Abstract
Background:
Loss-of-control (LOC)-eating post-operatively is a consistent predictor of suboptimal longer-term bariatric surgery outcomes. This randomized controlled trial (RCT) examined the effectiveness of two guided-self-help treatments (cognitive-behavioral therapy [gshCBT] and behavioral weight-loss [gshBWL]) to a control (CON) for reducing LOC-eating and weight.
Methods:
140 patients with recurrent LOC-eating six months after bariatric surgery were randomly assigned (5:5:2 ratio) to one of three conditions: gshCBT (N=56), gshBWL (N=60), or CON (N=24). Three-month treatments were delivered by trained allied-health clinicians to increase generalizability to bariatric-surgery settings. Independent assessments were performed by doctoral research-clinicians using established interviews/measures; post-outcomes were obtained for 89% of patients.
Results:
Mixed-models revealed significant improvements for LOC-eating frequency and weight-loss but no significant differences between treatments; race neither predicted (main-effect) nor moderated (interaction-effect) treatment outcomes. ITT categorical analyses of abstinence from LOC-eating (30% for gshCBT, 27% for gshBWL, 38% for CON) and proportion attaining 5% weight loss (20%, 22%, 17%) revealed no significant differences between treatments; non-White participants had higher proportion achieving LOC-eating abstinence but lower proportion attaining 5% weight-loss than White participants.
Conclusions:
In this 12-week RCT following bariatric surgery, significant LOC-eating reductions and weight-loss did not differ significantly between treatments. Race was associated with post-treatment categorical outcomes.
Keywords: Obesity, bariatric surgery, binge eating, loss-of-control eating, behavior therapy
Introduction
Bariatric surgery, the most effective treatment for severe obesity, results in overall impressive weight-loss and improved medical comorbidities (1,2,3). Laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric-bypass (RYGB), the two most commonly performed procedures (4), result in roughly 50% excess weight loss; RYGB shows slight advantages in weight loss and medical improvements over LSG (which is surgically easier to perform and has fewer short-term surgically-related complications) and both are superior to adjustable gastric banding (5). Although bariatric surgery produces overall impressive outcomes, there is marked heterogeneity in weight-losses (6) and weight-regain after surgery is associated with reoccurrence of medical comorbidities (7). These findings are concerning and suggest bariatric surgery alone is often not enough, highlighting the need for research to identify predictors and methods to enhance outcomes.
Given concerns about variability and durability of outcomes achieved with bariatric surgery, emerging research has followed two main paths. Investigators have begun to test behavioral/psychological interventions delivered either prior to or following bariatric surgery for improving targets such as eating behaviors and functioning in the hopes of also enhancing weight losses (3). These interventions, mostly brief and low-intensity, evaluated in small trials have yielded some encouraging, albeit mixed, findings that weight-losses can be improved (3,8,9). One consistent finding is that optimal time to deliver adjunctive interventions appears to be early in the post-surgery period although more research is needed to inform the refinement of preliminary treatments (8).
Investigators have also attempted to identify predictors of poor outcomes which could inform “which patients” need adjunctive interventions and “what behaviors” to specifically target. Research examining a broad range of pre-surgical patient variables has failed to reliably identify any potentially modifiable predictors of weight-loss outcomes following bariatric surgery (3). In contrast, research has identified one reliable post-surgery predictor of subsequent outcomes – “loss of control” (LOC) eating” (10,11,12; see 3).
LOC while eating is one of two core components of “binge eating” criterion for binge-eating disorder (BED) in Diagnostic and Statistical Manual of Mental Disorders–5th edition [DSM-5](13). The second component of “binge eating” is that the episode involves an unusually large amount of food which, following bariatric surgery, is physically difficult. However, eating smaller quantities of food while experiencing LOC remains possible following surgery. Emerging research with patients who have undergone bariatric surgery (14,15) along with considerable research with clinical/community samples across weight categories have documented that “loss of control” is the meaningful aspect of binge eating (regardless of quantity consumed) and is a strong predictor of eating-disorder psychopathology and psychological disturbance (16). Indeed, the salience of LOC eating for binge eating has been recognized officially in the recent version of International Classification of Diseases [ICD-11] (17). Research has found that LOC-eating following surgery (11), not binge-eating prior to surgery (18), is the most reliable modifiable predictor of poorer weight outcomes (12). Research is needed to identify treatments for LOC-eating following bariatric surgery.
This study was a randomized controlled trial (RCT) to evaluate effective treatments from the BED/obesity fields adapted for patients following bariatric surgery who are experiencing LOC-eating. In non-bariatric samples, certain psychological treatments, such as cognitive-behavioral therapy (CBT), are effective for binge-eating and produce significant durable improvements (19,20). Research has supported “scalable” guided self-help CBT (gshCBT) for binge eating (21,22). Preliminary findings suggest that brief-CBT delivered six months following bariatric surgery shows promise for reducing problematic eating (23). Although CBT treatments improve eating behaviors, they consistently fail to produce substantial weight-loss in patients with obesity (22,24). In contrast, RCTs have demonstrated that certain behavioral treatments (lifestyle behavioral weight loss (BWL)) and pharmacological methods, produce substantial reductions in binge eating but offer a potential advantage over CBT for weight-loss (22,24,25).
This RCT was designed to specifically test the effectiveness of two guided-self-help treatments (gshCBT and gshBWL) for improving LOC- eating, weight, and associated features following bariatric surgery. Because experimental conditions should demonstrate superiority over usual/standard care (26), a control (CON) condition with standard care was chosen to provide additional context. The study used allied-health clinicians to deliver treatments; this was intended as a methodologic match to the CON condition and to enhance generalizability to bariatric surgery settings which have allied-health clinicians in such roles. This strategy was supported by research suggesting that such scalable guided-self-help interventions can be delivered by generalist clinicians (27) and are cost-effective (28). Finally, the study aimed to examine whether race predicts/moderates or is associated with treatment outcomes given research findings given research findings that, relative to White patients, Black patients experience lower weight-loss following bariatric surgery (31) and treatments for BED despite being more likely to stop binge eating in analyses of data aggregated across RCTs (32).
Methods
Participants
Participants were 140 consecutively randomized patients recruited September 2014 to December 2017 interested in treatment for eating/weight concerns approximately six months following laparoscopic RYGB or SG. Participants were recruited from a single bariatric center-of-excellence at an academic medical center using flyers/mailings soliciting postoperative patients with eating concerns interested in a behavioral treatment study. Inclusion criteria included age 18–65 and regular LOC-eating (≥once weekly) during past 28 days (feeling unable to stop/control an eating episode at least once weekly regardless of quantity). Exclusion criteria, minimal to enhance generalizability, included medications known to effectively influence eating/weight, substance dependence, or severe psychiatric illness requiring acute care determined by clinical/diagnostic interviews during intake. Assessments were conducted independently from bariatric center. This study received Yale Institutional Review Board approval; participants provided written informed consent.
