Abstract
Background
We aim to evaluate practice and understand the impact of the first wave of the SARS‐CoV‐2 pandemic on heart transplantation in the UK.
Methods
A retrospective review of the UK Transplant Registry (UKTR) and a national survey of UK heart transplant centers have been performed. The early pandemic period is defined here as 1 March to 31 May 2020.
Results
There was geographic variation in the prevalence of COVID‐19 across the UK. All centers reported adaptations to maintain the safety of their staff, candidate, and recipient populations. The number of donors fell by 31% during the early pandemic period. Heart utilization increased to 35%, compared to 26% during the same period of 2019. The number of heart transplants was well maintained, across all centers, with 38 performed, compared to 41 during the same period of 2019, with no change in 30‐day survival. Twenty‐seven heart transplant recipients with confirmed COVID‐19 infection were reported during the study period.
Conclusion
All UK heart transplant centers have successfully adapted their programs to overcome the challenges of staff redeployment and ICU and hospital resource limitation, associated with the pandemic, whilst continuing heart transplant activity. On‐going evaluation of practice changes, with sharing of lessons learned, is required as the pandemic continues.
Keywords: Coronavirus pandemic, COVID‐19, Heart transplantation, organ donation, SARS‐CoV‐2
1. INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus‐type 2 (SARS‐CoV‐2), the causative agent for Coronavirus Disease 2019 (COVID‐19), was first diagnosed in the United Kingdom (UK) in January 2020 1 and has since led to unprecedented public health measures aimed at containing the spread of the virus and reducing the impact of a surge of cases on the National Health Service (NHS), including the first of two national lockdowns on 23 March 2020. 2 The number of daily deaths from COVID‐19 peaked in April 2020 and is once again rising, totaling around 50 000 at the time of writing. 3
As the pandemic evolved in the UK, guidance for solid‐organ donation was issued, at a national level, from National Health Service Blood and Transplant (NHSBT). 4 Individual organ programs and transplant centers employed local decision‐making, based on the needs of their patient population and the effect of the pandemic on their hospital.
The UK heart transplant waiting list population consists of a significant proportion of urgent, inpatient candidates, at risk of clinical deterioration and death, as a result of progression of their underlying pathology. The risk of both acquiring COVID‐19 and suffering from the more severe form of the infection is likely to be higher in these patients. With increasing hospital prevalence of COVID‐19 patients, maintaining the safety of this patient population becomes increasingly challenging. Transplanting these patients must also be balanced with the risk of COVID‐19 in the early post‐transplant period and its’ potential impact on outcomes. 5 Cardiovascular manifestations of COVID‐19 are reported 6 and specific concerns about the risk and severity of illness in the early post‐operative period, when the burden of immunosuppression is greatest, have been raised.
In addition to balancing the risks of deterioration of heart failure and COVID‐19 infection, the ability of a center to maintain heart transplant activity, during the pandemic, is dictated by the availability and safety of deceased organ donors, and hospital resource, including intensive care unit (ICU) capacity and appropriate staff. Early reports from heart transplant programs globally have highlighted a significant decrease in organ donation and heart transplant activity during the pandemic. 7
Although the challenges for solid heart transplantation outlined here are well recognized, 8 there is limited published data on the national and regional changes to clinical practice, during this time. The aim of this study was to understand the impact of the first wave of the SARS‐CoV‐2 pandemic on heart transplantation in the UK.
2. METHODS
2.1. Study population
All adult and pediatric heart transplant centers in the UK were included in the study. Analysis included all adult (aged 16 years and older) and pediatric organ donors, heart transplant waiting list candidates and heart transplant recipients.
2.2. Study design
A retrospective review of data submitted to the UK Transplant Registry (UKTR) from 1 January 2019 to 30 June 2020 was performed. The early pandemic period is defined here as 1 March to 31 May 2020. Month‐to‐month and center‐level variation in practice were reviewed from 1 January to 30 June 2020, to include the early pandemic period, and, where appropriate, compared to data from 1 January to 30 June 2019.
Eligible, potential heart donors are defined as those aged less than 65 years, with no absolute contra‐indications to organ donation. Heart utilization was calculated as number of donors transplanted from the number of donors (who donated at least 1 solid organ) from whom the heart was offered for transplantation. Analysis included heart transplant activity, early (30‐day) mortality for recipients transplanted during the pandemic period, heart transplant candidate listing and waiting list activity, including mortality, and COVID‐19 infection in heart transplant recipients. Prospective reporting to the UKTR, of all transplant recipients, or patients on the organ transplant waiting list, with confirmed COVID‐19 infection, was commenced on 17 March 2020.
