Abstract
Background
Coronavirus disease 2019 (COVID‐19) is associated with gastrointestinal and hepatic manifestation in up to one fifth of patients. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the etiologic agent of COVID‐19, infects gastrointestinal epithelial cells expressing angiotensin‐converting enzyme 2 (ACE2) receptors triggering a cascade of events leading to mucosal and systemic inflammation. Symptomatic patients display changes in gut microbiota composition and function which may contribute to intestinal barrier dysfunction and immune activation. Evidence suggests that SARS‐CoV‐2 infection and related mucosal inflammation impact on the function of the enteric nervous system and the activation of sensory fibers conveying information to the central nervous system, which, may at least in part, contribute symptom generation such as vomiting and diarrhea described in COVID‐19. Liver and pancreas dysfunctions have also been described as non‐respiratory complications of COVID‐19 and add further emphasis to the common view of SARS‐CoV‐2 infection as a systemic disease with multiorgan involvement.
Purpose
The aim of this review was to highlight the current knowledge on the pathophysiology of gastrointestinal SARS‐CoV‐2 infection, including the crosstalk with the gut microbiota, the fecal‐oral route of virus transmission, and the potential interaction of the virus with the enteric nervous system. We also review the current available data on gastrointestinal and liver manifestations, management, and outcomes of patients with COVID‐19.
Keywords: ACE2, COVID‐19, diarrhea, enteric nervous system, gastrointestinal, gut microbiota, pandemic, SARS‐CoV‐2
SARS‐CoV‐2 infects the gastrointestinal tract leading to a cascade of events including dysbiosis, enterocyte damage and inflammation, immune dysregulation, and enteric nervous system dysfunction possibly involved in GI symptom development.
Key Points.
SARS‐CoV‐2 is able to infect gastrointestinal tract and liver leading to cell damage and inflammation.
Several data support the hypothesis of a fecal‐oral route of SARS‐CoV‐2 transmission, but this still remains unproven.
Dysbiosis described in COVID‐19 patients may enhance inflammatory response and cytokine storm.
Data suggest that the enteric nervous system may be affected either directly or indirectly by SARS‐CoV‐2 leading to gut dysfunction.
Diarrhea and dysgeusia are the most reported gastrointestinal manifestations of COVID‐19.
No specific therapies have been investigated for gastrointestinal and hepato‐biliopancreatic manifestations, which are self‐limiting.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) initially described at the beginning of December 2019 in Wuhan, Hubei Province of China, has spread all over the world. 1 As of September 28, 2020, the World Health Organization reported more than 32 million cases and 995 thousands deaths from 235 countries related to this pandemic. 2 The clinical course of SARS‐CoV‐2 infection can span a wide range from asymptomatic to a rapidly progressing and life‐threatening disease, most commonly associated with a variety of symptoms, such as fever, cough, dyspnea, pneumonia, acute respiratory distress syndrome, systemic inflammatory response syndrome, and multiple organ failure. 3 Like many other coronaviruses, SARS‐CoV‐2 infects the gastrointestinal tract. 4 , 5 Accordingly, in COVID‐19 patients, beside respiratory manifestations, some patients complain of symptoms originating from the gastrointestinal tract, including nausea, vomiting, abdominal pain, and diarrhea. 1 Although the preferential route of infection of SARS‐CoV‐2 is through exhaled droplets, increasing evidence suggests that SARS‐CoV‐2 may be also transmitted by a fecal‐oral route. Taken together, this evidence provides a rational basis for interpreting the common occurrence of gastrointestinal symptoms reported by COVID‐19 infected patients. 6 , 7 We aimed at summarizing the current evidence on the pathophysiology of gastrointestinal SARS‐CoV‐2 infection, fecal‐oral route of virus transmission, the involvement of the enteric nervous system, clinical manifestations, treatments, and outcomes of patients with COVID‐19.
2. SARS‐COV‐2 AND THE GASTROINTESTINAL TRACT
2.1. Gastrointestinal life cycle
SARS‐CoV‐2 is a novel single‐stranded β‐coronavirus, the seventh coronavirus so far described infecting humans, with a genome similarity up to 80% to other highly infective coronaviruses like those of the acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV). 8 SARS‐CoV‐2 interacts with the host through its envelope spike glycoprotein 9 which binds the ACE2 receptor of the host (Figure 1A). The spike glycoprotein is composed of two subunits, namely S1 and S2, which favor, respectively, the binding of the virus to the cells and the fusion between the two cellular membranes. 10 This process is independent from the activity of the ACE enzyme since SARS‐CoV‐2 shows a high binding affinity to ACE2 receptors, reported to be comparable to that of SARS‐CoV. 11 , 12 After viral binding to ACE2 receptors, the transmembrane protease serine (TMPRSS)2 mediates the cleavage of spike glycoprotein, regulating the virus internalization into the host cell. 7 After internalization, the virus starts its replication using the cellular replication processes, which ends with new viral assemblies, viral secretion, and release of cytokine which contribute to symptom generation. 13
FIGURE 1.
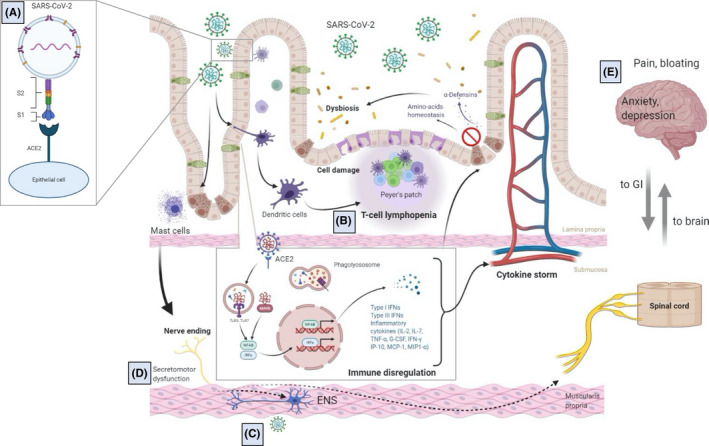
Schematic representation of the putative interplay between SARS‐CoV‐2 and the gastrointestinal tract. (A) SARS‐CoV‐2 enters epithelial cells through ACE2 receptors with the participation of TMMPRSS2. (B) Virus‐related epithelial damage is associated with increased intestinal permeability, mucosal immune dysregulation, tissue inflammation (eg, mast cell activation, T‐cell lymphopenia, cytokine release), and dysbiosis. (C) A potential direct SARS‐CoV‐2 infection of enteric and afferent nerves and/or inflammation may contribute to (D) secretomotor dysfunction and (E) symptom perception
ACE2 receptors have been reported to be highly expressed in several organs of the human body beyond the lungs, such as endothelial cells, renal tubular epithelium, testes, kidneys, brain, heart, and liver. 7 However, the highest expression of ACE2 in the human body occurs in the brush border of intestinal enterocytes. 14 , 15 ACE2 receptors and TMPRSS2 are abundantly expressed in gastric and intestinal epithelial cells and on the cilia of glandular epithelial cells, but not in esophageal squamous epithelial cells. 16 , 17 In addition, it appeared that SARS‐CoV‐2 was not able to infect goblet cells across culture conditions. 18 In other seminal experiments, human or bat intestinal tissues 19 exposed to nasopharyngeal secretions obtained from COVID‐19 patients were associated with rapid virus replication and a cytopathic response. Viral nucleocapsid proteins have been detected in the cytoplasm of gastric, duodenal, and rectal cells, but not in esophageal cells from a COVID‐19–infected patient with SARS‐CoV‐2 fecal shedding. 16 The affinity of SARS‐CoV‐2 for the gastrointestinal tract is highlighted by the fact that between 10% and 20% of COVID‐19 patients experience diarrhea as their first symptom before the onset of respiratory symptoms. 20 , 21 Furthermore, in a series of COVID‐19 patients from the United States, 48 out of 206 patients presented digestive symptoms alone without signs of systemic or respiratory involvement. 21 Taken together, these results support the hypothesis that SARS‐COV‐2 can infect and damage human gastrointestinal epithelial cells in vivo and that the gastrointestinal tract could be the primary site of SARS‐CoV‐2 infection in a subset of patients. 22
2.2. Fecal‐oral route of transmission
