, M.‐H. (2019). What are the known effects of yoga on the brain in relation to motor performances, body awareness and pain? A Narrative Review. Complementary Therapies in Medicine, 44, 129–142. [DOI] [PubMed] [Google Scholar]
- Sadeh, A. (2004). A brief screening questionnaire for infant sleep problems: Validation and findings for an internet sample. Pediatrics, 113, e570–e577. [DOI] [PubMed] [Google Scholar]
- Scholle, S. , Beyer, U. , Bernhard, M. et al (2011). Normative values of polysomnographic parameters in childhood and adolescence: Quantitative sleep parameters. Sleep Medicine, 12, 542–549. [DOI] [PubMed] [Google Scholar]
- Simola, P. , Liukkonen, K. , Pitkäranta, A. , Pirinen, T. , & Aronen, E. T. (2014). Psychosocial and somatic outcomes of sleep problems in children: A 4‐year follow‐up study. Child: Care, Health and Development, 40, 60–67. [DOI] [PubMed] [Google Scholar]
- Skeldon, A. C. , Derks, G. , & Dijk, D.‐J. (2016). Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Medicine Reviews, 28, 96–107. [DOI] [PubMed] [Google Scholar]
- Smyth, N. , Rossi, E. , & Wood, C. (2020). Effectiveness of stress‐relieving strategies in regulating patterns of cortisol secretion and promoting brain health. International Review of Neurobiology, 150, 219–246. [DOI] [PubMed] [Google Scholar]
- Sprang, G. , & Silman, M. (2013). Posttraumatic stress disorder in parents and youth after health‐related disasters. Disaster Medicine and Public Health Preparedness, 7, 105–110. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Sun, Z. , Wu, L. , Zhu, Z. , Zhang, F. , Shang, Z. , Jia, Y. , Gu, J. , Zhou, Y. , Wang, Y. , Liu, N. , & Liu, W. (2020). Prevalence and risk factors of acute posttraumatic stress symptoms during the COVID‐19 outbreak in Wuhan, China. Medrxiv, 2020, 2020.03.06.20032425 [Google Scholar]
- Symon, B. , & Crichton, G. E. (2017). The joy of parenting: Infant sleep intervention to improve maternal emotional well‐being and infant sleep. Singapore Medical Journal, 58, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras, E. M. , Rifas‐Shiman, S. L. , Oken, E. , Gunderson, E. P. , & Gillman, M. W. (2008). Short sleep duration in infancy and risk of childhood overweight. Archives of Pediatrics and Adolescent Medicine, 162, 305–311. 10.1001/archpedi.162.4.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren, S. (2018). Flexible imputation of missing data (2nd ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Werner, H. , Hunkeler, P. , Benz, C. , Molinari, L. , Guyer, C. , Häfliger, F. , Huber, R. , & Jenni, O. G. (2015). The Zurich 3‐step concept for the management of behavioral sleep disorders in children: A before‐and‐after study. Journal of Clinical Sleep Medicine, 11, 241–249. 10.5664/jcsm.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, K. P. , Linton, S. K. , Withrow, D. , Casiraghi, L. , Lanza, S. M. , Iglesia, H. D. L. , Vetter, C. , & Depner, C. M. (2020). Sleep in university students prior to and during COVID‐19 Stay‐at‐Home orders. Current Biology, 30, R797–R798. 10.1016/j.cub.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zreik, G. , Asraf, K. , Haimov, I. , & Tikotzky, L. (2021). Maternal perceptions of sleep problems among children and mothers during the coronavirus disease 2019 (COVID‐19) pandemic in Israel. Journal of Sleep Research, 30. 10.1111/jsr.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The datasets are available from the corresponding author on reasonable request and pending ethics approval.