To explore regional, center‐level variation in clinical practice and policy, during the pandemic, a national survey of UK transplant centers was performed.
2.3. Survey design and conduct
The survey was developed using an iterative process, to identify changes in heart transplantation clinical practice, including candidate assessment, recipient management, organ donation and activity, perceptions of the prevalence of COVID‐19 locally, and the impact of the transplant workforce and hospital resource. The final survey was approved following review from members of the NHSBT Cardiothoracic Advisory Group Clinical Audit Group (CTAG CAG). The NHS England Coronavirus specialty guide cardiothoracic escalation framework 9 was used to define phases in response to the pandemic.
The survey was conducted between 15 May and 18 June 2020. At each center, a cardiothoracic transplant clinical lead and transplant coordinator were identified to complete the survey. Participants were emailed a copy of the survey. Information required to complete the survey was gathered by the nominated persons, from members of the wider team. Additional telephone interview follow‐up was performed between 20 June and 13 July 2020, where further information was required, or to clarify specific survey responses.
2.4. Statistical analysis
Categorical variables are expressed as absolute numbers and their relative frequencies, with percentage change compared to previous year, where appropriate. Continuous variables are expressed as mean ± standard deviation (SD) if normally distributed, or as median and inter‐quartile range (IQR) if non‐normally distributed. Chi‐square and Fisher's exact tests were used for comparison between groups of categorical variables. Continuous variables were compared using Student's t test or Mann‐Whitney U test, where appropriate. Statistical significance was considered for P <.05. Missing values were excluded from p value calculation. All statistical analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA.)
2.5. Ethical approval
Data analyses were performed by NHSBT, who maintain the UK Transplant Registry on behalf of UK transplant centers, under the UK General Data Protection Regulation (GDPR), allowing NHSBT to use patient identifiable information for service evaluation, without additional patient consent. Survey response was voluntary, with appropriate information provided to inform consent to participation.
3. RESULTS
The survey return rate was 100%. Additional information and clarification of responses were performed by telephone interview with 3 of 7 UK heart transplant centers.
There was geographic variation in the prevalence of COVID‐19 infection across the UK noted in the survey responses. The impact of the pandemic in each center varied as a result of local prevalence of the virus, resultant hospital and ICU admission, designation as an ECMO (extra‐corporeal membrane oxygenation) center (2 centers) and designation of the center as “COVID‐light” (1 center). COVID‐light describes the physical separation of COVID‐19‐positive and ‐negative patients within designated areas of NHS hospitals or for an entire hospital, functioning as an NHS network hub. 10
Movement of each center through the pandemic phases is shown in Table 1. At the time of the survey, all centers considered themselves to be moving into the recovery phase; however, a date for return to “normal” (pre‐pandemic) service had not been identified in any center. All centers recognized that changes to clinical practice, adopted during the pandemic, would persist.
TABLE 1.
Movement of UK heart transplant centers through pandemic phases, during the early pandemic period March to May 2020. Local and hospital virus prevalence as assessed by individual centers and pandemic phases defined by NHS England 9
| Preparation | Escalation | Crisis compensated | Crisis uncompensated |
Resolution |
Recovery |
|
|---|---|---|---|---|---|---|
|
Center A High local prevalence |
Early March | Mid‐March | Mid‐March | Late March | Mid‐ May |
In progress |
|
Center B Low local prevalence Low hospital prevalence |
Early March | March | Not applicable | Not applicable | April | In progress |
|
Center C High local prevalence “COVID‐light” designation |
Early March | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
|
Center D ECMO center |
Early March | Mid‐March | Mid‐March | Late March | Early May |
In progress |
|
Center E High local prevalence Moderate hospital prevalence |
Early March | Mid‐March | Late March | Not applicable | Early May |
Late May |
|
Center F ECMO center |
Early March | Late‐March | Early April | Mid‐April | Mid May |
Late May |
|
Center G High local prevalence Low hospital prevalence |
February | Early March | Mid‐March | Not applicable | Mid‐May |
Late May |
3.1. Deceased organ donation and heart utilization
During the early pandemic period, the number of deceased donors, who donated at least 1 solid organ, where the heart was offered for transplantation, fell by 31%, from 159 in March to May 2019 to 109 in the same period of 2020. The characteristics of donors, from whom the heart was offered, during the early pandemic period, compared to those from the same period of 2019, are shown in Table 2. During the early pandemic period, there was a significant decrease in donor age (from a median age of 47 years in 2019 to 40 years during the early pandemic period, P =.0039) and the proportion of donors with a history of hypertension (31% in 2019 to 10% during the early pandemic period, P =.0002).