COVID‐19 is an infection with a predominant airborne route of transmission through salivary droplets. 3 Nonetheless, the possibility that SARS‐CoV‐2 could be transmitted via a fecal‐oral route was hypothesized early on after the description of the first cases of COVID‐19 reported in visitors of the Seafood Market in Wuhan. 1 Accordingly, it was hypothesized that SARS‐CoV‐2 gained access via the gastrointestinal tract and subsequently infected the organism following the consumption of meat of illegally traded bats and pangolins. In enterocyte organoids infected with SARS‐CoV‐2, the virus was primarily secreted apically. 18 If the same occurs in vivo, the virus cloud be excreted in the lumen of the intestine and eliminated with the feces. In support of the fecal‐oral route of transmission of SARS‐CoV‐2 and in line with the abovementioned gastrointestinal involvement in COVID‐19, viral RNA was found in the stool of up to 50% of patients with 23 at concentrations ranging from 103 to 105 copies/mL, up to 12 days from the initial assessment and even after nasopharyngeal swab became negative. 16 Several other reports described the detection of SARS‐CoV‐2 RNA in stool samples. 22 , 24 A comprehensive meta‐analysis 25 included 95 studies and 2149 patients. The authors 25 found that 934 patients (43%) had one or more SARS‐CoV‐2–positive sampling sites (stool or anal swab). Although viral RNA was detected in a mean of 25 days after symptom onset, patients may show fecal shedding up to 70 days after symptom onset, 26 even after viral clearance from the respiratory tract 16 and respiratory symptoms disappearance. 27
Taken together, this evidence suggests that a fecal‐oral route of viral transmission is plausible; however, there are still some concerns. First, the detection of SARS‐CoV‐2 in the feces is based on RT‐PCR techniques that may not be able to distinguish between viral fragments and a viable replicating virus. Indeed, while SARS‐CoV‐2 virus with infective potential was isolated from lungs or throat of COVID‐19 patients, in the feces they were either not found 28 or isolated only in a small proportion of patients (35%). 25 Second, even if SARS‐CoV‐2 RNA has been detected in gastrointestinal specimens from most patients with digestive symptoms, this association was not statistically significant. 25
2.3. The enteric nervous system as a potential entry route of SARS‐CoV‐2
Growing evidence indicates that SARS‐CoV2 infection is associated with neurological symptoms in a subgroup of patients with COVID‐19 and that neurological involvement can aggravate the course of the disease. 29 , 30 In both animal studies and in patients with neurological symptoms, coronaviruses show the ability to penetrate the cerebrospinal fluid 31 and damage the structure and function of the nervous system. 32 The mechanisms through which SARS‐CoV2 enters the central nervous system remain unknown. 33 The most plausible route of invasion is through the blood‐brain barrier. 31 Alternatively, it has been suggested that coronaviruses can migrate to the brain through sensory or motor nerve endings, achieving retrograde or anterograde neuronal transport through dynein and kinesin motor proteins. 34 Recently, Esposito et al 35 suggested that the enteric nervous system (ENS) could act as an entry route of SARS‐CoV‐2 to the brain and the virus would gain access to the brain via vagal and/or splanchnic nerves. A comparable mechanism of neurogenic transmission to the CNS was previously shown for herpes 36 and influenza viruses. 37 Previous reports showed that gastrointestinal inoculation of MERS‐CoV in mice, another β‐coronavirus sharing similarity with SARS‐CoV‐2, was associated with brain infection. 38 A recent histochemical study on small and large intestinal specimens and choroid plexus, and adjacent brain parenchyma obtained post‐mortem in COVID‐19 patients, supports the anatomical plausibility for SARS‐CoV‐2 neuro‐invasion through the ENS. 39 Indeed, ACE2 and TMPRSS2 were abundantly expressed in the perikarya of enteric neurons and glial cells, both in the myenteric and submucous plexus. Enteric neurons showed different levels of ACE2 staining intensity, suggesting a differential expression between neuronal subtypes (Figure 2).
FIGURE 2.
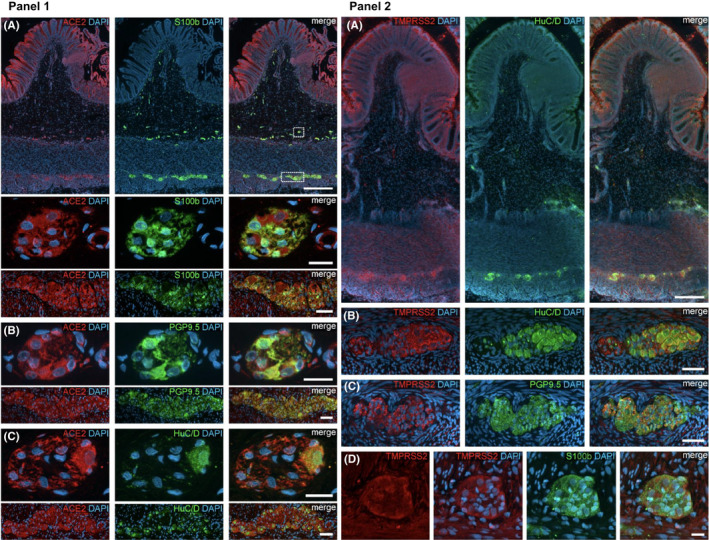
Panel 1: ACE2 expression in the human ENS of the large intestine. (A) Overview of the entire gut wall of a colon segment with immunofluorescence stainings for ACE2 (red), the glial marker S100b (green), and with the nuclear marker DAPI (blue). The white rectangles indicate the location of the high‐power magnification micrographs below showing a representative submucous and myenteric ganglion. (B, C) Show representative submucous and myenteric ganglia stained for ACE2 (red), DAPI (blue), and the neuronal markers PGP9.5 (B, red) or HuC/D (C, red). The ACE2 staining can be found in neurons and glial cells and is considerably stronger in the colon compared to the small intestine. The overview is a standard epifluorescence image; details are maximum intensity projections of optical sections by structured illumination. Scale bars: overview 250 mm; details 50 mm. Panel 2: TMPRSS2 expression in the human ENS. (A) Overview of the entire gut wall of a colon segment with immunofluorescence stainings for TMPRSS2 (red), the neuronal marker HuC/D (green), and the nuclear marker DAPI (blue). (B, C) Show representative large intestinal myenteric ganglia stained for TMPRSS2 (red), DAPI (blue), and the neuronal markers HuC/D (B, red) or PGP9.5 (C, red). (D) Representative myenteric ganglion in the small intestine stained for TMPRSS2 (red), the glial marker S100b (green), and the nuclear marker DAPI (blue). Note that TMPRSS2 stainings were markedly stronger in enteric ganglia in the colon (A–C) than in the small intestine (D). The overview is a standard epifluorescence image; details are maximum intensity projections of optical sections by structured illumination. Scale bars: (A) 250 mm; (B–D) 50 mm. Figure adapted with permission from Deffner F et al. Front Neuroanat 2020; 14:596439; Copyright © 2020 Deffner, Scharr, Klingenstein, Klingenstein, Milazzo, Scherer, Wagner, Hirt, Mack and Neckel
3. PATHOGENESIS
3.1. Epithelial cell damage
Intestinal mucosal biopsies obtained during endoscopy from one COVID‐19 symptomatic patient revealed normal macroscopic findings, except for mild lymphocyte and plasma cell infiltration and interstitial edema. 16 If confirmed in larger series, this evidence would suggest that SARS‐COV‐2 infection is not associated with gross pathology detectable with routine diagnostic techniques but may require more sophisticated assessment of tissue damage and dysfunction. A direct consequence of SARS‐CoV‐2 infection may be the reduction in the epithelial cell functional mass. In line with this, epithelial cell damage has been shown in both bat and human enteroids which developed progressive cytopathic effect after SARS‐CoV‐2 inoculation. 19 In addition, Uzzan et al, 40 assessed plasma concentrations of the amino acid citrulline, a surrogate marker of enterocyte mass and function. 41 Compared to COVID‐19 patients without gastrointestinal symptoms, those with symptoms (ie, nausea, vomiting, and loss of appetite) had lower plasma citrulline levels and low plasma citrulline was inversely correlated with inflammatory markers, including C‐reactive protein and ferritin. 41
3.2. Inflammation
SARS‐CoV‐2 infection is associated with innate and adaptive immune cell responses in the infected host. 42 These include the release of interleukin (IL)‐2, IL‐7, tumor necrosis factor (ΤΝF)‐α, macrophage and monocyte products, such as granulocyte colony‐stimulating factor, interferon (IFN)‐γ‐inducible protein 10, monocyte chemoattractant protein 1, and macrophage inflammatory protein 1‐α. 43 In the intestinal tract, this may lead to tissue inflammation, malabsorption, and diarrhea 44 (Figure 1). To date, there are limited data on gastrointestinal inflammation in COVID‐19 patients. However, cytokine production at this level is plausible since SARS‐CoV‐2 infection of enterocyte organoids engages a strong IFN response along with a milder induction of IP‐10/CXCL10 and other cytokine genes. 18 , 45 In addition, the finding of increased fecal calprotectin levels in COVID‐19 patients provides indirect evidence of gut inflammation. 46 , 47 Effenberger et al 47 reported higher concentrations of fecal calprotectin in patients with diarrhea compared to those with previous diarrhea or without this manifestation. Interestingly, fecal calprotectin concentrations significantly correlated with serum interleukin 6, suggesting that gastrointestinal involvement in SARS‐CoV‐2 may contribute to systemic inflammation. 47 However, the same authors 47 found that gastrointestinal inflammation did not correlate with SARS‐CoV‐2 RNA stool shedding, thus weakening the hypothesis of direct cause‐effect mechanism.
