Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a retrovirus having genome size of around 30 kb. Its genome contains a highly conserved leader sequence at its 5′ end, which is added to all subgenomic mRNAs at their 5′ terminus by a discontinuous transcription mechanism and regulates their translation. Targeting the leader sequence by RNA interference can be an effective approach to inhibit the viral replication. In the present study an in‐silico prediction of highly effective siRNAs was performed to target the leader sequence using the online software siDirect version 2.0. Low seed‐duplex stability, exact complementarity with target, at least three mismatches with any off‐target and least number of off‐targets, were considered as effective criteria for highly specific siRNA. Further validation of siRNA affinity for the target was accomplished by molecular docking by HNADOCK online server. Our results revealed four potential siRNAs, of which siRNA having guide strand sequence 5′GUUUAGAGAACAGAUCUACAA3′ met almost all specificity criteria with no off‐targets for guide strand. Molecular docking of all predicted siRNAs (guide strand) with the target leader sequence depicted highest binding score of −327.45 for above‐mentioned siRNA. Furthermore, molecular docking of the passenger strand of the best candidate with off‐target sequences gave significantly low binding scores. Hence, 5′GUUUAGAGAACAGAUCUACAA3′ siRNA possess great potential to silence the leader sequence of SARS‐CoV‐2 with least off‐target effect. Present study provides great scope for development of gene therapy against the prevailing COVID‐19 disease, thus further research in this concern is urgently demanded.
Keywords: leader sequence, off‐target effect, RNAi therapy, SARS‐CoV‐2, siRNA
1. INTRODUCTION
Current reports on COVID‐19 by WHO depicted 90 759 370 cases which includes 1 963 169 deaths as on 14 January 2021. In turn, the absence of any potential treatment and vaccine in clinical use with no side effects increases the severity of the disease and put up an urgent demand for effective treatment (https://covid19.who.int/). Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the etiological agent of the current COVID‐19 pandemic, is an enveloped virus which belongs to betacoronavirus genus and has 50% and 80% homology with Middle East Respiratory Syndrome virus and SARS‐CoV, respectively. It has a positive sense 30 kb RNA genome which is single‐stranded. 1 The characteristic transcript of coronavirus contains a 3′ polyA tail and 5′ cap. After entering the host cell, the virus translates non‐structural proteins (nsps) from the 2 ORFs (Open reading frames) 1a and 1b. Polypeptide 1a of 440‐500 kDa produced from ORF1a cleaves to form 11 nsps. Furthermore, single frameshift of ribosome occurs at the upstream of the stop codon of ORF1a immediately and allows for the continuation of translation of ORF1b thereby yielding a long polypeptide of 740‐810 kDa which cleaves to form 15 nsps. 2 The cleavage of polypeptides is performed by proteases nsp5 and nsp3 that contain a 3C like protease and papain enzyme‐like protease domain, respectively. 3 For the replication of viral genome and its transcription, nsp12 encodes a protein having an activity of RNA‐dependent RNA polymerase. 4 RNA intermediates of negative sense are produced to act as a template for positive‐sense genomic RNA synthesis and for the synthesis of subgenomic RNAs. The packaging of the genomic RNA is done by utilizing the structural proteins to form new virions. Small subgenomic RNAs translate into structural proteins like envelope (E), spike (S), nucleocapsid (N), and membrane (M) proteins along with some accessory proteins. Six accessory proteins are translated by SARS‐CoV‐2, which includes 10, 8, 7a, 7b, 6, and 3a as per the current annotation. 5 Each SARS‐CoV‐2 RNA have a leader sequence in common, of around 70 nucleotides which gets fused to the body of the sequence present in the part at the downstream of the genome (Figure 1). The known models made it evident that the fusion of body sequence to the leader sequence happen while the negative strand is being synthesized at the small transcription regulatory sequence motifs located in the immediate vicinity of the ORFs. This regulatory sequence has conserved core sequence of 6‐7 nucleotides bordered by some variable sequences. 6 During the synthesis of the negative strand, RNA‐dependent RNA polymerase, when encounters the transcriptional regulatory sequence, pauses and switch template to leader transcriptional regulatory sequence which leads to discontinuous transcription resulting in fusion of the leader to body sequence. Now these negative sense strand intermediates which have fused leader sequence transcribes into positive‐sense strand mRNAs. 7 This leader sequence fused to subgenomic RNAs (5′ACCUUCCCAGGUAACAAACCAACCAACUUUCGAUCUCUUGUAGAUCUGUUCUCUAAACGAAC 3′) is nearly identical to the leader sequence or the 5′ untranslated region of the viral genome. 8
FIGURE 1.