TABLE 2.
Characteristics of donors, (*who donated at least 1 solid organ) where the heart was offered from 1 March to 31 May 2020 and 1 March to 31 May 2019
|
Deceased donors where the heart was offered * for donation 1 March to 31 May 2020 2019 |
% change or p value | ||
|---|---|---|---|
|
Number |
109 | 159 | ‐31% |
| Donation after brainstem death (DBD), n (%) |
92 (84%) |
131 (82%) |
0.7896 |
| Donation after circulatory death (DCD), n (%) |
17 (16%) |
28 (18%) | |
|
Donor age (years), median (IQR) |
40 (30‐51) |
47 (37‐54) |
0.0039 |
|
Pediatric donor, n (%) |
7 (6.4%) | 6 (4%) | 0.4827 |
|
Donor sex male, n (%) |
60 (55%) | 91 (57%) | 0.8187 |
|
Donor BMI (kg/m2) Median (IQR) |
25 (22.2‐28.3) |
26 (22.6‐29) |
0.5410 |
|
Cause of death, n (%) Intracranial Trauma Other |
98 (90%) 4 (4%) 7 (6%) |
141(89%) 5 (3%) 13 (8%) |
0.8476 |
|
Donor past smoker, n (%) Yes Unknown |
69 (64%) 1 |
96 (61%) 1 |
0.6981 |
|
Donor past diabetes, n (%) Yes Unknown |
2 (2%) 1 |
9 (6%) 1 |
0.2176 |
|
Donor past cardiac disease, n (%) Yes unknown |
6 (6%) 0 |
9 (6%) 4 (2.5%) |
>0.999 |
|
Donor past hypertension, n (%) Yes unknown |
11 (10%) 0 |
47 (31%) 5 (3%) |
0.0002 |
| Number of donors transplanted |
38 38 heart transplant |
42 39 heart transplant 3 heart‐lung transplant |
‐10% |
|
Utilization rate Transplanted/offered x 100 % |
35% |
26% |
+35% |
In the early pandemic period, hearts from 38 donors were implanted, a utilization rate of 35%. In March to May 2019, 42 UK donors were utilized for heart transplantation (39 heart‐only recipients and 3 heart‐lung recipients), a utilization rate of 26% (Table 3).
TABLE 3.
The number of deceased donors, who donated at least 1 solid organ, where the heart was offered for transplantation, the number of deceased donors from whom the heart was retrieved and the number of heart transplants performed, during the early pandemic period, compared to 1 March to 31 May 2019
| Heart |
Deceased donors where heart was offered 1 March to 30 June 2020 (19) |
Heart retrieved 1 March to 30 June 2020 (19) |
Heart transplanted 1 March to 30 June 2020 (19) |
Utilization rate % |
||||
|---|---|---|---|---|---|---|---|---|
|
2020 |
2019 |
|||||||
| March | 47 | (61) | 20 | (20) | 18 | (19) | ||
| April | 25 | (49) | 12 | (12) | 12 | (12) | ||
| May | 37 | (49) | 10 | (11) | 8 | (10) | ||
| Total | 109 | (159) | 42 | (43) | 38 | (41) | 35% | (26%) |
Survey participants reported that, where donor offers were received, they were confident that the risk of COVID‐19 in the donor was low, as a result of national policy for donor testing.
In addition to national guidance for solid‐organ donation, reducing donor age limit for DBD to less than 60 years and DCD less than 50 years, in survey responses, all centers describe center‐level changes to heart donor acceptance criteria during the pandemic. Changes described included consideration of lower age limits for potential donors and increased caution when considering travel and ischemic times.
3.2. National cardiothoracic organ retrieval service (NORS)
In survey responses, all centers describe changes to practice to maintain social distancing and the safety of retrieval team members. These include attendance at donor hospitals by the minimum number of team members required, additional transportation for travel, wearing of facemasks throughout and providing all equipment, including personal protective equipment (PPE), to remove the need to find and use equipment from donor hospitals. The primary concerns expressed were with regards to exposure of retrieval team members to COVID‐19 in the foreign environment of the donor hospital. Redeployment of retrieval team members to the ICU for the care of COVID‐19 patients was reported by 3 (of 6) cardiothoracic NORS teams.