3.3. Enteric nervous system dysfunction
Evidence is accumulating to support the biological plausibility for a direct or indirect involvement of the ENS in SARS‐CoV‐2 infection. Indeed, coronaviruses have a strong neuro‐invasive potential as shown in previous studies after the outbreak of SARS‐CoV‐1. These studies showed that viral particles could be detected in the brain, where they were located almost exclusively in neurons. 48 It has been suggested that SARS‐CoV‐2 infection of the central nervous system could occur via neuronal, pericellular, hematogenous, lymphatic, and Trojan routes (infecting migrating leukocytes). Studies in human brain organoids showed that SARS‐CoV‐2 infects neuronal cells within 2 days of exposure. In addition, SARS‐CoV‐2 exposure altered the distribution of tau from axons to soma, hyperphosphorylation, and apparent neuronal death. 49 Given the ability of SARS‐CoV‐2 to infect the gastrointestinal tract, the abundant neural network supplying the alimentary canal, and the fact that both ACE2 and TMPRSS2 are abundantly expressed in the enteric nerves (Figure 2), 39 the possibility of a ENS neuro‐invasion, dysfunction, and damage should be of great concern.
In addition to a putative direct effect of the virus on enteric nerves, inflammatory and immune activation in the intestine may cause alterations in the ENS, enteroglial cells, and intestinal smooth muscle 35 , 50 which may be involved in symptom generation. 51 Concerning nausea, vomiting, and loss of appetite, several other hypotheses beyond the damage and inflammation of the gastrointestinal tract have been postulated such as the presence of the virus in the dorsal vagal complex and in the area postrema which may elicit symptoms at early stages of the infection. 52 The activation of ENS reflexes and secretomotor responses may be viewed as a defense mechanism to expel the pathogen. However, like in many other gastrointestinal infections, the price to be paid for this is represented by symptom development and eventually long‐lasting derangements of gut sensory‐motor functions in susceptible individuals. 53 , 54
3.4. ACE2 receptors
In addition to the well‐known activity of ACE2 in the renin‐angiotensin system (RAS), 55 this enzyme is also involved in the regulation of intestinal amino acid homeostasis and the expression of antimicrobial peptides which may contribute to the regulation of gut microbiota. The dietary amino acid tryptophan is able to modulate ACE2 function. 55 In laboratory animals, anorexia and malnutrition and reduction in tryptophan intake correlated to ACE2 dysfunction leading to altered expression of gut antimicrobial peptides, followed by gut dysbiosis and intestinal inflammation. 55 Accordingly, ACE2 knockout mice display increased susceptibility to intestinal inflammation induced by epithelial cell damage. 55 As ACE2 receptor downregulation has been reported in previous SARS coronavirus‐induced lung injury, 56 ACE2 dysfunction has been postulated to participate to the development of COVID‐19–related gastrointestinal symptom generation.
3.5. Altered gut microbiota
SARS‐CoV‐2 has been shown to be associated with an altered microbial community, 57 , 58 which in turn could participate in the COVID‐19 systemic inflammatory response and cytokine storm. 24 Moreover, ACE2 downregulation by SARS‐CoV‐2 59 may produce itself changes in gut microbiota since this receptor normally acts as regulator of immunity. Previous data in patients with influenza showed changes in gut microbiota, which in turn reduced host immune response leading to greater lung damage. 60 Changes in gut microbiota composition and the potential benefit of microbiota modulation in COVID‐19 have been recently investigated. 61 An early report from Hong Kong 62 compared the fecal microbiota of 15 patients with COVID‐19 with that of 6 patients with community‐acquired pneumonia and 15 healthy individuals. The results showed enrichment of opportunistic pathogens in COVID‐19 (ie, Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii) along with depletion in commensals (ie, Eubacterium, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae taxa). 62 A subsequent study 63 evaluated gut microbiota composition of patients with active replication of SARS‐CoV‐2. 64 Fecal samples associated with signatures of high SARS‐CoV‐2 infectivity showed enrichment of Collinsella aerofaciens, Collinsella tanakaei, Streptococcus infantis, and Morganella morganii, which have been previously linked to opportunistic infections. 65 A further study 66 investigating gut microbiota of COVID‐19 patients found an enrichment in Streptococcus, Rothia, Veillonella, Actinomyces, and Erysipelatoclostridium, and all these genera, except the latter, were correlated with C‐reactive protein and D‐dimer levels suggesting a possible correlation between changes in fecal microbiota and systemic inflammation. 66 Although all these studies suffer from small sample size and lack of appropriate control groups, taken together, these results suggest the presence of an altered gut microbial community in patients with SARS‐CoV‐2 infection susceptibility and its association with gastrointestinal and systemic inflammation in COVID‐19.
3.6. Liver injury
A possible pathogenic mechanism explaining hepatobiliary manifestations is represented by the entrance of the virus into the hepatocyte mediated by ACE2 receptor, which is expressed in the liver, causing SARS‐CoV‐2–mediated immunologic injury. 7 Indeed, a number of other mechanisms have been called into question, namely hypoxic injury as a consequence of respiratory failure, the systemic inflammatory response (ie, cytokine storm), 67 the exacerbation of a pre‐existent liver disease, and the injury caused by the drugs used for treating the infection and its manifestations (eg, antiviral therapies, antibiotics, monoclonal antibodies, acetaminophen). 68 Some insights into the mechanisms leading to liver damage derive from studies focusing on post‐mortem histopathological alterations of the liver. 69 , 70 More in depth, liver findings were described in a series of 40 autopsy cases from the United States. 69 Histologically, macro‐vesicular steatosis, mild acute hepatitis, and minimal‐to‐mild portal inflammation were the most common findings. Viral PCR was detected in 11/20 (50%) patients, even if at very low levels in most cases, and its presence was not associated with ALT levels. Taking all these data together, viral‐mediated injury seems to be the most plausible mechanism of liver damage.
4. CLINICAL FEATURES
4.1. Common gastrointestinal manifestations
Beside the respiratory and systemic manifestation of COVID‐19, such as fever, dyspnea, cough, pneumonia, fatigue, headache, rhinorrhea, anosmia, and dysgeusia, symptoms involving the gastrointestinal tract have been also widely described (Figure 3). 1 , 3 , 71 Among these symptoms, diarrhea is the most commonly reported, however with a wide prevalence according the published literature ranging from 3% to 96%. 72 , 73 , 74 Dysgeusia has also been frequently described as an early and sometimes unique symptom of COVID‐19 with a prevalence ranging from 71% to 88.8%. 75 A recent meta‐analysis 76 including 78 studies with 12797 patients assessing the occurrence of gastrointestinal symptoms in COVID‐19 patients concluded that digestive symptoms are seen in up to 1 in 5 infected patients. Among digestive symptoms, the weighted pooled prevalence of diarrhea was 12.4% [95% confidence interval (CI), 8.2% to 17.1%], nausea and/or vomiting 9.0% (95% CI, 5.5% to 12.9%), anorexia 22.3% (95% CI, 11.2% to 34.6%), and abdominal pain 6.2% (95% CI, 2.6% to 10.3%). However, these data need further validation due to the high data heterogeneity, diverse study designs, methodology pitfalls, such as the absence of use of validated questionnaires or definitions for symptoms assessment, lack of controls and the lack of evaluation of previous gastrointestinal chronic disease or the influence of concomitant therapies with potential adverse events on the gastrointestinal tract. A previous metanalysis 77 that adjusted the results for pre‐existing gastrointestinal conditions, showed, as expected, a lower rate of digestive symptom occurrence. The adjusted reported pooled prevalence was 8.7% (95% CI, 5.4% to 13.9%) for diarrhea, 8.0% (95% CI, 3.0% to 19.8%) for anorexia, and 5.1% (95% CI, 2.3% to 11.0%) for nausea. Several metanalyses have reported a higher prevalence of gastrointestinal symptoms in severe vs. mild cases of COVID‐19, 77 , 78 that is, anorexia 31.4% vs. 14.9%, diarrhea 11.1% vs 5.5%, vomiting 5.1% vs 2.5%, and abdominal pain 8.1% vs 1.8%, suggesting that digestive symptoms severity and frequency raise in parallel with advanced stages of the disease.
