Dear Editor,
The World Health Organization identified a novel coronavirus in city of Wuhan in China that caused diseases of COVİD‐19. The virus spread very quickly and has to date resulted in the deaths of thousands of people. Hydroxychloroquine (HCQ) has recently entered into clinical use the treatment of COVID‐19 effect, both by inhibiting the viral entry into the cell and in the following stage. 1 HCQ is a drug with multiple effects that has long been in use for its antimalarial and antirheumatic features. 2 Previous studies have reported several dermatological side effects of HCQ, including drug eruption, pruritus, psoriasis, and alopecia. 3 Psoriasis is a chronic and systemic inflammatory skin disease with multiple comorbidities that can be induced or exacerbated by several drugs, including HCQ, which is a synthetic antimalarial drug. 4
We present here a case of psoriasis in a suspected COVID‐19 patient that exacerbated following HCQ treatment, and that was treated successfully and rapidly with the IL‐12/IL‐23 inhibitor. To the best of our knowledge, this is the second case of psoriasis exacerbation following HCQ treatment for suspected COVID‐19 to be reported on since the outbreak.
A 40‐year‐old male patient presented with a diffuse rash, pruritus and joint pain that had been increasing for 20 days. It was ascertained that the patient had presented at the hospital approximately 1 month previously with fever, cough and sore throat, when blood tests, lung tomography, and polymerase chain reaction (PCR) tests were requested due to suspicions of COVID‐19 infection. An initial assessment revealed lesions consistent with COVID‐19 infection on a lung tomography, and so treatment with azithromycin 500 mg daily and HCQ 200 mg BID was initiated. After a few days of treatment, the patient's rash and pruritus increased in severity and spread further. The lung tomography was considered consistent with lobar pneumonia, and PCR tests at 3‐day intervals were negative. The patient was hospitalized due to suspected COVID‐19, and was discharged after treatment. This patient was not evaluated as a COVID‐19 infection.
He had plaque psoriasis with occasional exacerbation since the age of 10 years. A dermatological examination revealed very diffuse, very thick, erythematous plaque with squamae on the upper, and lower extremities, and the trunk (Figure 1A‐C). After treatment with subcutaneous (sc) ustekinumab 90 mg was initiated, the lesions were observed to have regressed distinctively at the follow‐up visit 1 month later (Figure 2A‐C).
FIGURE 1.
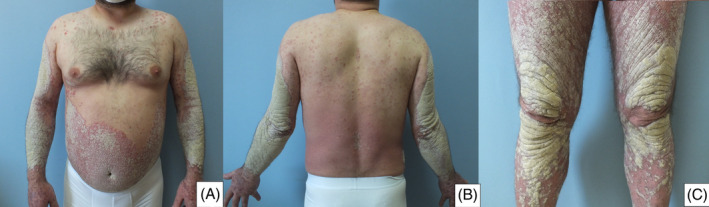
A‐C, Erythematous plaque with squamae on the upper and lower extremities and the trunk
FIGURE 2.
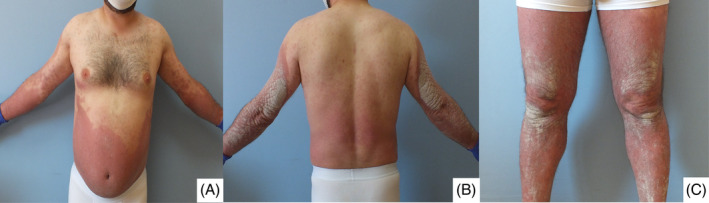
A‐C, The lesions were observed to have regressed distinctively at the follow‐up visit 1 month later
HCQ may result in the exacerbation, relapse or induction of psoriasis. 5 A recent case report featuring a 71‐year‐old patient with psoriasis, who was diagnosed with COVID‐19 and underwent 4‐day HCQ treatment, experienced disease exacerbation with psoriatic plaque. 6
There have been several mechanisms suggested for hydroxychloroquine to cause psoriasis, as (a) the stimulation of hyperproliferation and irregular keratinization in epidermal cells following the inhibition of epidermal transglutaminase, (b) keratinocyte growth and differentiation upon increased IL‐17, IL‐23, and (c) overproduction of TNF‐a. 5 , 7
Infections also play a major role in the etiopathogenesis of psoriasis. COVID‐19 or lobar pneumonia may cause cytokine dysregulations and trigger the exacerbation of psoriasis. Whether the triggering factor of psoriasis in this case is mainly related to the presence of infection cannot be ignored. İncreased stress upon learning of the COVID‐19 diagnosis—a fatal disease—may have contributed to the trigger. We did not find any reports that azithromycin may exacerbate psoriasis. 8 There were no reports of any severe cases of COVID‐19 infection linked to biological agents, and we administered ustekinumab 90 mg sc to our patient effectively. 9
Further studies will guide whether hydroxychloroquine is indeed a single trigger or initiator in the etiopathogenesis of psoriasis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
Data is available upon request.
REFERENCES
- 1. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine is effective in inhibiting SARS‐CoV2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang WM, Wang KY, Wang T, Jin HZ, Fang K. Hydroxychloroquine‐induced psoriasis‐form Erythroderma in a patient with systemic lupus erythematosus. Chin Med J (Engl). 2018;131:1887‐1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020;83:563‐578. [DOI] [PubMed] [Google Scholar]
- 4. Balak DM, Hajdarbegovic E. Drug‐induced psoriasis: clinical perspectives. Psoriasis (Auckl). 2017;7:87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sachdeva M, Mufti A, Maliyar K, Lytvyn Y, Yeung J. Hydroxychloroquine effects on psoriasis: a systematic review and a cautionary note for COVID‐19 treatment. J Am Acad Dermatol. 2020;83:579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kutlu Ö, Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: will cases of psoriasis increase after COVID‐19 pandemic? Dermatol Ther. 2020;33:e13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCoy S, Nagaraja V, Paviol S, Gudjonsson JE, Kahlenberg JM. Exacerbation of psoriasis due to hydroxychloroquine. Arch Med. 2015;7:1‐3. [Google Scholar]
- 8. Saxena VN, Dogra J. Long‐term oral azithromycin in chronic plaque psoriasis: a controlled trial. Eur J Dermatol. 2010;20:329‐333. [DOI] [PubMed] [Google Scholar]
- 9. Carugno A, Gambini DM, Raponi F, et al. COVID‐19 and biologics for psoriasis: a high‐epidemic area experience‐Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