Participants had a mean age of 45.6 (SD=10.9) years, were predominately female (n=119, 85.0%), and diverse in ethnicity (n=15 (10.7%) identified as Hispanic/Latino/a) and race [(n=79 (56.4%) White, n=44 (31.4%) Black, n=11 (7.9%) “Other”, n=3 (2.1%) Bi/Multiracial, n=2 (1.4%) American-Indian/Alaska-Native, and n=1 (0.7%) Native-Hawaiian/Other Pacific-Islander]. Table 1 shows categorization of White/Non-White used in statistical models. Of the 140 participants, 123 (87.9%) had LSG, 17 (12.1%) had RYGB. At study enrollment, mean time since surgery was 6.4 (SD=1.5; range 4–10) months, and percent weight-loss was 20.3 (SD=7.9).
Table 1.
Demographic and Clinical Characteristics of 140 Randomized Patients Overall and Separately for Treatment Groups
| Overall N=140 | Control n=24 | gshBWL n=60 | gshCBT n=56 | Test Statistic | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | F | ||
| Age | 45.6 | 10.9 | 45.3 | 7.2 | 43.0 | 10.9 | 48.6 | 11.7 | 4.03 | 0.020 |
| Months since surgery | 6.4 | 1.5 | 6.5 | 1.7 | 6.4 | 1.5 | 6.3 | 1.4 | 0.13 | 0.731 |
| Weight loss at enrollment (%) | 20.3 | 7.9 | 20.9 | 8.0 | 20.8 | 8.4 | 19.5 | 7.4 | 0.54 | 0.586 |
| n | % | n | % | n | % | n | % | Chi-Square | p-value | |
| Gender (Female) | 119 | 85 | 21 | 87.5 | 48 | 80.0 | 50 | 89.3 | 2.10 | 0.350 |
| Race1 | 0.64 | 0.727 | ||||||||
| White | 75 | 54.7 | 12 | 54.5 | 35 | 58.3 | 28 | 50.9 | ||
| Non-White | 62 | 45.3 | 10 | 45.5 | 25 | 41.7 | 27 | 49.1 | ||
| Hispanic | 15 | 10.7 | 3 | 12.5 | 5 | 8.3 | 7 | 12.5 | ||
| Education | 5.60 | 0.061 | ||||||||
| At least college degree | 56 | 40.0 | 14 | 58.3 | 25 | 41.7 | 17 | 30.4 | ||
| Some college | 55 | 39.3 | 6 | 25.0 | 23 | 38.3 | 26 | 46.4 | ||
| High school | 25 | 17.9 | 4 | 16.7 | 10 | 16.6 | 11 | 19.7 | ||
| Less than high school degree | 4 | 2.8 | 0 | 0.0 | 2 | 3.4 | 2 | 3.6 | ||
| Binge-Eating Disorder (lifetime, pre-surgical) | 65 | 46.4 | 10 | 41.7 | 29 | 48.3 | 26 | 46.4 | 0.31 | 0.858 |
| Surgery | ||||||||||
| Bypass | 17 | 12.1 | 3 | 12.5 | 8 | 13.3 | 6 | 10.7 | 0.19 | 0.910 |
| Sleeve | 123 | 87.9 | 21 | 87.5 | 52 | 86.7 | 50 | 89.3 | ||
Note: N=140. BWL = behavioral weight loss (guided self-help); CBT = cognitive behavioral therapy (guided self-help).
Test statistic = chi-square for categorical variables and ANOVAs for dimensional variables. M= Mean; SD = standard deviation.
BED = binge-eating disorder.
denotes that chi-square was performed for two collapsed categories given low frequencies of some variables (i.e., white versus non-white [excluded n=1 Native Hawaiian or Other Pacific Islander and n=2 American Indian or Alaska Native] and college graduate versus less than college degree).
Diagnostic and Repeated Outcomes Measures
Trained and monitored doctoral research-clinicians (blinded to treatment; see below) administered the Eating Disorder Examination-Bariatric-Surgery-Version (EDE-BSV)(33,34) interview at baseline and post-treatment (end-of-treatment without time lapse). EDE-BSV, a semi-structured investigator-based interview, assessed frequency of LOC-eating and eating-disorder psychopathology (reflected in Global Score). EDE-BSV, the modified version of the EDE interview which has good psychometric properties in studies of obesity and BED(35,36), was adapted specifically for bariatric research, including the Longitudinal Assessment Bariatric Surgery (LABS) study(12,33,34). In the present study, interrater reliability, assessed with N=20 cases, was excellent for both LOC-eating frequency (ICC=0.90) and Global Score (ICC=0.95).
Weight/Height Variables.
Measured height and measured weight (baseline, monthly, post-treatment) obtained using a high-capacity digital scale were used to calculate BMI and %TWL during treatment ([(Session 1 Weight)−(Post Weight)]/[(Session 1 Weight)]*100). Per bariatric-surgery guidelines (37), percent weight-loss at time of enrollment was computed: ([(Preoperative Weight)−(Postoperative Weight)]/[(Preoperative Weight)]*100).
Self-report measures were completed at baseline, monthly during treatment, and post-treatment. Beck Depression Inventory (BDI-II)(38) is a psychometrically-sound measure of depressive symptom levels. Godin Leisure Time Exercise Questionnaire (GLTEQ)(39) assesses mild, moderate, and vigorous physical activity, has good test-retest reliability, and has been validated against objective measurements(40). Medical Outcomes Study Short-Form Health Survey (SF-36)(41), assesses health-related quality-of-life, includes two summary scores for physical (SF-PCS) and mental (SF-MCS) health, and has good validity and reliability(42). Scores are transformed and computed as t-scores with mean of 50 and standard deviation of 10.
Treatment Conditions
Treatments were delivered over 12 weeks by trained (by C.M.G. and V.I.) allied-health clinicians (e.g., masters’-level nurses with various specialties including mental/behavioral health and nutrition, APRNs, graduate students in clinical psychology or public health in behavioral/social science) who were monitored (weekly supervision focused on quality and fidelity of sessions including review of audiotapes) throughout the study to maintain adherence to manualized treatment protocols described below. Using “generalist” allied-health clinicians to deliver the scalable guided-self-help treatments, per previous research(22,27), served as a methodologic match to the CON control condition and increases generalizability to bariatric surgery settings. The CON condition comprised standard care available at the Yale bariatric center-of-excellence including support groups and nutrition education following national guidelines(29,30) delivered by allied-health professionals. Although all participants had access to the bariatric center’s standard care, participants assigned to the CON condition were specifically encouraged to continue working with the bariatric center’s clinicians and resources by the study research-clinicians assigned to them for baseline and monthly assessments, which was intended as a partial “control-for-attention.” Experimental treatments (gshCBT, gshBWL) were closely matched to each other in number of sessions and time, session structure and in-between session tasks, and self-care materials.
Guided-Self-Help Cognitive-Behavioral Therapy
Guided-Self-Help Cognitive-Behavioral Therapy (gshCBT) followed manualized protocols(21) used in RCTs for BED adapted for use for patients following bariatric surgery. gshCBT, delivered via 6 individual sessions (25–30 minutes) over 12 weeks, was keyed to a self-care manual along with standard bariatric nutrition education provided to participants. gshCBT follows six steps to assess and modify maladaptive eating-related behaviors and cognitions (e.g., shape/weight concerns) hypothesized to maintain the disordered eating. Clinicians “guide” the gshCBT by addressing patients’ questions about the CBT approach, helping with difficulties around following behavioral steps and cognitive-restructuring exercises, and reinforcing importance of self-monitoring, record keeping, and goal-setting.