FIGURE 3.
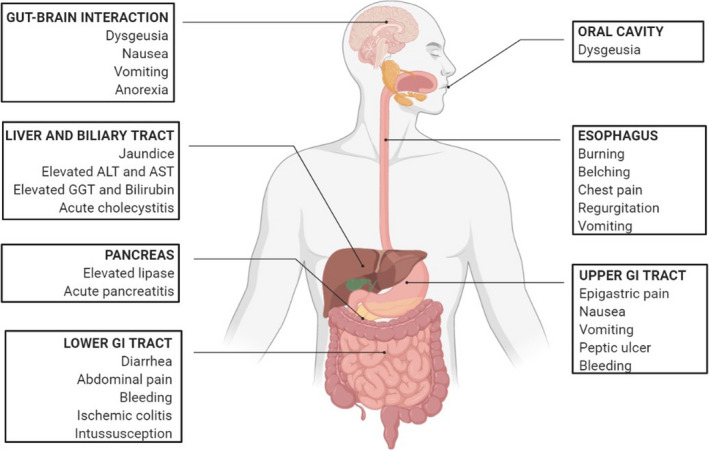
Gastrointestinal, hepatic, biliary, and pancreatic manifestation of COVID‐19
4.2. Uncommon gastrointestinal manifestations
Several case reports described a wide range of less frequent gastrointestinal manifestations. Among these, gastrointestinal bleeding has been described in several papers. 79 , 80 , 81 Little is known on the potential mechanisms involved. These may include inflammation‐induced coagulopathy and thrombo‐inflammation and a direct damage of the virus on the gastrointestinal mucosa. 82 However, since bleeding occurred mainly during hospitalization a multifactorial etiology has been postulated. A rather high prevalence of peptic ulcer disease complicated by bleeding was noticed in patients with a moderate‐to‐severe acute respiratory distress syndrome caused by COVID‐19. 83 , 84 Notably, most patients admitted to hospital were given thromboprophylaxis, which may represent an additional risk factor for bleeding. COVID‐19–induced coagulopathy associated with increased D‐dimer and fibrinogen levels may predispose to a high risk of micro‐ and macro‐circulatory thrombosis which may explain the occurrence of another complication, namely ischemic colitis. 85 It has been hypothesized that COVID‐19–associated immune activation in the gastrointestinal tract may lead to Peyer's patch hypertrophy and mesenteric lymphadenopathy, which can act as a primary point for intussusception, an event reported in case series of COVID‐19 patients. 86 Finally, disorders of gastrointestinal motor function up to severe motility derangement and pseudo‐obstruction have been reported in the critically‐ill COVID‐19 patient with high degree of systemic and intestinal inflammation. 87
4.3. Inflammatory bowel disease
Theoretically, patients with inflammatory bowel disease (IBD) may be more susceptible to SARS‐CoV‐2 infection due to the chronic intestinal inflammatory state and the use of immunosuppressant agents. 88 Previous reports showed that compared to controls, patients with IBD showed a sustained higher ACE2 expression in the mucosa of the ileum and colon and higher soluble circulating levels of ACE2, independently of the presence of inflammation, 55 , 89 possibly related to higher expression of IFN‐γ which promotes ACE2 expression. Moreover, trypsin‐like proteases, which are responsible of S protein cleavage and SARS‐CoV‐2 internalization, have been reported to be upregulated in IBD patients. 90 However, to date there is no evidence supporting an increased susceptibility to SARS‐CoV‐2 infection due to ACE2 and TMPRSS2 upregulation. 88 According to another hypothesis IBD patients could be protected from the infection due to higher levels of circulating ACE2 soluble receptors which bind SARS‐CoV‐2, thus competing with cell bindings and preventing or limiting the infection. 91 , 92 A large multicentric Western collaborative study reported a cumulative incidence of SARS‐CoV‐2 infection in patients with IBD of 0.4% (97 out of 23879), comparable to that of the general population (0.4%), 93 thus excluding an increased or reduced risk of COVID‐19 for these patients. The authors 93 also found that corticosteroids increased the risk of hospitalization [odds ratio (OR) 7.6], whereas monoclonal antibodies therapy reduced the risk of pneumonia and hospitalization (OR 0.1 and 0.3, respectively). Taken together, these data suggest that the risk of SARS‐CoV‐2 infection of patients with IBD seems comparable to that of the general population.
4.4. Celiac disease
Celiac disease, an autoimmune gluten‐related intestinal disease, is associated with increased risk of infections, including influenza 94 and pneumonia. 95 Based on this evidence, it could be speculated that celiac disease is associated with increased risk of COVID‐19. A recent a cross‐sectional large‐scale study showed that COVID‐19 patients do not have a significant difference in the odds of having a positive test for SARS‐CoV‐2 as compared with control subjects (9.4% vs. 8.1%; OR 1.18; 95% CI, 0.75–1.84). Furthermore, no differences in the odds of COVID‐19 were found in patients with or without histological confirmation of celiac disease, symptomatic or without symptoms, and adopting or not adopting gluten‐free diet. 96
Similar results were obtained from a recent real‐life study of a cohort of celiac disease patients during the SARS‐CoV‐2 outbreak in Italy. 97 All together, these data suggest that subjects with celiac disease are not associated with an increased risk of COVID‐19; however, longitudinal prospective studies are needed to better understand whether the risk of contracting COVID‐19 changes over time and additional precautions to prevent virus exposure are necessary in these subjects.
4.5. Hepatic manifestations
Liver impairment in patients with COVID‐19, defined by the alteration of blood liver enzymes, is a common finding, and it has been reported since the description of the first case series from China. 98 Liver test abnormalities (ie, altered transaminases and/or bilirubin) were found to be common in most reports, ranging from 16% to 53% of the series. 1 , 20 , 74 , 98 , 99 In most cases, transaminases were more commonly increased in patients with severe COVID‐19, especially those requiring admission to the intensive care unit. 1 Also, severe liver alteration was uncommon, and transaminase alterations were not necessarily associated with a worse outcome. 99 Later, a systematic review and meta‐analysis reported all published data from Asian populations until April 4, including a total of 1948 individuals. 100 The pooled prevalence of liver injury was 12% (18% when considering altered alanine aminotransferase [ALT]), while that of liver comorbidities was 3%. Also, patients displaying gastrointestinal symptoms were more likely to have liver injury, and this corroborates the possible spread of the virus from the gastrointestinal tract to the portal vein, until the liver. Finally, liver injury was more likely to occur in patients with severe COVID‐19, as previously hypothesized. 98 Later, more data regarding liver injury emerged (Table 1). 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 According to another retrospective series of 2273 patients who tested positive for SARS‐CoV‐2, transaminase alterations were common, but mild in most of the cases. 104 In a multivariable analysis, severe acute liver injury was significantly associated with increased blood inflammatory markers (ferritin and interleukin 6). Also, patients with severe liver injury showed higher rates of intensive care unit admission, acute kidney injury, and mortality.
TABLE 1.