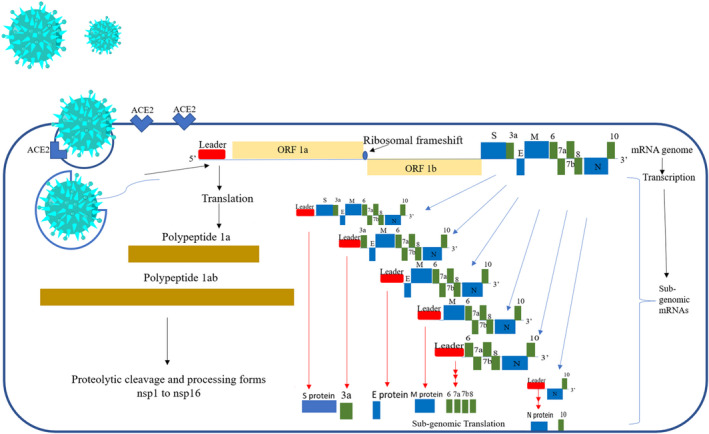
Genomic structural organization of SARS‐CoV‐2 genome depicting leader sequence (red), viral ORF 1a and 1b (light brown), structural genes (blue) and non‐structural genes (green), that translates into polypeptides 1a and 1ab (dark brown), structural proteins (blue) and non‐structural proteins (green) respectively 6
RNA interference or RNAi is a highly specific post‐transcriptional mechanism of gene silencing. Utilization of double‐stranded DNA for silencing the expression of a gene has proved itself highly effective compared to single antisense or sense strand. 9 Double‐stranded RNA mediates gene silencing in a homology‐dependent manner and may modulate the expression of genes in viral systems. 10 , 11 Small interfering RNA (siRNA) is a double stranded RNA of around 21 nucleotides, which has shown immense potentials in numerous therapeutic and gene function studies. The strand of siRNA which has complementarity with the target gene is the guide strand and the other strand is the passenger strand. 12 The perfect complementarity of the guide strand of siRNA with the target highly enhances the specificity of the silencing mechanism. A drawback associated is silencing of off‐target genes or unintended gene down regulation, due to the complementarity of seven nucleotides of seed region of siRNA with the off‐target gene. Studies have reported that melting temperature or thermodynamic stability of the duplex of seed siRNA sequence (2‐8 nucleotide of siRNA guide strand from 5′ end) and the target gene mediates the off‐target binding effect. Thus, a siRNA which have perfect complementarity only with the target gene and have low thermostability of seed‐target duplex (Tm less than 21.5°C) can effectively eliminate the off‐target binding of the siRNA. Also, selection of an siRNA having a minimum of 2 mismatches with any other off‐target sequence can further reduce the probability of siRNA to bind to the undesired off‐target sequence. 13 , 14 Along with seed region the effectiveness of non‐seed region of guide strand has also been reported in mediating off target effect but a negative correlation of T m value and GC content with downregulation or off target effect was found. 15
Several studies have shown scope for RNAi (RNA interference) in the treatment of viral infections. Numerous researchers have reported an effective reduction of target viral gene expression by corresponding siRNAs. RNAi‐based therapies have been conducted in various cell and animal disease models of polio, Rous sarcoma, HIV, HCV, and HBV viral diseases and have shown a significant reduction in expression of genes involved in viral replication. 16 , 17 , 18 , 19 , 20 Efforts have also been made to inhibit essential SARS‐CoV genes, including those which mediate replication of the virus, by siRNAs. 21 Li et al, in their study demonstrated the high potential of siRNA complementary to leader sequence in decreasing the expression of viral genes and hence viral replication in 293T and VeroE6 cell lines compared to the siRNA generated to reduce spike protein expression. 22 Very recently, Chen et al in their computational study identified nine potential siRNA target sequences with least off‐target effect, against different proteins of SARS‐CoV‐2. Therefore, all these studies portray the great potential of siRNAs therapy to fight against the deadly disease of COVID‐19. 23 , 24
The leading role of the leader sequence of SARS‐CoV‐2 makes it a highly operative target which can be focused to develop therapies against COVID‐19. Hence, this study deals with the in silico investigation of a potential siRNA against the leader sequence of SAS‐CoV‐2 and its validation by molecular docking approach.