Guided-Self-Help Behavioral Weight Loss
Guided-Self-Help Behavioral Weight Loss (gshBWL) followed manualized protocols used previously for BED(21) based initially on the LEARN (lifestyle, exercise, attitudes, relationships, and nutrition) program and adapted for use for patients following bariatric surgery. gshBWL, delivered via 6 individual sessions (25–30 minutes) over 12 weeks, was keyed to a self-care manual along with standard bariatric nutrition education provided to participants. gshBWL focuses on making gradual and modest lifestyle changes during the challenging post-operative period, including re-establishing acceptable eating and nutrition patterns given the restrictions of surgery, gradually increasing physical activity, and problem-solving social/interpersonal contexts to sustain changes.
Treatment Randomization and Blind Assessment Protocols
Randomization followed an allocation ratio of 5:5:2 to the three treatments (gshCBT, gshBWL, and CON, respectively). Randomization with unequal proportions to treatments was used to attempt to increase efficiency/power by reducing numbers of participants to certain treatments such as controls; this strategy, demonstrated for instances of three treatments(43), was used previously in RCT for BED(21). Restricted randomization procedure using randomly permutated blocks of 12 was used to approximate the allocation ratio across treatments. The randomization schedule, created by the biostatistician, was kept blind from research-clinicians and participants until starting treatment. Study-coordinator assigned participants to treatment conditions in the order they were enrolled by research-clinicians after completing all assessments and meeting eligibility. Independent assessors performing outcome evaluations were blind to specific treatments and participants were reminded not to disclose which treatments they received.
Statistical Analyses
Sample size was based on power calculations for primary aim hypothesis of greater LOC reductions and abstinence rates for the gshCBT and greater weight-loss for the gshBWL over the CON condition based on relevant data from (non-bariatric) RCTs for BED/obesity cited above, considering clinically-meaningful effect sizes, and performing sensitivity analyses for different outcomes. A target sample of N=120 (which was exceeded with N=140) allocated in a 5:5:2 ratio yielded >80% power to detect meaningful effect sizes (f=0.32, d=0.64) for main outcomes at two-sided alpha level of 0.05.
Analyses designed to compare treatments were “intent-to-treat” (ITT) and were performed for all randomized patients. Baseline characteristics (demographic and clinical variables) for the treatment groups were compared using chi-square analyses for categorical variables and ANOVAs for continuous measures.
The two primary treatment outcome variables were LOC-eating and weight-loss, each considered using complementary (categorical and continuous) approaches. LOC-eating was analyzed continuously (pre- to post-treatment) as frequency and categorically (end-point) as proportion achieving abstinence. Weight-loss was analyzed continuously (percent weight loss from baseline) and categorically (end-point) as proportion attaining 5% weight loss. Abstinence from LOC eating was defined as zero episodes during the past 28 days (regardless of size/quantity assessed by the EDE-BSV). For the categorical analyses, in instances of treatment dropouts or missing data, pre-treatment baseline data were carried forward (i.e., failure imputed). Secondary treatment outcomes were eating-disorder psychopathology (EDE-BSV global score), depression (BDI-II total), physical activity (GLTEQ total score), and functioning (SF36 Mental and Physical Scale total scores) tested continuously.
For analyses of continuous measures, ITT approach was followed with all available data (baseline, month 1, month 2, and posttreatment) used in mixed models analyses without imputation. Mixed effects models allow for different numbers of observations per subject, use all available data on each subject, and are unaffected by randomly missing data. They also provide flexibility in modeling the correlation structure of the data. In each model, we included fixed effects of time, treatment condition, treatment-by-time interaction, race, and all possible interactions. In the mixed models, treatment-by-time interaction is the primary test for the effectiveness of the treatments on outcome variables. In the mixed models, race was included in the analyses as a main effect (“predictor”) and as an interaction effect with treatment (“moderator”). Examining race as a predictor/moderator (a priori analysis) seemed indicated given findings in the obesity literature regarding racial differences in outcomes; for example, recent studies reported findings that, relative to White patients, Black patients have less weight-loss following bariatric surgery (31) and following treatments for BED (32) despite being more likely to stop binge eating in aggregated RCT data (32). Type of surgery (LSG, RYGB) main effects were also added to the models run on the entire data set (N=140). We also performed sensitivity analyses restricted only to the sample of individuals with LSG (N=123); no differences in results were observed and therefore they are not reported. Variables not conforming to normality were transformed prior to analysis. In each model the best-fitting variance-covariance structure was selected based on the Schwartz’ Bayesian Criterion (BIC). Significant main/interaction effects were explained by tests of simple effects or pairwise comparisons.
For analyses of categorical outcomes (end-point post-treatment analyses of LOC-eating abstinence and attaining 5% weight loss) chi-square tests were performed overall, and separately by race.
Results
Randomization and Participant Characteristics
Figure 1 shows the flow of participants throughout the study. Of the 140 randomized patients, N=56 received gshCBT, N=60 received gshBWL, and N=24 received CON. Treatment groups did not differ significantly in sex, race/ethnicity, education, history of DSM-5-defined BED diagnosis, type of bariatric surgery procedure (LSG, RYGB), time since surgery, or percent total weight loss at time of enrollment; only age differed significantly (p=.02; ηp2=.056), with gshCBT being older than gshBWL (Table 1). Treatment groups did not differ significantly on pretreatment levels of any outcome variables (Table 2): primary outcomes (LOC-eating (F(2,140)=0.46,p=.63) and weight (F(2,140)=0.36,p=.70)) and secondary outcomes (EDE Global (F(2,140)=0.45,p=.64), BDI-II (F(2,140)=1.04,p=.36), GLTEQ (F(2,140)=0.12,p=.90), SF36 (F(2,140)=1.27,p=.29)).
Figure 1.