3.3. Heart transplant activity and post‐transplant outcomes
All centers reported a local and individual case‐by‐case, decision‐making process, based on local, hospital‐level guidance, with consideration of intensive care unit capacity, and clinician decision‐making based on individual candidate perceived risk. The risk considerations included the urgency of the candidate (and the risk of remaining on the waiting list) and the risk of COVID‐19 in the early post‐transplant period.
Thirty‐eight heart transplants were performed in the UK during the early pandemic period, March to May 2020, compared to 41 for the same period of 2019, a reduction in activity of 10%. Trends in donors offered for heart transplantation and heart transplant activity are shown in Figure 1. Activity was distributed across all UK heart transplant centers.
FIGURE 1.
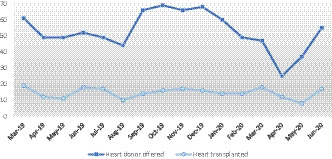
Number of deceased donors (who donated at least 1 solid organ) offered for heart transplantation and heart transplants performed, 1 March 2019 to 30 June 2020
Recipient characteristics are shown in Table 4. The proportion of patients transplanted, by urgency, remained unchanged. The 30‐day survival for those patients transplanted during the early pandemic period was 92% (76.9‐97.3), which compares to 90.0% (75.5‐96.1) for the same period of 2019 (P =.7830).
TABLE 4.
Recipient characteristics for heart transplants performed from 1 March to 31 May 2020, compared to 1 March to 31 May 2019
|
Heart transplant recipients 1 March to 31 May |
P value | ||
|---|---|---|---|
|
2020 (n = 38) |
2019 (n = 41) |
||
|
Donation after brainstem death (DBD), n (%) |
33 (87%) |
31 (76%) |
0.3247 |
| Donation after circulatory death (DCD), n (%) |
5 (13%) |
10 (24%) | |
|
Donor age (years), median (IQR) |
30 (22‐35) |
37 (24‐46) |
0.0867 |
| Pediatric donor, n (%) | 5 (13%) | 5 (12%) |
>0.999 |
|
Donor sex male, n (%) |
23 (61%) | 26 (63%) | 0.9742 |
|
Donor BMI (kg/m2), Median (IQR) |
23 (21.6‐26.5) |
24 (20.5‐27) |
0.6376 |
|
Cause of death, n (%) Intracranial Trauma Other |
35 (92%) 1 (3%) 2 (5%) |
35 (85%) 1 (2%) 5 (12%) |
0.7135 |
|
Donor history of hypertension, n (%) Yes Unknown |
2 (5%) 0 |
3 (8%) 4 (9%) |
0.6745 |
|
Donor past smoker, n (%) Yes Unknown |
23 (61%) 0 |
26 (67%) 2 (5%) |
0.7466 |
|
Donor past diabetes, n (%) Yes Unknown |
0 0 |
1 (3%) 2 |
>0.999 |
|
Donor past cardiothoracic disease, n (%) Yes Unknown |
1 (3%) 0 |
0 4 (10%) |
>0.999 |
|
Total ischemic time (hours), median (IQR) |
3.4 (2.9‐4.8) | 3.6 (2.9‐4.8) | 0.7730 |
|
Recipient age (years), Median (IQR) |
34 (13‐54) | 44 (28‐59) | 0.1436 |
|
Pediatric recipient, n (%) Yes |
10 (26%) | 4 (10%) | 0.0772 |
|
Recipient sex male, n (%) |
23 (61%) | 23 (56%) | 0.8646 |
|
Urgency status, n (%) Routine Urgent Super‐urgent |
6 (16%) 25 (66%) 7 (18%) |
4 (10%) 30 (73%) 7 (17%) |
0.6902 |
|
30‐day survival % (95% CI) |
91.9 (76.9‐97.3) | 90.0 (75.5‐96.1) | 0.7830 |
3.4. Pediatric heart transplantation
There was no significant difference in the proportion of pediatric donors offered for heart transplantation, during the early pandemic period (6.4%), compared to the same period of 2019 (4%, P =.4827). There was an increase in the proportion of pediatric recipients transplanted during the early pandemic, 26% compared to 10% during the same period of 2019, although this did not reach statistical significance (P =.0772). Of the 2 UK pediatric heart transplant centers, 80% of the early pandemic period activity was focused in 1 center.