Main studies reporting liver test abnormalities or liver injury in non‐Asian populations published since April 2020
| First author | Country | Total no. of patients | Definition of liver involvement | No. of patients (%) with liver involvement/injury | Summary of the main findings |
|---|---|---|---|---|---|
| Goyal et al 101 | USA | 375 | ALT >40 U/L | 120 (32) | Liver involvement more common in mechanically ventilated patients |
| Richardson et al 102 | USA | 5700 | ALT >60 U/L | 2176 (39) | Acute hepatic injury occurred in 56 cases, and this was associated with greater mortality |
| Singh et al 103 | USA | 2780 | ALT >50 U/L |
60 (46.1; with LD) 390 (50.6; no LD) |
250 patients (9%) had a pre‐existing liver disease, and this was associated with increased hospitalization and mortality |
| Phipps et al 104 | USA | 2273 | ALT >50 U/L | 537 (24) | Liver injury was mild in most cases; severe liver injury was uncommon but was associated with unfavorable outcomes (admission to the ICU, death) |
| Lenti et al 107 | Italy | 100 | ALT or GGT >50 U/L | 58/93 (62.4) | Greater mortality and need for ICU in patients with altered liver function tests who develop ARDS; patients with pre‐existing liver disease had no worse outcomes |
| Ponziani et al 108 | Italy | 515 | ALT or AST >45 U/L or GGT >61 U/L | 161 (31.3) | Liver involvement was mild in most cases, and was not associated with increased risk of mortality, but with the need for admission to the ICU; no cases of severe liver injury were reported |
| Schattenberg et al 106 | Germany | 44 | ALT >50 U/L | 6/38 (15.8%) | AST was more commonly increased compared to ALT; severe liver injury occurred in 9% of the cases; patients with pre‐existing liver disease had no worse outcomes |
| Medetalibeyoglu et al 105 | Turkey | 554 | ALT or AST >40 U/L | 153 (27.6) | Moderate‐to‐severe pneumonia and need for ICU admission more common in patients with altered transaminases |
Abbreviations: ALT, alanine aminotransferase; ARDS, acute severe respiratory distress syndrome; aspartate aminotransferase; AST; gamma‐glutamyl transpeptidase; GGT; ICU, intensive care unit; LD, liver disease.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4.5.1. COVID‐19 and pre‐existing liver disease
Data regarding the outcome of COVID‐19 in patients with a pre‐existing liver disease are still scant. In the largest studies from the United States focusing on this issue, 250/2780 patients (9%) with COVID‐19 were affected by a liver disease, and liver cirrhosis was reported in 50 patients. 103 The most commonly reported liver diseases were fatty liver disease and non‐alcoholic steatohepatitis. After propensity matching, the risk of death was increased (risk ratio 3.0) compared to patients with no known liver disease, as well as the risk of hospitalization. Given the observational nature of the study, the possible causes of this finding were not further investigated.
4.5.2. COVID‐19 in liver transplant patients
The magnitude of the impact of COVID‐19 in patients with a transplanted liver is yet to be clearly defined. According to a series from an Italian liver transplant center, three out of 111 transplanted patients died from severe COVID‐19. All of them were elderly male and were transplanted more than 10 years before. On the contrary, three out of 40 patients who had been recently transplanted and were on immunosuppressants seem to have developed a milder disease. Hence, Bhoori et al. 109 suggested not to withdraw immunosuppressants in these patients. According to a report from the United States, 110 out of 38 transplanted patients with COVID‐19, seven (18%) died after a median symptom onset time of 19 days. In these patients, acute kidney injury was also noticed in more than half of the cases, and it might have represented the most important contributor of the unfavorable outcome. Upon admission, liver function tests were within the limit of normal in most cases. Unlike the study by Bhoori et al, 109 Lee et al 110 are cautious regarding the continuation of immunosuppressants. To conclude, liver function test alterations are very common, and usually mild, in patients with COVID‐19. A pre‐existent liver disease may predispose to worse outcomes.
4.6. Biliary and pancreatic manifestations
Several reports identified the presence of acute acalculous cholecystitis in COVID‐19 patients. 111 , 112 , 113
No definitive data are available for explaining cholecystitis origin, which may be bloodstream‐related or due to SARS‐CoV‐2 direct infection on bile ducts through ACE2 receptor binding, which levels are higher even in this district. 114 However, a case report on a resected gallbladder after cholecystitis highlighted the presence of SARS‐CoV‐2 within the tissue, thus confirming viral presence. 113 Pancreatic involvement, defined as an increase in serum amylase and/or lipase or overt acute pancreatitis, has also been described in few reports or case series. 115 , 116 , 117 , 118 In the largest retrospective case series, elevated serum lipase was found in 14/83 cases (16.8%), and this was correlated with higher rates of admission to the intensive care unit and need for intubation. 117 However, the number of cases described is rather small and most patients were severely obese. Indeed, the lack of proper diagnostic imaging is another strong limit. Finally, given the importance of the spleen‐liver axis in maintaining the immunological homeostasis, 119 even the role of the spleen in contributing to the clinical picture of COVID‐19 has been explored in a few studies. In particular, spleen atrophy, mainly affecting the white pulp, was observed in post‐mortem cases of COVID‐19. 120 A study exploring spleen function in 66 COVID‐19 patients admitted to an internal medicine ward found a high prevalence of IgM memory B‐cell depletion, and this was associated with greater mortality and development of superimposed bacterial infections. 121 However, more data are still needed in order to ascertain the role of SARS‐CoV‐2 in causing direct liver damage, pancreatic, and splenic involvement.
5. DIAGNOSIS
In patients with COVID‐19 and gastrointestinal symptoms, careful history should be taken in order to assess whether symptoms developed with COVID infection or were pre‐existent. In patients presenting to the clinician with acute onset of gastrointestinal symptoms, particularly diarrhea, information regarding high‐risk contact exposure and the presence of other symptoms should be investigated. 122 The onset of gastrointestinal symptoms should be carefully assessed as they may precede respiratory symptoms of a few days. In clinical settings with limited resources, patients with both respiratory and gastrointestinal symptoms should be prioritized for SARS‐CoV‐2 testing. 73
No strong evidence is available for supporting routine stool testing for SARS‐CoV‐2. 122 Commonly used laboratory tests for the management of COVID‐19 patients are reported in Table 2. A recent metanalysis including 60 studies showed that SARS‐CoV‐2 RNA was detected in stool samples from 48.1% patients and, more importantly, viral RNA was found also in stool collected after respiratory samples turned negative. 123 Only 1% had gastrointestinal positivity alone (ie, rectal swabs or fecal assays) in the absence of a positive test from other sites (sputum and oral, nasopharyngeal, or throat swab). 25 These findings suggest that only in rare cases with negative nasopharyngeal swabs, would stool and rectal swab testing be of value, particularly in patients with gastrointestinal symptoms, increasing the possibility of obtaining a diagnosis of SARS‐CoV‐2 infection. Moreover, fecal or rectal testing may be helpful for monitoring the infection and the viral shedding since 49 out of 54 studies (91%) with serial RNA evaluations, reported persistent positivity for SARS‐CoV‐2 RNA after respiratory testing turned negative, with a mean time of delayed positivity of 12.5 days. However, further validation studies are need before these tests are included in the clinical algorithm of COVID‐19. In addition, although SARS‐CoV‐2 RNA can be detected in biopsy samples from the esophagus, stomach, duodenum, and rectum taken during endoscopy, 16 , 21 to date, there is no indication for invasive assessments in COVID‐19 patients complaining of gastrointestinal symptoms. 122
TABLE 2.
Non‐invasive commonly used biomarkers for gastrointestinal, liver and pancreatic COVID‐19 involvement evaluation
| Sample | Parameter | Variation | Meaning |
|---|---|---|---|
| Sputum | SARS‐CoV‐2 RNA | + |
COVID‐19 diagnosis Positive rate higher than throat swabs (from 28.6% 146 to 72% 147 ) |
| Blood | Blood count and coagulation | ||
| Neutrophils | ↑ | Associated with diarrhea and deaths 148 | |
| Lymphocyte | ↑ | Associated with diarrhea and deaths 148 | |
| Hemoglobin | ↓ | GI bleeding 149 | |
| D‐dimer | ↑ | Associated with ischemic colitis 85 | |
| Inflammatory markers | |||
| C‐reactive protein | ↑ | Associated with GI symptoms 150 | |
| Interleukin‐6 | ↑ |
Associated with diarrhea and death 148 Correlation with fecal calprotectin 47 |
|
| Interleukin‐10 | ↑ | Associated with diarrhea and death 148 | |
| Tumor necrosis factor‐α | ↑ | Associated with diarrhea and death 148 | |
| Ferritin | ↑ | Associated with severe clinical course and thrombotic complications 151 | |
| Interferon‐γ | ↑ |
Stimulate the production of inflammatory cytokines. Enhance hyper‐inflammation and exacerbates the severity of the disease. 152 |
|
| Hepato‐biliopancreatic tests | |||
|
Alanine aminotransferase Aspartate aminotransferase |
↑ | In 19% of patients, associated with severe clinical course 100 | |
|
Gamma‐glutamyltransferase Alkaline Phosphatase |
↑ |
In 32.7% 153 of patients. Associated with liver injury, longer hospital stay, 153 and intensive care unit admission 154 |
|
| Lactate dehydrogenase | ↑ | Associated with GI symptoms 150 and severe/critical clinical course 155 | |
| Bilirubin | ↑ | Associated with severe clinical course 156 | |
| Albumin | ↓ | Associated with GI symptoms 155 | |
| Lipase | ↑ |
In 12.1% of patients, no association with poor outcomes or symptoms 157 |
|
| Glucose | ↑ | Associated with severe/critical clinical course 155 | |
| Intestinal cells integrity tests | |||
| Citrulline | ↓ |
Associated with systemic inflammation and GI symptoms 40 Associated with ↑ C reactive protein and Ferritin and ↓ Albumin 40 |
|
| Feces | SARS‐CoV‐2 RNA | + | From 29% 147 to 58.1% 159 of patients; pooled results: 43.7% 160 |
| Fecal calprotectin | ↑ | Associated with diarrhea 47 | |
| Fecal occult blood | + | In 38% of patients with GI symptoms 161 | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
5.1. Liver and pancreatic testing
Despite the absence of established guidelines, there seems to be enough evidence to support monitoring of liver function through serum blood markers. In particular, we would suggest testing, in all hospitalized patients, transaminases, total and fractionated bilirubin, gamma‐glutamyl transpeptidase, alkaline phosphatase, coagulation, and serum albumin. In those patients showing alterations at baseline, or with a worsening clinical picture, additional testing during hospital stay could be useful as a prognostic marker. 106 , 107 The role of diagnostic imaging in this setting has not yet been established, as well as the potential role of a liver biopsy. Pancreatic enzymes (ie, amylase, lipase) should only be tested in case of suspicious of acute pancreatitis.