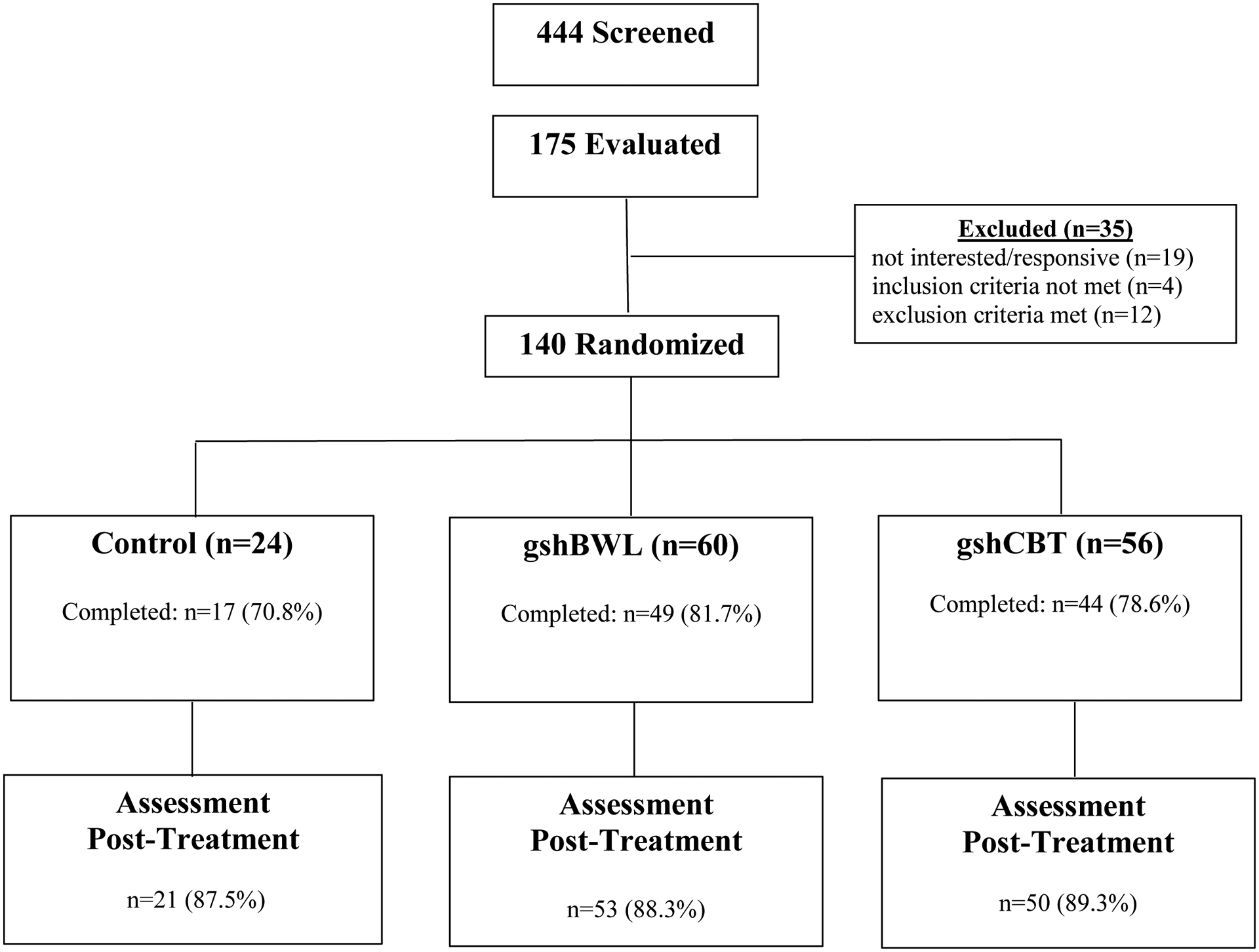
Flow of participants throughout study.
Table 2.
Clinical Variables Across Treatment Groups Pre- and Post-Treatment Following Bariatric Surgery
| Control n=24 | gshBWL n=60 | gshCBT n=56 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | N | M | SD | |
| EDE LOC eating | |||||||||
| Pre-Treatment | 24 | 17.0 | 16.9 | 60 | 23.4 | 33.7 | 56 | 22.1 | 23.2 |
| Post-Treatment | 20 | 4.0 | 6.6 | 52 | 6.8 | 9.8 | 49 | 6.4 | 11.7 |
| Change | 20 | −13.2 | 15.7 | 52 | −15.8 | 33.1 | 49 | −15.9 | 21.4 |
| Weight | |||||||||
| Pre-Treatment | 24 | 218.5 | 55.7 | 60 | 228.4 | 50.2 | 56 | 223.0 | 50.8 |
| Post-Treatment | 20 | 215.7 | 64.0 | 52 | 218.1 | 47.8 | 48 | 223.4 | 55.5 |
| Change | 20 | −2.6 | 8.3 | 52 | −4.7 | 8.1 | 48 | −3.4 | 7.8 |
| BMI | |||||||||
| Pre-Treatment | 24 | 36.5 | 7.8 | 60 | 36.8 | 6.1 | 56 | 36.9 | 6.6 |
| Post-Treatment | 20 | 35.8 | 9.2 | 52 | 35.5 | 6.2 | 48 | 36.8 | 7.2 |
| Change | 20 | −0.4 | 1.4 | 52 | −0.8 | 1.3 | 48 | −0.6 | 1.3 |
| %TWL during treatment | |||||||||
| Pre-Treatment | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Post-Treatment | 20 | −1.6 | 4.2 | 52 | −2.1 | 3.8 | 48 | −1.7 | 3.5 |
| EDE Global Score | |||||||||
| Pre-Treatment | 24 | 1.9 | 1.0 | 60 | 2.1 | 0.8 | 56 | 2.1 | 1.0 |
| Post-Treatment | 20 | 1.7 | 1.0 | 52 | 1.6 | 1.0 | 49 | 1.6 | 1.0 |
| Change | 20 | −0.4 | 0.8 | 52 | −0.5 | 0.9 | 49 | −0.5 | 0.8 |
| BDI-II | |||||||||
| Pre-Treatment | 24 | 9.6 | 8.2 | 60 | 10.9 | 9.4 | 56 | 12.8 | 10.6 |
| Post-Treatment | 21 | 7.4 | 8.2 | 53 | 7.6 | 8.6 | 50 | 9.9 | 11.2 |
| Change | 21 | −2.7 | 5.6 | 53 | −2.9 | 8.9 | −2.8 | 8.9 | |
| GLTEQ | |||||||||
| Pre-Treatment | 24 | 35.4 | 22.2 | 60 | 35.4 | 22.2 | 56 | 32.6 | 26.4 |
| Post-Treatment | 21 | 52.7 | 53.1 | 53 | 52.7 | 53.1 | 50 | 41.9 | 48.2 |
| Change | 21 | 16.6 | 55.0 | 53 | 8.5 | 41.4 | 50 | 10.6 | 42.3 |
| SF36-MFS | |||||||||
| Pre-Treatment | 24 | 76.8 | 17.4 | 60 | 74.9 | 16.2 | 56 | 68.5 | 20.1 |
| Post-Treatment | 21 | 82.5 | 16.0 | 53 | 74.0 | 18.1 | 49 | 68.9 | 23.2 |
| Change | 21 | 7.2 | 14.1 | 53 | −1.1 | 16.3 | 49 | 0.9 | 17.5 |
| SF36-PFS | |||||||||
| Pre-Treatment | 24 | 78.5 | 26.1 | 60 | 80.8 | 24.4 | 56 | 73.0 | 29.2 |
| Post-Treatment | 21 | 80.2 | 26.3 | 53 | 84.8 | 18.9 | 49 | 71.0 | 29.6 |
| Change | 21 | 4.8 | 11.0 | 53 | 2.5 | 17.1 | 49 | −0.4 | 21.8 |
Note: N=140. Data reported are raw data. BWL = behavioral weight loss (guided self-help); CBT = cognitive behavioral therapy (guided self-help). M = mean; SD = standard deviation. EDE = eating disorder examination interview; BDI-II = beck depression inventory-II; GLTEQ = godin leisure-time exercise questionnaire; SF36-MFS = medical outcomes study short form health survey-mental health functioning; SF36-PFS = medical outcomes study short form health survey-physical health functioning.