3.5. Mechanical circulatory support
Long‐term bridging implants fell by 46%, from 28 in March to May 2019 to 15 during the early pandemic period. There was a small increase in the number of short‐term bridging device implants, from 24 in 2019 to 26 in the early pandemic period, an increase of 8%. Mechanical circulatory support use, by type/indication, by month of the early pandemic period, compared to 2019, is shown in Table 5.
TABLE 5.
Monthly mechanical circulatory support, by device type/indication, during the early pandemic period compared to 1 March to 31 May 2019
| Mechanical circulatory support | Long‐term bridging implants 2020 (19) | Short‐term bridging implants 2020 (19) | Primary graft dysfunction (PGD) 2020 (19) | Total | % change | |||
|---|---|---|---|---|---|---|---|---|
| March | 4 | (10) | 11 | (7) | 4 | (2) | ||
| April | 5 | (11) | 5 | (5) | 1 | (3) | ||
| May | 5 | (7) | 10 | (12) | 1 | (1) | ||
| Total | 15 | (28) | 26 | (24) | 6 | (6) | 47 (58) | ‐19% |
3.6. The heart transplant waiting list
The number of new registrations to the heart transplant waiting list, by urgency, is shown in Table 6.
TABLE 6.
Monthly new registrations to the UK heart transplant waiting list during the early pandemic period, 1 March to 31 May 2020 and 2019
| New registrations to the heart transplant waiting list | Non‐urgent 2020 (19) | % change | Urgent 2020 (19) | % change | Super‐Urgent 2020 (19) | % change |
Total 2020 (19) |
% change | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| March | 6 | (16) | 16 | (13) | 5 | (3) | |||||
| April | 17 | (20) | 9 | (19) | 3 | (5) | |||||
| May | 7 | (12) | 7 | (21) | 4 | (0) | |||||
| Total | 30 | (48) | ‐38% | 32 | (53) | ‐45% | 12 | (8) | +50% | 74 (109) | ‐32% |
In survey responses, all but essential transplant assessments were suspended, in all centers, at the start of the early pandemic period. As centers moved through the phases of the pandemic, transplant assessment began, at reduced capacity, with reduced inpatient stay, to facilitate social distancing. One center (pediatric) reported the use of online platforms for virtual transplant assessment. This facilitated the re‐starting of transplant assessment, earlier. Respondents from this center indicated that they intend to continue this method of transplant assessment, reducing travel and hospital attendance for patients and families.
The outcome for patients on the heart transplant waiting list during the early pandemic period, compared to 2019, is shown in Table 7. Confirmed COVID‐19 infection was reported in 9 candidates during the study period.
TABLE 7.
Total number of active patients and outcome of the heart transplant waiting list 1 March to 31 May 2020, compared to 1 March to 31 May 2019
| Outcome on the UK heart transplant waiting list | Total number active on the transplant waiting list 2020 (19) |
Died 2020 (19) |
Removed 2020 (19) |
Transplanted 2020 (19) |
||||
|---|---|---|---|---|---|---|---|---|
| March | 336 | (288) | 3 | (1) | 6 | (8) | 18 | (19) |
| April | 344 | (306) | 2 | (0) | 4 | (2) | 12 | (12) |
| May | 346 | (314) | 0 | (4) | 2 | (9) | 8 | (10) |
| Total | 5 | (5) | 12 | (19) | 38 | (41) | ||
3.7. Recipient management
All centers reported that active efforts had been made to reduce hospital attendance for heart transplant recipients. At the time of the survey, in all cases, these changes were planned to continue. Face‐to‐face follow‐up, routine assessment, and investigations have been reduced to only those within the first 6 months of transplant in one center, and those within the first 12 months of transplant in the remaining 6 centers. All centers have implemented telephone consultation follow‐up, with outpatient clinic attendance only where clinical concerns have been identified. Where outpatient attendance has continued, measures have been implemented to decrease the number of patients in attendance, to reduce the use of waiting areas and implement social distancing measures. One center reported the use of online video conferencing platforms to conduct recipient follow‐up.
All centers stated that no changes were made to immunosuppression therapy in long‐term heart transplant recipients, who remained well. Where COVID‐19 infection was suspected or confirmed, all centers reported cessation, or dose reduction, of Mycophenolate Mofetil (MMF) or Azathioprine, on an individual patient basis.