6. OUTCOME AND PROGNOSIS
6.1. Association of symptoms with severity and mortality
Since gastrointestinal symptoms mainly occur before respiratory and systemic involvement, 124 , 125 several authors evaluated whether digestive symptoms occurrence represents an unfavorable prognostic factor. 76 , 100 , 126 In a pivotal metanalysis on 4 studies, Gul et al 127 concluded that COVID‐19 patients with gastrointestinal symptoms had a higher risk of acute respiratory distress syndrome, but not mortality. 127 Accordingly, pooled data from various studies confirmed that COVID‐19 mortality in patients with gastrointestinal symptoms was comparable to the overall COVID‐19 mortality, accounting for 0.4% (95% CI, 0% to 1.1%). 76 , 100 , 126 On the other hand, the occurrence of gastrointestinal symptoms may be useful in predicting nasopharyngeal swab positivity (OR 1.7). 73
7. SHOULD WE EXPECT A WAVE OF POST–SARS‐COV‐2 FUNCTIONAL GASTROINTESTINAL DISORDERS?
Acute infection gastroenteritis of bacterial, protozoan, and viral nature is currently the strongest known risk factor for the development of irritable bowel syndrome (IBS) and functional dyspepsia. 54 A systematic review and meta‐analysis showed that >10% of patients with infectious enteritis develop IBS. 128 A recent large community survey suggests that viral gastroenteritis could be one of the most frequent form of post‐infection IBS. 129 Risk factors for post‐infection functional syndromes included female gender, severe enteritis, the presence of psychological distress, and the use of antibiotics during the infection. 128
Given the ability of SARS‐CoV‐2 to infect the gastrointestinal tract, leading to tissue damage and inflammation, and based on the large use of antibiotics in COVID‐19 patients, it seems reasonable to speculate that this combination would lead to a wave of post–COVID‐19 functional gastrointestinal disorders, including IBS. 130 Other factors support this hypothesis: first, COVID‐19 course has a median length of about 12 days, 131 thus hypothetically conferring more than a 10‐fold increase in the risk of post‐infectious IBS; 132 second, COVID‐19 is associated with psychological impairment, including anxiety and depression, 133 which may contribute to the development of functional gastrointestinal disorders.
Putative pathophysiological mechanisms underlying long‐term gut dysfunction and symptom generation after SARS‐CoV‐2 infection of the gastrointestinal tract may include the persistence of gut dysbiosis seen in post–COVID‐19 (see above) which, in turn, could contribute to maintain a chronic state of intestinal low‐grade inflammation, increased permeability, and bile acid malabsorption. In addition, SARS‐CoV‐2 infection is associated with T helper cell 17 and mast cell activation contributing to COVID‐19–related cytokine storm, 134 which resembles that observed in septic complications of intestinal bacterial translocation. 135 Mast cell activation could be a direct effect of viral entrance into the cell as mast cells express ACE2 and TMPRSS2 required for SARS‐CoV‐2 life cycle. 134 Previous studies demonstrated that mast cell infiltration and mediator release in proximity to mucosal innervation may contribute to abdominal pain perception in IBS patients. 136 , 137 Although these data suggest that mast cells could play a role in gastrointestinal symptom development in COVID‐19, further studies are now needed to confirm this hypothesis. Taken together, these data suggest that a sequence of events, including SARS‐CoV‐2 infection of epithelial cells, inflammatory cells, and enteric neurons may lead to long‐lasting changes in gastrointestinal function, leading to symptom generation including nausea, vomiting, diarrhea, and abdominal pain in susceptible individuals.
8. MANAGEMENT
To date, no specific drugs have been reported for the treatment of gastrointestinal symptoms in COVID‐19 patients. However, since the pathophysiological mechanisms underlying digestive symptoms are similar to those reported for respiratory symptoms, it may be reasonable thinking about a beneficial effect of these drugs for the gastrointestinal tract. For example, several monoclonal antibodies inhibit ACE2 receptors, creating an interference for virus binding, 138 whereas other molecules act on virus internalization mechanisms. 7 On this line, it has been reported an amelioration of diarrhea after antiviral treatment. 139 It is worth noticing that several drugs currently used for COVID‐19 treatment may also cause gastrointestinal symptoms. 122 Indeed, chloroquine, hydroxychloroquine, and lopinavir/ritonavir may induce nausea, vomiting, abdominal pain, and diarrhea in up to 30% of patients. 140 , 141 , 142 Moreover, antibiotics and antivirals used for COVID‐19 treatment may cause dysbiosis and diarrhea. The China National Health Commission has been among the first to recommend probiotics in severe COVID‐19 patients to ameliorate gut microbial homeostasis, to prevent bacterial infections, and to likely obtain antiviral effect. 143 Indeed, probiotics may favor the innate and adaptive immune response, interfere with virus lifecycle through the production of antiviral metabolites. 144 Thus, symptomatic treatments for each gastrointestinal symptom may be advised, 122 in addition to specific nutritional recommendations and micronutrients supplementation. 145 No specific treatment for treating liver injury exists. 122
9. CONCLUSIONS AND FUTURE PERSPECTIVES
Although gastrointestinal manifestations represent a tangible and important phenotypic expression of SARS‐CoV2 infection, several aspects still need to be clearly defined, including (1) a transmission via the fecal‐oral route; (2) a contribution of gut microbiota to severity and progression of the disease; (3) the long‐term consequences of the infection on digestive functions; (4) the role of gastrointestinal symptoms as predictors of severity; and (5) efficacy of therapies directed to gastrointestinal and liver manifestations. Understanding the relative importance of each of these factors and their interactions is needed to better understand the complex pathophysiology and management of gastrointestinal symptoms in COVID‐19.
CONFLICT OF INTERESTS
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
GM, VS, ADS, and GB designed the review; GM, MVL, CC, MRB, and GB performed literature search and drafted the manuscript; and all authors critically revised and approved the final version of the manuscript.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus disease (COVID‐19). https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed September 28, 2020.
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Openshaw PJ. Crossing barriers: Infections of the lung and the gut. Mucosal Immunol. 2009;2:100‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saif LJ. Bovine respiratory coronavirus. Vet Clin North Am ‐ Food Anim Pract. 2010;26:349‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou P, Lou YX, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, Mü MA, Drosten C, Pö S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak‐ An update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade‐Long Structural Studies of SARS Coronavirus. J Virol. 2020;94:e00127‐e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS‐CoV‐2 Spike Glycoprotein. Cell. 2020;181:281‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F. Gastrointestinal implications in COVID‐19. J Investig Med. 2020;68:1397‐1401. [DOI] [PubMed] [Google Scholar]
- 14. ACE2 protein expression summary ‐ The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000130234‐ACE2. Accessed November 18, 2020.