As Figure 1 shows, treatment completion rates, defined as at least five of six treatment sessions for active treatment groups (gshBWL and gshCBT) and attendance at three monthly assessment sessions for CON group) did not differ significantly (gshCBT (78.6%), gshBWL (81.7%), and CON (70.8%) (χ2(2)=1.20, p=.55)) nor did post-treatment assessment completion rates (gshCBT (89.3%), gshBWL (88.3%), and CON (87.5%) (χ 2(2)=0.10, p=.97)). Post-treatment assessment completion rates did not differ significantly by race overall: 88.7% (N=55/62) for non-white and 84.0% (N=63/75) for white participants (chi-square (df=1)=0.63, p=0.43) or by group: CON: 90.0% (n=9/10) for non-white and 75.0% (n=9/12) for white participants (Fisher’s Exact Test p-value =0.59); gshBWL group: 88.0% (n=22/25) for non-white and 85.7% (n=30/35) for white participants (Fisher’s Exact Test p=1.00); gshCBT: 88.9% (n=24/27) for non-white and 85.7% (n=24/28) for white participants (Fisher’s Exact Test p=1.00). Thus, end-point categorical analyses are unlikely to be confounded by missing data.
LOC-Eating Outcomes
Mixed models analyses of LOC-eating frequency (Table 2), which was log-transformed, revealed overall significant reductions from baseline to post-treatment across conditions (F(3,312)=10.96, p<.0001) but no significant differences between treatments (F(2,148)=0.15,p=0.86) nor significant treatment-by-time interactions (F(3,312)=0.52, p=0.79). No significant effects were observed for surgery type or for racial group (p-values > 0.10).
Figure 2 summarizes findings for LOC-eating abstinence at post-treatment across the treatment conditions (gshCBT (30.4%), gshBWL (26.7%), and CON (37.5%)) which did not differ significantly (χ 2(2)=0.96, p=.62;phi=.08). Overall, abstinence rates differed significantly by race (White (17.3%) versus Non-White (43.6%) participants (χ 2(1)=11.28, p=.001;phi=.29). Figure 2 also summarizes LOC-eating abstinence rates by race across the three treatment conditions. For gshCBT (N=55), abstinence rates for White (17.9%) and non-White (44.4%) participants differed significantly (χ 2(1)=4.55, p=.03;phi=.29). For gshBWL (N=60), abstinence rates for White (14.3%) and non-White (44.0%) participants differed significantly (χ2(1)=6.58,p=.01;phi=.33). For CON (N=22), abstinence rates for White (25.0%) and non-White (40.0%) participants did not differ significantly (Fisher’s Exact Test p=.65; phi=.16).
Figure 2.
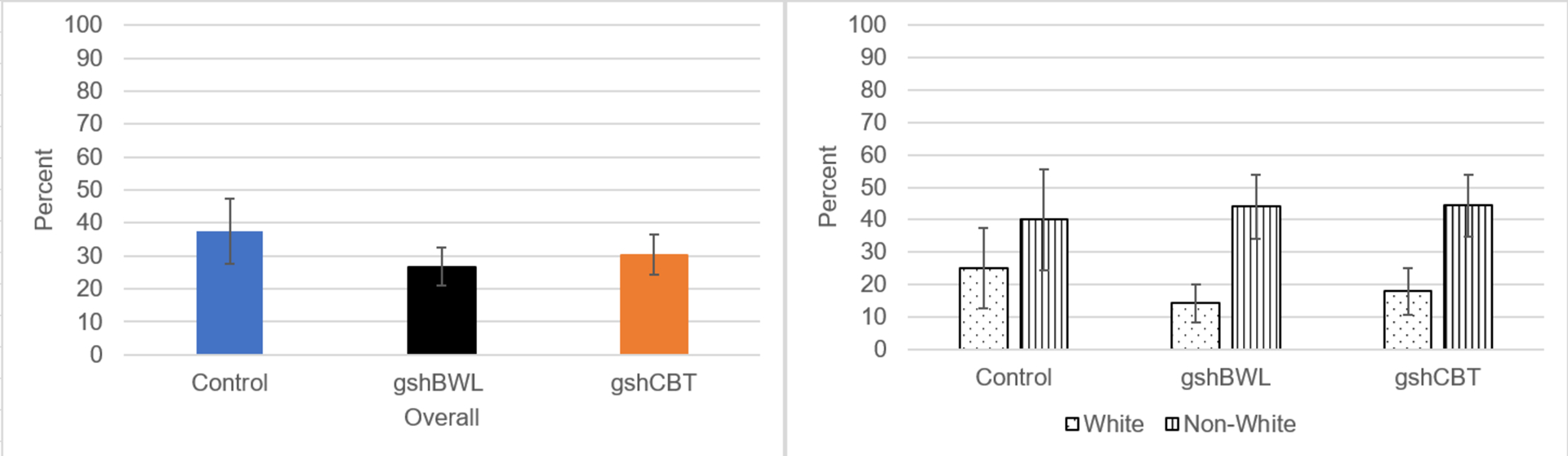
Percentage of LOC Eating Abstinence Overall and by Race Across Treatment Groups
Note: Left Panel summarizes LOC-eating abstinence rates across treatments which did not differ significantly (χ 2(2)=0.96, p=.62). Right Panel shows LOC-eating abstinence rates were significantly lower for White than non-White participants in gshCBT (χ 2(1)=4.55, p=.03) and gshBWL (χ2(1)=6.58, p=.01) but not in CON (Fisher’s Exact Test p=.65).
For context, descriptive data (Mean (SD)) for LOC-eating frequency for pre-treatment, post-treatment, and change, respectively for CON (17.1 (14.6), 6.4 (9.1), −10.6 (11.9) for White participants and 19.9 (21.0), 2.3 (2.6), −17.1 (SD 20.2) for non-White participants), for gshBWL (20.1 (19.6), 6.7 (8.8), −11.5 (19.3) for White participants and 27.9 (47.1), 6.9 (11.2), −21.5 (45.7) for non-White participants), and for gshCBT (25.0 (24.0), 9.2 (14.4), −14.8 (23.0) for White participants and 19.8 (22.6), 3.9 (8.0), −17.4 (20.5) for non-White participants).
Weight-Loss Outcomes
Table 2 summarizes findings for percent weight-loss across treatment conditions (and shows BMI values for descriptive context). Mixed models analyses of percent weight-loss (log-transformed) revealed overall significant reductions from baseline to posttreatment (F(3, 113)=11.89, p<.0001) but no significant differences between treatments (F(2, 130)=0.55, p=0.58) nor significant treatment-by-time interactions (F(5,133)=0.43, p=0.86). No significant effects were observed for surgery type or for racial group (all p>.08).