3.8. COVID‐19 in heart transplant recipients
Twenty‐seven heart transplant recipients with confirmed COVID‐19 infection were reported, during the study period. Median (IQR) age of COVID‐19‐positive recipients is 51 years (24‐61). Mean (SD) time from transplant was 8 (8.2) years. One COVID‐19‐positive recipient was transplanted during the early pandemic period. For this patient, pre‐operative COVID‐19 testing of the donor and candidate was negative, and the recipient had been discharged following post‐operative recovery, re‐presenting to hospital 54‐days later, with symptoms of COVID‐19. Outcome data are available for 18 of 27 patients, with 7 deaths reported.
4. DISCUSSION
Heart transplant activity has continued during the early period of the COVID‐19 pandemic in the UK, facilitated by the proportion of urgent and super‐urgent candidates requiring transplantation during this period, favorable changes to donor demographics, as a result of national policy for organ donation during this time, and an increase in utilization. At the beginning of the early pandemic period, national guidance placed restrictions on donor age, limiting DBD to age less than 60 years. Pre‐COVID, the upper age limit for DBD heart donors in the UK was 65 years. These restrictions were gradually lifted between April and June 2020 and have not been re‐implemented as the pandemic has continued. As a result, the number of deceased donors from whom the heart was offered for transplantation fell by 31%, mirroring the experience of other solid‐organ transplant programs globally. Donors offered for heart transplantation in the UK during the pandemic were significantly younger and not hypertensive, compared to those offered during the same period of 2019. With this, the number of donors utilized for heart transplant, as a proportion of those offered, increased from 26% March to May 2019 to 35% during the early pandemic period. In pre‐pandemic practice, between 2015 and 2018, the UK heart utilization has sat around 19% each year. Heart transplant activity has been well maintained, across all centers, during this early pandemic period, compared to the same period of 2019. Small month‐to‐month and year‐to‐year variation in transplant numbers is normally seen, and between 2015 and 2018, on average, 200 heart transplants have been performed per year, or 15 to 20 per month.
In survey responses, staff reported confidence, which increased throughout the early pandemic period that where deceased donors were offered for heart transplantation, the risk of COVID‐19 in the donor was low. This was facilitated by the development of national policy for donor screening and testing, candidate testing, and donor management practice. 11 Potential donors are epidemiologically screened for history of symptoms, positive test or contact with a positive, and/or symptomatic person. PCR testing for SARS‐CoV‐2 is performed in all donors using nose and throat swab, as well as endotracheal aspirate or bronchoalveolar lavage (BAL) specimens. Potential recipient screening is performed in the same way. Where possible, potential donors are transferred to COVID‐light areas within the hospital. COVID‐light describes the physical separation of COVID‐19‐positive and ‐negative patients within designated areas within hospitals or for an entire hospital, function as an NHS network, 10 and facilitates safe continuation of healthcare service for non‐COVID‐related disease.
As yet, there is no published evidence of transmission of COVID‐19 from a donor to a recipient, although the number of transplants performed from COVID‐19‐positive donors remains low. The first single case report of a COVID‐19‐positive donor successfully resulting in lung transplantation has been published, 12 and, as the pandemic continues, and the number of people within the general population who have previously tested positive for COVID‐19 increases, new questions, including time since exposure and confidence in viral testing, will need to be answered, to ensure safe and on‐going utilization of donors. National guidance in the UK will no doubt to evolve as the pandemic continues. Currently donors who have previously tested positive and recovered (with a period of 28 days from full clinical recovery) may be considered for organ donation, with liaison with microbiology/virology.
Within all healthcare practice in the UK at this time, concerns regarding a surge in COVID‐19 cases, and the subsequent demands on ICU capacity and staff resource, lead to local decision‐making surrounding the continuation of non‐essential services. This included elective and non‐urgent cardiothoracic surgical activity, in line with national guidance for the temporary suspension of non‐essential services throughout the NHS. In UK heart transplant practice, a significant proportion of candidates are listed for urgent or super‐urgent allocation. Over the last 5 years, urgent and super‐urgent heart transplant candidates have made up approximately 12% of the total heart transplant waiting list and 70%‐80% of the annual heart transplant activity. 13 During the early pandemic period, the proportion of activity represented by recipient urgency has remained un‐changed. As the majority of heart transplant activity is performed for candidates in the urgent inpatient allocation category, the balance of risk was shifted in favor of transplantation, to a greater degree than that experienced within some other solid‐organ transplant programs, including lung transplantation.
In considering the risks to urgent heart transplant candidates of remaining on the waiting list versus transplant during this time, individual clinicians, including those in ICU, were able to make decisions regarding their capacity at the time of organ offering. Transplant clinicians balanced the risks to the candidate of remaining on the waiting list versus nosocomial COVID‐19 infection, on an individual candidate basis. This facilitated the continuation of activity and all UK adult heart transplant centers continued activity despite noted variation in the geographic spread of the virus and additional burden on ICU and staff capacity seen in some centers.