- 15. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158:1831‐1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Kang Z, Gong H, et al. Digestive system is a potential route of COVID‐19: an analysis of single‐cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010‐1018. [Google Scholar]
- 18. Lamers MM, Beumer J, Van Der VJ, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science. 2020;369:50‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS‐CoV‐2. Nat Med. 2020;26:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 20. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han C, Duan C, Zhang S, et al. Digestive Symptoms in COVID‐19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao F, Sun J, Xu Y, et al. Infectious SARS‐CoV‐2 in feces of patient with severe COVID‐19. Emerg Infect Dis. 2020;26:1920‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323:1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scaldaferri F, Ianiro G, Privitera G, et al. The thrilling journey of SARS‐CoV‐2 into the intestine: from pathogenesis to future clinical implications. Inflamm Bowel Dis. 2020;26:1306‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Doorn AS, Meijer B, Frampton CMA, Barclay ML, de Boer NKH. Systematic review with meta‐analysis: SARS‐CoV‐2 stool testing and the potential for faecal‐oral transmission. Aliment Pharmacol Ther. 2020;52:1276‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua C, Miao Z, Zheng J, et al. Epidemiological features and viral shedding in children with SARS‐CoV‐2 infection. J Med Virol. 2020;92:2804‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 29. Li Z, Liu T, Yang N, et al. Neurological manifestations of patients with COVID‐19: potential routes of SARS‐CoV‐2 neuroinvasion from the periphery to the brain. Front Med. 2020;14:533‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92:552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Felice FG, Tovar‐Moll F, Moll J, Munoz DP, Ferreira ST. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) and the Central Nervous System. Trends Neurosci. 2020;43:355‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cataldi M, Pignataro G, Taglialatela M. Neurobiology of coronaviruses: Potential relevance for COVID‐19. Neurobiol Dis. 2020;143:105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swanson PA, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS‐CoV2 neuroinvasion? Brain Behav Immun. 2020;87:93‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khoury‐Hanold W, Yordy B, Kong P, et al. Viral Spread to Enteric Neurons Links Genital HSV‐1 Infection to Toxic Megacolon and Lethality. Cell Host Microbe. 2016;19:788‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park CH, Ishinaka M, Takada A, et al. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol. 2002;147:1425‐1436. [DOI] [PubMed] [Google Scholar]
- 38. Zhou J, Li C, Zhao G, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3(11):eaao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deffner F, Scharr M, Klingenstein S, et al. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of Neuroinvasion by SARS‐CoV2. Front Neuroanat. 2020;14:596439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uzzan M, Soudan D, Peoch K, Weiss E, Corcos O, Treton X. Patients with COVID‐19 present with low plasma citrulline concentrations that associate with systemic inflammation and gastrointestinal symptoms. Dig Liver Dis. 2020;52:1104‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328‐339. [DOI] [PubMed] [Google Scholar]
- 42. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology. 2020;158:1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced Host Response to SARS‐CoV‐2 Drives Development of COVID‐19. Cell. 2020;181:1036‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garg M, Royce SG, Lubel JS. Letter: intestinal inflammation, COVID‐19 and gastrointestinal ACE2—exploring RAS inhibitors. Aliment Pharmacol Ther. 2020;52:569‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Effenberger M, Grabherr F, Mayr L, et al. Faecal calprotectin indicates intestinal inflammation in COVID‐19. Gut. 2020;69:1543‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramani A, Müller L, Ostermann PN, et al. SARS ‐CoV‐2 targets neurons of 3D human brain organoids. EMBO J. 2020;39:e106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akiho H. Cytokine‐induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bamias G, Dinarello CA, Rivera‐Nieves J. Innate Cytokines Dictate the Fate of Acute Intestinal Inflammation. Gastroenterology. 2015;148:248‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID‐19. ACS Chem Neurosci. 2020;11:1520‐1522. [DOI] [PubMed] [Google Scholar]
- 53. Spiller RC. Role of nerves in enteric infection. Gut. 2002;51:759‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barbara G, Grover M, Bercik P, et al. Rome foundation working team report on post‐infection irritable bowel syndrome. Gastroenterology. 2019;156:46‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xing X‐C, Qi B, Deng K. SARS‐CoV‐2 RNA Detection in Gastrointestinal Sample Displays Poor Performance. Gastroenterology. 2020;S0016–5085(20):34776‐34784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota‐mediated Th17 cell‐dependent inflammation. J Exp Med. 2014;211:2397‐2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Villapol S. Gastrointestinal symptoms associated with COVID‐19: impact on the gut microbiome. Transl Res. 2020;226:57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zuo T, Zhang F, Lui GCY, et al. Alterations in Gut Microbiota of Patients With COVID‐19 During Time of Hospitalization. Gastroenterology. 2020;159(3):944‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zuo T, Liu Q, Zhang F, et al. Depicting SARS‐CoV‐2 faecal viral activity in association with gut microbiota composition in patients with COVID‐19. Gut. 2020;70:276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The Architecture of SARS‐CoV‐2 Transcriptome. Cell. 2020;181(4):914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu H, Zhu J, Hu Q, Rao X. Morganella morganii, a non‐negligent opportunistic pathogen. Int J Infect Dis. 2016;50:10‐17. [DOI] [PubMed] [Google Scholar]
- 66. Gu S, Chen Y, Wu Z, et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71(10):2669‐2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID‐19: An overview of the involvement of the chemokine/chemokine‐receptor system. Cytokine Growth Factor Rev. 2020;53:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID‐19. World J Gastroenterol. 2020;26:4753‐4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID‐19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Y, Liu S, Liu H, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang L, Tu L. Implications of gastrointestinal manifestations of COVID‐19. Lancet Gastroenterol Hepatol. 2020;5:629‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: early experience from California. Gastroenterology. 2020;159:775‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and coronavirus disease 2019: A case‐control study from the United States. Gastroenterology. 2020;159:373‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lozada‐Nur F, Chainani‐Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID‐19: Possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:344‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tariq R, Saha S, Furqan F, Hassett L, Pardi D, Khanna S. Prevalence and Mortality of COVID‐19 Patients With Gastrointestinal Symptoms: A Systematic Review and Meta‐analysis. Mayo Clin Proc. 2020;95:1632‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zarifian A, Zamiri Bidary M, Arekhi S, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;93(1):336‐350. 10.1002/jmv.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suresh Kumar VC, Mukherjee S, Harne PS, et al. Novelty in the gut: A systematic review and meta‐analysis of the gastrointestinal manifestations of COVID‐19. BMJ Open Gastroenterol. 2020;7:e000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carvalho A, Alqusairi R, Adams A, et al. SARS‐CoV‐2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID‐19 Disease. Am J Gastroenterol. 2020;115:942‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: A descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin TA, Wan DW, Hajifathalian K, et al. Gastrointestinal bleeding in patients with coronavirus disease 2019: A matched case‐control study. Am J Gastroenterol. 2020;115:1609‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aurelio M, Federico DG, Vincenzo LM, et al. Upper gastrointestinal bleeding in COVID‐19 inpatients: Incidence and management in a multicenter experience from Northern Italy. Clin Res Hepatol Gastroenterol. 2020;S2210–7401(20):30216‐30223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Melazzini F, Lenti MV, Mauro A, De Grazia F, Di Sabatino A. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115:1139‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chan KH, Lim SL, Damati A, et al. Coronavirus disease 2019 (COVID‐19) and ischemic colitis: An under‐recognized complication. Am J Emerg Med. 2020;38:2758‐2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moazzam Z, Salim A, Ashraf A, Jehan F, Arshad M. Intussusception in an infant as a manifestation of COVID‐19. J Pediatr Surg Case Reports. 2020;59:101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kaafarani HMA, El Moheb M, Hwabejire JO, et al. Gastrointestinal Complications in Critically Ill Patients With COVID‐19. Ann Surg. 2020;272:e61‐e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Neurath MF. COVID‐19 and immunomodulation in IBD. Gut. 2020;69:1335‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin‐angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut. 2020;69:841‐854. [DOI] [PubMed] [Google Scholar]
- 90. Jablaoui A, Kriaa A, Mkaouar H, et al. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front Cell Infect Microbiol. 2020;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Monteleone G, Ardizzone S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid‐19 Infection? J Crohns Colitis. 2020;14:1334‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Batlle D, Wysocki J, Satchell K. Soluble angiotensin‐converting enzyme 2: A potential approach for coronavirus infection therapy? Clin Sci. 2020;134:543‐545. [DOI] [PubMed] [Google Scholar]