Figure 3 shows rates of attaining 5% weight-loss at post-treatment across the three treatment conditions: gshCBT (19.6%), gshBWL (21.7%), and CON (16.7%); these rates did not differ significantly (χ 2(2)=0.28, p=.87;phi=.04). Overall rates of attaining 5% weight-loss differed significantly by race (White (26.7%) versus Non-White (11.3%) participants (χ 2(1)=5.07, p=.02;phi=.19). Figure 3 also shows rates of attaining 5% weight-loss at post-treatment by race across the treatment conditions. For gshBWL, rates of 5% weight-loss attainment for White (31.4%) and non-White (8.0%) participants differed significantly (χ 2(1)=4.72, p=.03;phi=.28). In contrast, rates of 5% weight-loss attainment with gshCBT did not differ for White (25.0%) and non-White (11.1%) participants (Fisher’s Exact Test p=.30;phi=.18) nor did the rates differ by race in the CON condition (White (16.7%) versus non-White (20.0%); Fisher’s Exact Test p=1.00;phi=.04).
Figure 3.
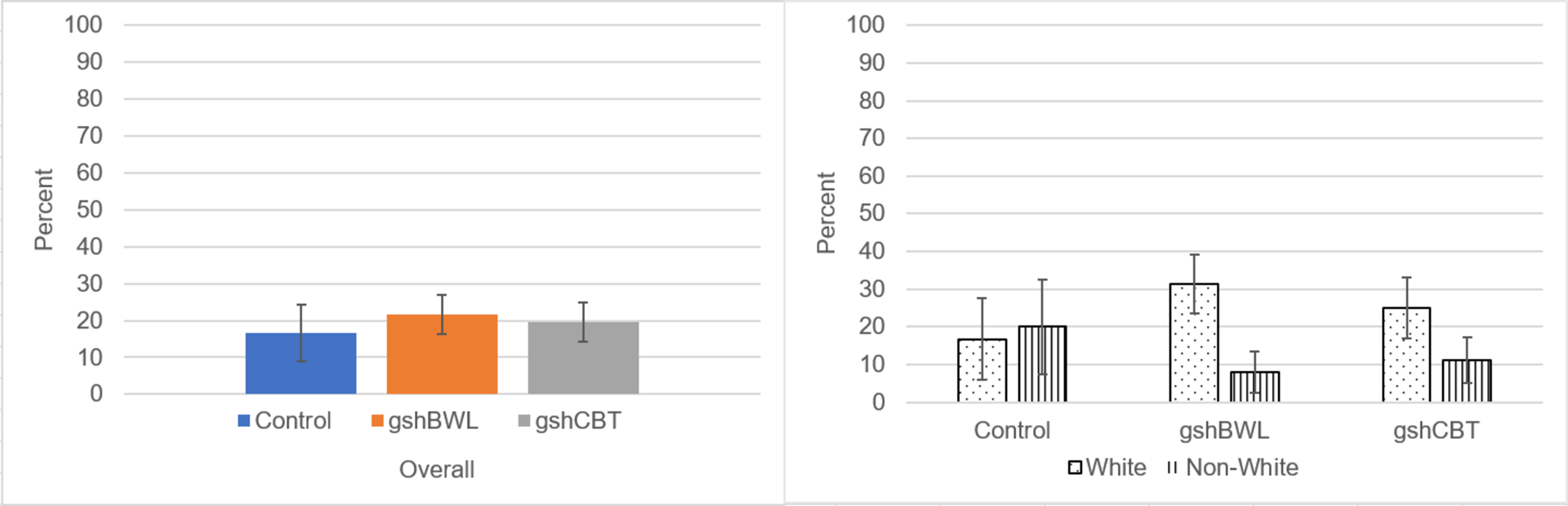
5% Weight Loss Overall and by Race Across Treatment Groups
Note: Left Panel summarizes proportion achieving 5% weight loss across treatments which did not differ significantly (χ 2(2)=0.28, p=.87). Right Panel shows LOC-eating abstinence rates were significantly higher for White than non-White participants in gshBWL (χ 2(1)=4.72, p=.03) but did not differ significantly in either CON (Fisher’s Exact Test p=1.00) or gshCBT (Fisher’s Exact Test p=.30).
For context, descriptive data (Mean (SD)) for weight for pre-treatment, post-treatment, and change, respectively for CON (211.7 (57.6), 201.8 (66.9), −3.2 (6.9) for White participants and 224.4 (59.1), 226.2 (67.4), −2.7 (10.4) for non-White participants), for gshBWL (228.1 (51.8), 213.7 (45.4), −6.5 (8.2) for White participants and 228.9 (48.9), 224.2 (51.4), −2.4 (7.4) for non-White participants), and for gshCBT (222.7 (54.1), 223.4 (61.6), +3.3 (8.0) for White participants and 221.8 (48.5), 223 (51.2), +2.2 (5.3) for non-White participants).
Secondary Outcomes: Eating-Disorder Psychopathology, Depression, Physical Activity, and Social Functioning Measures
Table 2 shows descriptive analyses for secondary (continuous) outcomes of eating-disorder psychopathology (EDE global score), depression (BDI-II), physical activity (GLTEQ), and social functioning (SF-36 composite scales). Mixed models revealed significant main effects for time in EDE global (F(1,117)=24.49, p<.0001), BDI (F(3, 112)=8.86, p<.0001), and SF-36 Mental Functioning Scale (F(2, 132)=3.27, p=0.04) scores; main effects for time were not significant for either GLTEQ or SF-36 Mental Functioning scores. Mixed models revealed no significant differences between treatments nor any significant treatment-by-time interactions on any of these secondary outcomes (all p-values >0.05). No significant effects were observed for race on any of these outcomes, except for a borderline significant main effect on EDE Global (F(1,129)=3.82, p=0.05) with White participants having greater ED psychopathology scores.
Discussion
The objective was to evaluate effective treatments from the BED and obesity fields adapted for use for patients who have undergone bariatric surgery and are experiencing recurrent LOC-eating postoperatively. This 12-week RCT compared the effectiveness of two guided-self-help treatments (gshCBT and gshBWL) and a control (CON) condition comprising standard care from the bariatric center. Overall, significant reductions in LOC-eating frequency and weight-losses were observed that not differ significantly across the treatments. Rates of abstinence from LOC-eating were 30% (for gshCBT), 27% (for gshBWL), and 38% (for CON) and the proportion of patients achieving 5% weight loss were 20%, 22%, and 17%, respectively; these categorical post-treatment findings also did not differ across treatments. Mixed analyses also revealed significant reductions (changes pre- to post-treatment) in dimensional secondary outcomes (levels of eating-disorder psychopathology, depression, and mental functioning) that did not differ significantly across treatments. Although mixed models of treatment changes in continuous variables revealed that race neither predicted nor moderated treatment outcomes, analyses of treatment endpoints for categorical variables revealed non-White participants had higher proportion attaining abstinence from LOC-eating but lower proportion achieving 5% weight loss than White participants.