Pediatric heart transplant activity represents around 30 transplants per year in the UK, or 2.6 transplants per month. During the early pandemic period, heart transplant activity included 10 pediatric recipients over the 3‐month period, a borderline significant increase on 2019, and higher than the monthly activity averages over the last 5 years. At the same time, the median age of donors decreased (to 40 years) but there was no change in the proportion of pediatric donors. The apparent driver for the increase in pediatric heart transplant activity was the availability of pediatric units to continue transplantation at a time when adult transplant centers were stretched due to COVID‐19 cases. Pediatric heart transplant centers (2 in the UK) were relatively better protected from COVID‐19, a combination of prevalence of the disease in the hospital, but also environment, facilities and ability to protect some ICU capacity and working areas from COVID.
With the maintained heart transplant activity, there was little change in waiting list outcomes during the early pandemic period. A reduction in the number of long‐term bridging mechanical circulatory support devices is noted. The number of new registrations to the waiting list did fall and similar trends, with reduced waiting list additions, have been reported in the USA. 7 Transplant assessment for routine patients was paused during the early pandemic period and, as described in the survey responses, was recommencing in May/June 2020 with reduced capacity, reduced inpatient stay and in some cases, increased use of online platforms, to facilitate social distancing and reduced face‐to‐face contact. One pediatric heart transplant center reported the positive impact of this change in practice, with reduced travel and hospital attendance for patients and families. Continued investment and development in technology to facilitate consultations provide an area for quality improvement.
Cardiovascular manifestations of COVID‐19 have been reported, 6 and concerns that the risk of contracting the disease, and experiencing the more severe form of illness in the immune‐compromised recipient population, have been raised. Analysis of COVID‐19 in the UK solid‐organ recipient population has been published 14 and, more specifically, in a small series of heart transplant recipients. 15 , 16 A relatively small number of heart recipients with COVID‐19 were reported to UKTR during the study period, and information on patient outcomes is limited. The number of deaths reported here, in the COVID‐19 recipient group, is high; however, we use caution in drawing conclusions from this, due to the reliability of reporting patient numbers and outcomes. In addition, the small number of positive recipients may be, in part, due to strict shielding advice given to solid‐organ recipients in the UK and there is some published evidence of strong adherence to isolation measures for thoracic transplant recipients during the pandemic. 17 . Understanding the risk factors, clinical characteristics and severity of COVID‐19 in this patient population are of fundamental importance, but cannot be addressed by this study. Further work is required to better understand COVID‐19, clinical course, and management in the solid‐organ transplant population.
All UK heart transplant centers have successfully adapted their programs to overcome the challenges of staff sickness, redeployment, and resource limitation, associated with the SARS‐CoV‐2 pandemic, successfully maintaining activity, during this time. Changes to outpatient follow‐up and monitoring have been reported in the survey and mirror those adopted in other transplant programs. 18 The positive impact of some of these changes has been noted and measures to maintain social distancing, reduced hospital attendance, and technology‐enhanced communication are continuing.
This early pandemic period has been followed by a second wave of COVID‐19 cases and it is increasingly evident that the SARS‐CoV‐2 pandemic will continue, with lasting effects on health and social care and the economy. As the number of COVID‐19 cases again declines, it is important to consider the backlog of elective and non‐urgent patients, and the subsequent pressure this will inflict on the healthcare service. To facilitate on‐going transplant activity, some protection must be offered to our ICU and staff resource. The approval of the first vaccine against the virus will, it is hoped, protect patients, their families, and healthcare workers, from the virus. Delivery of the vaccine to vulnerable groups, including those on the solid‐organ transplant waiting list, will allow these patients to remain active candidates. Immunosuppressed patients have not been included in vaccine trials and their immune response to the vaccine remains uncertain. What we have learnt during the first wave of the pandemic will, it is hoped, enable us to respond to subsequent waves. Continually reviewing and evaluating practice during this time is fundamental in ensuring the safe continuation of heart transplantation.