- 93. Allocca M, Chaparro M, Gonzalez HA, et al. Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID‐19: A Large Multinational Cohort Study. J Clin Med. 2020;9:3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mårild K, Fredlund H, Ludvigsson JF. Increased risk of hospital admission for influenza in patients with celiac disease: A nationwide cohort study in Sweden. Am J Gastroenterol. 2010;105:2465‐2473. [DOI] [PubMed] [Google Scholar]
- 95. Zingone F, Abdul Sultan A, Crooks CJ, Tata LJ, Ciacci C, West J. The risk of community‐acquired pneumonia among 9803 patients with coeliac disease compared to the general population: a cohort study. Aliment Pharmacol Ther. 2016;44:57‐67. [DOI] [PubMed] [Google Scholar]
- 96. Zhen J, Stefanolo JP, Temprano MP, et al. The risk of contracting COVID‐19 is not increased in patients with celiac disease. Clin Gastroenterol Hepatol. 2020;S1542–3565(20):31398‐31407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zingone F, D’Odorico A, Lorenzon G, Marsilio I, Farinati F, Savarino EV. Risk of COVID‐19 in celiac disease patients. Autoimmun Rev. 2020;19:102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5:667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID‐19 in the New York City Area. JAMA. 2020;323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID‐19: Prevalence and association with clinical outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Medetalibeyoglu A, Catma Y, Senkal N, et al. The effect of liver test abnormalities on the prognosis of COVID‐19. Ann Hepatol. 2020;19:614‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schattenberg JM, Labenz C, Wörns MA, et al. Patterns of liver injury in COVID‐19 – a German case series. United Eur Gastroenterol J. 2020;8:814‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lenti MV, Borrelli de Andreis F, Pellegrino I, et al. Impact of COVID‐19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med. 2020;15:1399‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ponziani FR, Del Zompo F, Nesci A, et al. Liver involvement is not associated with mortality: results from a large cohort of SARS‐CoV‐2 positive patients. Aliment Pharmacol Ther. 2020;52:1060‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lee BT, Perumalswami PV, Im GY, et al. COVID‐19 in Liver Transplant Recipients: An Initial Experience From the US Epicenter. Gastroenterology. 2020;159(3):1176‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mattone E, Sofia M, Schembari E, et al. Acute acalculous cholecystitis on a COVID‐19 patient: a case report. Ann Med Surg. 2020;58:73‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ying M, Lu B, Pan J, et al. COVID‐19 with acute cholecystitis: A case report. BMC Infect Dis. 2020;20:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Balaphas A, Gkoufa K, Meyer J, et al. COVID‐19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73:1566‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shinohara T, Otani A, Yamashita M, et al. Acute Pancreatitis During COVID‐19 Pneumonia. Pancreas. 2020;49:e106‐e108. [DOI] [PubMed] [Google Scholar]
- 116. Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease‐19 (COVID‐19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13:237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barlass U, Wiliams B, Dhana K, et al. Marked Elevation of Lipase in COVID‐19 Disease: A Cohort Study. Clin Transl Gastroenterol. 2020;11:e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Meyers MH, Main MJ, Orr JK, Obstein KL. A Case of COVID‐19–Induced Acute Pancreatitis. Pancreas. 2020;49:e108‐e109. [DOI] [PubMed] [Google Scholar]
- 119. Tarantino G, Scalera A, Finelli C. Liver‐spleen axis: Intersection between immunity, infections and metabolism. World J Gastroenterol. 2013;19:3534‐3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Duarte‐Neto AN, Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement in COVID‐19 patients assessed with ultrasound‐guided minimally invasive autopsy. Histopathology. 2020;77:186‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lenti M, Aronico N, Pellegrino I, et al. Depletion of circulating IgM memory B cells predicts unfavourable outcome in Covid‐19. Sci Rep. 2020;10:20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sultan S, Altayar O, Siddique SM, et al. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID‐19, Meta‐Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID‐19. Gastroenterology. 2020;159(1):320‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong Cohort: systematic review and meta‐analysis. Gastroenterology. 2020;159:81‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Buscarini E, Manfredi G, Brambilla G, et al. GI symptoms as early signs of COVID‐19 in hospitalised Italian patients. Gut. 2020;69:1547‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal Manifestations of SARS‐CoV‐2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta‐analysis. Gastroenterology. 2020;159:81‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gul F, Lo KB, Peterson J, McCullough PA, Goyal A, Rangaswami J. Meta‐analysis of outcomes of patients with COVID‐19 infection with versus without gastrointestinal symptoms. Baylor Univ Med Cent Proc. 2020;33:366‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta‐analysis. Gastroenterology. 2017;152(5):1042‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tam CC, Rodrigues LC, Viviani L, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut. 2012;61:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schmulson M, Ghoshal U, Barbara G. Managing the inevitable surge of post‐COVID‐19 functional gastrointestinal disorders. Am J Gastroenterol. 2020;116:4‐7. [DOI] [PubMed] [Google Scholar]
- 131. Gandhi RT, Lynch JB, del Rio C. Mild or Moderate Covid‐19. N Engl J Med. 2020;383:1757‐1766. [DOI] [PubMed] [Google Scholar]
- 132. Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: Postal survey of patients. Br Med J. 1997;314:779‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Al‐Ajlouni YA, Park SH, Alawa J, et al. Anxiety and depressive symptoms are associated with poor sleep health during a period of COVID‐19‐induced nationwide lockdown: a cross‐sectional analysis of adults in Jordan. BMJ Open. 2020;10:e041995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Theoharides TC. COVID‐19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. BioFactors. 2020;46:306‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693‐702. [DOI] [PubMed] [Google Scholar]
- 137. Barbara G, Wang B, Stanghellini V, et al. Mast cell‐dependent excitation of visceral‐nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26‐37. [DOI] [PubMed] [Google Scholar]
- 138. Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus‐specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Song Y, Liu P, Shi XL, et al. SARS‐CoV‐2 induced diarrhoea as onset symptom in patient with COVID‐19. Gut. 2020;69:1143‐1144. [DOI] [PubMed] [Google Scholar]
- 140. fda, cder . ARALEN ® CHLOROQUINE PHOSPHATE, USP. www.cdc.gov. Accessed October 7, 2020.
- 141. Plaquenil® Hydroxychloroquine Sulfate . USP warning physicians should completely familiarize themselves with the complete contents of this leaflet before prescribing hydroxychloroquine. https://products.sanofi.us/plaquenil/plaquenil.pdf
- 142. Fda . Kaletra (lopinavir and ritonavir) Label. Available at www.fda.gov/medwatch. Accessed October 7, 2020.
- 143. Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sundararaman A, Ray M, Ravindra PV, Halami PM. Role of probiotics to combat viral infections with emphasis on COVID‐19. Appl Microbiol Biotechnol. 2020;104:8089‐8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Barazzoni R, Bischoff SC, Breda J, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS‐CoV‐2 infection. Clin Nutr. 2020;39:1631‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lai T, Xiang F, Zeng J, et al. Reliability of induced sputum test is greater than that of throat swab test for detecting SARS‐CoV‐2 in patients with COVID‐19: A multi‐center cross‐sectional study. Virulence. 2020;11:1394‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang L, Han C, Zhang S, et al. Diarrhea and altered inflammatory cytokine pattern in severe coronavirus disease 2019: Impact on disease course and in‐hospital mortality. J Gastroenterol Hepatol. 2020. 10.1111/jgh.15166. [DOI] [PubMed] [Google Scholar]
- 149. Gadiparthi C, Perisetti A, Sayana H, Tharian B, Inamdar S, Korman A. Gastrointestinal Bleeding in Patients with Severe SARS‐CoV‐2. Am J Gastroenterol. 2020;115:1283‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Clinical characteristics of coronavirus disease (COVID‐19) patients with gastrointestinal symptoms: A report of 164 cases. Dig Liver Dis. 2020;52:1076‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: Bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136:489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sonkar C, Kashyap D, Varshney N, Baral B, Jha HC. Impact of Gastrointestinal Symptoms in COVID‐19: a Molecular Approach. SN Compr Clin Med. 2020;2(12):2658‐2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Shao T, Tong Y, Lu S, et al. Gamma‐glutamyltransferase elevation is frequent in patients with COVID‐19: A clinical epidemiologic study. Hepatol Commun. 2020;4:1744‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cai Q, Huang D, Yu H, et al. COVID‐19: Abnormal liver function tests. J Hepatol. 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69:1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid‐19: A pooled analysis. Liver Int. 2020;40:1787‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. McNabb‐Baltar J, Jin DX, Grover AS, et al. Lipase Elevation in Patients With COVID‐19. Am J Gastroenterol. 2020;115:1286‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID‐19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wu B, Yu T, Huang Z. Nucleic Acid Detection of Fecal Samples from Confirmed Cases of COVID‐19. Chin J Zoonoses. 2020;36:359‐361. 10.3969/j.issn.1002-2694.2020.00.027. [DOI] [Google Scholar]
- 160. Wong MC, Huang J, Lai C, Ng R, Chan FKL, Chan PKS. Detection of SARS‐CoV‐2 RNA in fecal specimens of patients with confirmed COVID‐19: A meta‐analysis. J Infect. 2020;81:e31‐e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Du L, Cao X, Chen J, et al. Fecal occult blood and urinary cytology tests for rapid screening of inflammatory infection in the gastrointestinal and urological systems in patients with Coronavirus disease 2019. J Clin Lab Anal. 2020;e23626. [DOI] [PMC free article] [PubMed] [Google Scholar]