This is the first RCT post-bariatric surgery specifically targeting LOC-eating. These acute results suggest reducing LOC-eating post-surgery does not appear to lead to meaningful improvements in weight loss. Comparisons to the literatures is offered descriptively for context but needs to be viewed cautiously given differences across studies in methodologies, comparison groups, therapists, patient groups and clinical settings. One small pilot study(23) with 16 patients of a similar low-intensity CBT also delivered 6 months post-bariatric surgery reported statistically significant reductions in binge-eating-related symptoms (not specifically LOC-eating), weight, and depression but did not have any control group. Reviews(3,8,9) have concluded that some small RCTs of behavioral/psychosocial interventions post-bariatric surgery have reported statistically significant results although weight losses, like those in our RCT, tend to be modest and mixed across trials. More broadly, our gshCBT abstinence rates for LOC-eating are comparable to binge-eating abstinence rates reported by Peterson(44) though lower than those we reported for patients with BED who had not undergone bariatric surgery(21); overall, these rates fall within the range across RCTs for gshCBT(19,20) for BED. The observed gshBWL outcomes for LOC are favorable to those reported for gshBWL for BED(21) but inferior to those for traditional BWL for BED(21,22,24). Finally, while some research has found scalable versions of CBT can be effectively delivered by “generalist” clinicians(26), other research in generalist medical settings has not(45).
Collectively, these findings suggest that longer and more intensive interventions may be needed to provide greater benefit to patients with recurrent LOC-eating following bariatric surgery. As a parallel, inspection across RCTs at our center for BED suggests that intensive CBT and BWL treatments(24,25) outperform scalable guided-self-help versions(21). In this postoperative group, the statistically significant weight loss (a function of time, not specific treatments) was not clinically meaningful. Importantly, the amount of post-surgical weight-loss at 6–9 months (time of RCT enrollment) was only 20.3%; this is dampened relative to what is typically reported (5,6); moreover, early postoperative weight loss predicts maximal weight-loss (6). Thus, it appears that this subgroup with recurrent LOC-eating postoperatively derives less benefit from bariatric surgery and perhaps requires more intensive adjunctive specialist treatments. Future research should investigate the utility of pharmacological treatments and employ stepped care approaches to assist non-responders to initial treatments(25).
Race was associated with different end-point outcomes reflecting clinically-meaningful categories. Specifically, non-White participants had higher proportion attaining LOC-eating abstinence (43.6% vs 17.3%) but lower proportion achieving 5% weight loss (11.3% versus 26.7%) than White participants. These findings are consistent with previous studies that Black patients have lower weight losses with bariatric surgery(31) and with treatments for BED(32) despite being more likely to achieve abstinence from binge eating(32). Although analyses did not reveal moderation effects for race, White patients had lower LOC-eating abstinence rates than non-White patients if receiving either gshCBT or gshBWL (17.9% or 14.3% versus 44.4% or 44.0%, respectively). The racial difference was less salient in the CON condition; although in the same direction (25% versus 40.0%) it was not significant perhaps reflecting less statistical power as fewer participants were allocated to CON. In terms of attaining 5% weight loss, White patients were significantly more likely than non-White patients to exceed 5% weight loss if receiving gshBWL (31.4% versus 8.0%) but the trends in the same direction for gshCBT and CON treatments did not achieve statistical significance.
We note potential strengths and weaknesses. Strengths include randomized controlled comparison of two scalable treatments and control condition (standard-of-care at bariatric center-of-excellence), doctoral research-clinicians performing blinded independent assessments, and rigorous assessment methods for primary outcomes. Self-report for secondary outcomes (e.g., physical activity) represents a limitation and future studies such consider objective measures. Relatively small sample size, particularly for the CON, represents a potential limitation in terms of statistical power to detect small differences. RCT designs with CON group receiving standard-care tend to have lower effect-sizes than efficacy trials with wait-list controls; although CON did not differ in assessment/completion rates from other treatments, lack of data on frequency/types of standard-care obtained is a limitation. For example, it is not known whether the CON participants attended support/nutrition groups more than is typical (perhaps due to study encouragement) and this might potentially have contributed to limiting the differences with the treatment groups. Findings may not generalize to different centers, to groups differing in sociodemographic characteristics, or to different treatment delivery methods (which range from pure self-help to intensive versions delivered by “specialists”). Our findings pertain only to acute outcomes following 12 weeks of treatment, and longer-term analyses are needed to address questions regarding durability, maintenance, and longer-term effects, as well as whether reducing LOC-eating enhances longer-term weight outcomes.
Study Importance Questions.
What is already known about this subject?
Postoperative loss-of-control (LOC) eating predicts poorer bariatric surgery outcomes.
Optimal time for adjunctive psychosocial interventions is following bariatric surgery, but research has yet to test treatments specifically for LOC eating after bariatric surgery.
What are the new findings in your manuscript?
Reductions in LOC-eating and weight-loss were statistically significant over time, but the three treatments did not differ significantly from each other.
LOC-eating abstinence rates following treatments were 30% for gshCBT, 27% for gshBWL, and 38% for the control condition.
How might your results change the direction of research or the focus of clinical practice?
LOC eating following bariatric surgery is challenging to treat and may require intensive specialist treatments.
Future research should investigate more intensive forms of psychological, behavioral, and pharmacological treatments to reduce LOC eating.
Funding:
This research was supported, in part, by National Institutes of Health grants R01 DK098492. Funders played no role in the content of this paper.
Footnotes
Clinicaltrials.gov registration number: NCT02259322 (Loss of Control Eating Following Weight Loss Surgery). URL: https://clinicaltrials.gov/ct2/show/NCT02259322
Potential conflicts of interest: The authors declare no conflicts of interest. Drs. Grilo, Ivezaj and Gueorguieva report several broader interests which did not influence this research or paper. Dr. Grilo’s broader interests include: Consultant to Sunovion and Weight Watchers; Honoraria for lectures, CME activities, and presentations at scientific conferences and Royalties from Guilford Press and Taylor & Francis Publishers for academic books. Dr. Ivezaj reports broader interests including Honoraria for journal editorial roles and lectures. Dr. Gueorguieva discloses royalties from book “Statistical Methods in Psychiatry and Related Fields” published by CRC Press, a United States patent application 20200143922by Yale University: Chekroud, AM., Krystal J., Gueorguieva, R., & Chandra A.. “Methods and Apparatus for Predicting Depression Treatment Outcomes”, and consulting fees as a member of the Working Group for PTSD Adaptive Platform Trial of Cohen Veterans Bioscience.
Data Sharing Statement: (1) Will individual deidentified participant data be available? No. (2) What other documents will be available? No. (3) With whom will data be shared? De-identified data will be provided in response to reasonable written request to achieve goals in an approved written proposal.