AUTHOR CONTRIBUTIONS
Gillian Hardman involved in concept/design, data analysis, data interpretation, and drafting article. Ruth Sutcliffe involved in concept/design, data collection, and approval of article. Rachel Hogg and Lisa Mumford involved in data analysis and statistics. Laura Grocott Lorraine Jerrett, Sarah‐Jane Mead‐Regan, Jane Nuttall, Stephanie Dunn, Philip, and Richard Quigley Seeley involved in data collection and approval of article. Stephen Clark approved the article. Johnathan R Dalzell critically reviewed the article. Nawwar Al‐Attar, Jayan Parameshwar, Andrew J Fisher, Karen Booth, and John H Dark involved in design, data interpretation, and critical revision of article.
Hardman G, Sutcliffe R, Hogg R, et al. Heart transplantation in the UK during the first wave of the SARS‐CoV‐2 pandemic. Clin Transplant. 2021;35:e14261. 10.1111/ctr.14261
On behalf of the NHS Blood and Transplant Cardiothoracic Advisory Group Clinical Audit Group
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. UK Government . Coronavirus (COVID‐19) in th UK. 2020. [Available from: https://coronavirus.data.gov.uk/cases. [accessed May 5, 2020]
- 2. UK Government . Coronavirus (COVID‐19): 23 March 2020 2020 [Available from: https://www.gov.uk/government/speeches/pm‐address‐to‐the‐nation‐on‐coronavirus‐23‐march‐2020. [accessed May 10, 2020]
- 3. UK Government . Coronavirus (COVID‐19) in the UK 2020 [Available from: https://coronavirus.data.gov.uk/details/deaths.[accessed December 10, 2020]
- 4. NHSBT ODT Clinical . COVID‐19: Advice for Clinicians 2020 [Available from: https://www.odt.nhs.uk/covid‐19‐advice‐for‐clinicians/. [accessed May 10, 2020]
- 5. Lima B, Gibson GT, Vullaganti S, et al. COVID‐19 in recent heart transplant recipients: Clinicopathologic features and early outcomes. Transpl Infect Dis. 2020:e13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS‐CoV‐2 Infection and Cardiovascular Disease: COVID‐19 Heart. Heart Lung Circ. 2020;29(7):973‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFilippis EM, Sinnenberg L, Reza N, et al. Trends in US Heart Transplant Waitlist Activity and Volume During the Coronavirus Disease 2019 (COVID‐19) Pandemic. JAMA Cardiol. 2020;5(9):1048‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeFilippis EM, Farr MA, Givertz MM. Challenges in Heart Transplantation in the Era of COVID‐19. Circulation. 2020;141(25):2048‐2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NHS Improvement . Coronavirus Specialty guides ‐ management of Cardiothoracic surgery patients 2020 [Available from: https://www.england.nhs.uk/coronavirus/wp‐content/uploads/sites/52/2020/03/specialty‐guide‐cardiothoracic‐surgery‐v1‐20‐march‐2020.pdf. [accessed May 5, 2020]
- 10. Royal Colege of Surgeons of England . Elective surgery during COVID‐19 2020 [Available from: https://www.rcseng.ac.uk/news‐and‐events/news/archive/survey‐results‐elective‐surgery‐under‐covid/. [accessed December 10, 2020]
- 11. NHSBT ODT clinical . POL304/2 ‐ SARS‐CoV‐2 Assessment and screening in Organ Donors and Recipients 2020 [Available from: https://nhsbtdbe.blob.core.windows.net/umbraco‐assets‐corp/20342/pol304.pdf. [accessed October 30, 2020]
- 12. Ceulemans LJ, Van Slambrouck J, De Leyn P, et al. Successful double‐lung transplantation from a donor previously infected with SARS‐CoV‐2. The Lancet Respiratory Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NHSBT ODT clinical . Annual Report on Cardiothoracic Transplantation 2018/19 (1 April 2009‐31 March 2019). 2019. [acccessed November 15, 2020]
- 14. Ravanan R, Callaghan CJ, Mumford L, et al. SARS‐CoV‐2 infection and early mortality of wait‐listed and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Latif F, Farr MA, Clerkin KJ, et al. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivinius R, Kaya Z, Schramm R, et al. COVID‐19 among heart transplant recipients in Germany: a multicenter survey. Clin Res Cardiol. 2020;1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett D, De Vita E, Ventura V, et al. Impact of SARS‐CoV‐2 outbreak on heart and lung transplant: A patient‐perspective survey. Transpl Infect Dis. 2020:e13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIlvennan CK, Allen LA, Devore AD, Granger CB, Kaltenbach LA, Granger BB. Changes in Care Delivery for Patients With Heart Failure During the COVID‐19 Pandemic: Results of a Multicenter Survey. J Card Fail. 2020;26(7):635‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