References
- 1.Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. [DOI] [PubMed] [Google Scholar]
- 2.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarwer DB, Heinberg LJ. A review of the psychosocial aspects of clinically severe obesity and bariatric surgery. Am Psychol 2020;75:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spaniolas K, Kasten KR, Brinkley J, Sippey ME, Mozer A, Chapman WH, Pories WJ. The changing bariatric surgery landscape in the USA. Obes Surg. 2015;25:1544–1546. [DOI] [PubMed] [Google Scholar]
- 5.Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Annals of surgery 2013;257:791–797. [DOI] [PubMed] [Google Scholar]
- 6.Manning S, Pucci A, Carter NC, Elkalaawy M, Querci G, Magno S, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David LA, Sijercic I, Cassin SE. Preoperative and post-operative psychosocial interventions for bariatric surgery patients: a systematic review. Obesity Rev. 2020;21:e12926. [DOI] [PubMed] [Google Scholar]
- 9.Stewart F, Avenell A. Behavioural interventions for severe obesity before and/or after Bariatric surgery: a systematic review and meta-analysis. Obesity Surg. 2016;26:1203–1214. [DOI] [PubMed] [Google Scholar]
- 10.Colles SL, Dixon JB, O’Brien PE. Grazing and loss of control related to eating: two high-risk factors following bariatric surgery. Obesity. 2008;16:615–22. [DOI] [PubMed] [Google Scholar]
- 11.White MA, Kalarchian M, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: a prospective 24-month follow-up study. J Clin Psychiatry 2010;71:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devlin MJ, King WC, Kalarchian MA, et al. Eating pathology and associations with long-term changes in weight and quality of life in the longitudinal assessment of bariatric surgery study. Int J Eat Disorder 2018;51:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5), Fifth edition edn: Washington DC, 2013. [Google Scholar]
- 14.Ivezaj V, Barnes RD, Cooper Z, Grilo CM. Loss-of-control eating after bariatric/sleeve gastrectomy surgery: Similar to binge-eating disorder despite differences in quantities. Gen Hosp Psychiatry 2018;54:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivezaj V, Lydecker J, Wiedemann AA, Duffy AJ, Grilo CM. Does bariatric binge-size matter? Conceptual model and empirical support. Obesity. 2020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mond JM, Latner JD, Hay PH, Owen C, Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: another nail in the coffin of a problematic distinction. Behav Res Ther 2010;48:661–669. [DOI] [PubMed] [Google Scholar]
- 17.Stein DJ, Szatmari P, Gaebel W, Berk M, Vieta E, Maj M, et al. Mental, behavioral and neurodevelopmental disorders in the ICD-11: an international perspective on key changes and controversies. BMC Med 2020;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White MA, Masheb RM, Rothschild B, Burke-Martindale C, Grilo CM. The prognostic\significance of regular binge eating in extremely obese gastric bypass patients: 12-month postoperative outcomes. J Clin Psychiatry 2006;67:1928–1935. [DOI] [PubMed] [Google Scholar]
- 19.Grilo CM. Psychological and behavioral treatments for binge-eating disorder. J Clin Psychiatry 2018;78(S1): 20–24. [DOI] [PubMed] [Google Scholar]
- 20.Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, Schmidt R. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J Consult Clin Psychol 2019;87:91–105. [DOI] [PubMed] [Google Scholar]
- 21.Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther 2005;43:1509–1525. [DOI] [PubMed] [Google Scholar]
- 22.Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Arch Gen Psychiatry 2010;67:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sockalingam S, Casin S, Wnuk S, Du C, Jackson T, Hawa R, Parikh SV. A pilot study on telephone cognitive behavioral therapy for patients six-months post-bariatric surgery. Obes Surg. 2017;27:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grilo CM, Masheb RM, Wilson GT, Gueorguieva R, White MA. Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. J Consult Clin Psychology 2011;79:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grilo CM, White MA, Masheb RM, Ivezaj V, Morgan PT, Gueorguieva R Randomized controlled trial testing the effectiveness of adaptive “SMART” stepped-care treatment for adults with binge-eating disorder comorbid with obesity. Am Psychol 2020:75:204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med. 2011;73:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBar LL, Striegel-Moore RH, Wilson GT, Perrin N, Yarborough BJ, Dickerson J, Lynch F, Rosselli F, Kraemer HC. Guided self-help treatment for recurrent binge eating: replication and extension. Psychiatr Serv. 2011;62:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch FL, Striegel-Moore RH, Dickerson JF, Perrin N, Debar L, Wilson GT, Kraemer HC. Cost-effectiveness of guided self-help treatments for recurrent binge eating. J Consult Clin Psychol. 2010;78:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, Hjelmesæth J, Kinzl J, Leitner DR, Makaronidis JM, Schindler K, Toplak H, Yumuk V. Obesity management task force of the European Association for the Study of Obesity released practical recommendations for the post-bariatric surgery medical management. Obes Surg. 2018;28:2117–2121 [DOI] [PubMed] [Google Scholar]
- 30.Mechanick JI, Kushner RF, Sugerman H., et al. American Association Clinical Endocrinologists,The Obesity Society, and American Society for Metabolic and Bariatric Surgery medical guidelines for clinical practice for the periopetrative nutritional and nonsurgical support of the bariatric surgery patient. Obesity. 2009;17:S3–S7219927143 [Google Scholar]
- 31.Wood MH, Carlin AM, Ghaferi AA, Varban OA, Hawasli A, Bonham AJ, Birkmeyer NJ, Finks JF. Association of race with bariatric surgery outcomes. JAMA Surg. 2019;154:e190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lydecker JA, Gueorguieva R, Masheb R, White MA, Grilo CM. Examining race as a predictor and moderator of treatment outcomes for binge-eating disorder: analysis of aggregated randomized controlled trials. J Consult Clin Psychol 2019;87:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Zwaan M, Hilbert A, Swan-Kremeier L, Simonich H, Lancaster K, Howell LM, et al. Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2010;6: 79–85. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JE, Selzer F, Kalarchian MA, Devlin MJ, Strain GW, Elder KA, et al. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis. 2012;8:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grilo CM, Masheb RM, Wilson GT. Different methods for assessing the features of eating disorders in patients with binge eating disorder: a replication. Obesity Res 2001;9:418–422. [DOI] [PubMed] [Google Scholar]
- 36.Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord 2004;35:80–85. [DOI] [PubMed] [Google Scholar]
- 37.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis 2015;11: 489–506. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clin Psychol Rev 1998;8:77–100. [Google Scholar]
- 39.Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J App Sports Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 40.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using Caltrac accelometer and five questionnaires. Med Sci Sports Exer. 1994;26:376–382. [PubMed] [Google Scholar]
- 41.Ware JE, Sherbourne CD. The Mos 36-Item Short-Form Health Survey (SF-36): I. Conceptual-framework and item selection. Med Care 1992;30: 473–483. [PubMed] [Google Scholar]
- 42.McHorney CA, Ware JE Jr, Lu JR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994: 40–66. [DOI] [PubMed] [Google Scholar]
- 43.Woods SW, Sholomskas DE, Shear MK, Gotrman JM, Barlow DH, Goddard AW, Cohen J. Efficient allocation of patients to treatment cells in clinical trials with more than two treatment conditions. Am J Psychiatry. 1998;155:1446–1448. [DOI] [PubMed] [Google Scholar]
- 44.Peterson CB, Mitchell JE, Crow SJ, Crosby RD, Wonderlich SA. The efficacy of self-help group treatment and therapist-led group treatment for binge eating disorder. Am J Psychiatry. 2009;166:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grilo CM, Masheb RM, White MA, Gueorguieva R, Barnes RD, Walsh BT, McKenzie K, Genao I, Garcia R. Treatment of binge eating disorder in racially and ethnically diverse obese patients in primary care: randomized placebo-controlled clinical trial of self-help and medication. Behav Res Ther. 2014;58:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