This study will lead to development of a highly effective gene therapy based on RNAi approach against the ongoing pandemic of COVID‐19 by contributing an efficient siRNA sequence‐specific for leader sequence, efficient enough to down regulate the replication of SARS‐CoV‐2 virus effectively by targeting the majority of the genes of the virus.
2. METHODOLOGY
2.1. Prediction of siRNA
The leader sequence (5′ACCUUCCCAGGUAACAAACCAACCAACUUUCGAUCUCUUGUAGAUCUGUUCUCUAAACGAAC 3′) was obtained from SARS‐CoV‐2 genome (GenBank: MW040697.1) from NCBI database. To find probable siRNAs for the leader sequence, this sequence was subjected to online siRNA finding tool siDirect version 2.0 (http://siDirect2.RNAi.jp/). The max T m value for seed‐target duplex stability was kept at 21.5°C to reduce off‐target effect. Homo sapiens non‐redundant database was selected for the analysis of off‐target sequences to avoid siRNA prediction against human RNAs. The leading criteria considered for siRNA prediction consists of selection of such sequence, which do not have a seed sequence having exact complementarity with any of the off‐target sequence. Moreover three conditions were considered which has to be fulfilled for effective siRNA prediction: presence of A/U in guide strand 5′ terminus, presence of G/C in passenger strand 5′ terminus, minimum of 4 A/U in the seven base pairs at 5′ end of guide strand. The siRNA having minimum T m value of seed target duplex, no off‐target for the overall guide strand (including both seed as well as non‐seed regions) and least number of off‐targets for the passenger strand was considered best. 14 To confirm null guide strand off‐target effect, the region of leader sequence targeted by best siRNA was evaluated by Invitrogen BLOCK iT RNAi Designer tool (Thermo fisher Scientific https://rnaidesigner.thermofisher.com/rnaiexpress/) where BLASTn of viral leader sequence with the gene sequences of species Homo sapiens was performed, to find similar or identical sequences present in database of Invitrogen. The region targeted by best siRNA was evaluated for BLAST matches with the human gene database as any effective similarity with this region will lead to complementarity with the siRNA guide strand and hence off‐target effect. Moreover BLAST of the best siRNA targeted region as well as whole leader sequence was done using NCBI Human genomic and transcript database excluding XM/XP models. The similarity scores were evaluated based on E‐values where E‐value less than 0.01 was considered significant for matches.
2.2. Analysis of siRNA to leader sequence binding affinity by molecular docking
To evaluate the structural binding potential of siRNAs with the leader sequence, molecular docking was performed by using online server HNADOCK developed by School of Physics, Huang University of Science and Technology. 25 The leader sequence and the sequences of siRNAs guide strands were given as input for evaluating the affinity of siRNAs for the target leader sequence. The siRNA scoring the highest binding score for leader sequence, having the lowest T m value of seed duplex and having the least number of passenger strand off‐targets was further considered for off‐target effect analysis by molecular docking.
2.3. Analysis of off‐target effect of best predicted siRNA by molecular docking
As all the predicted siRNAs were not having any off‐target for their whole guide strand sequences, so, predicted passenger strand off‐target sequences and the corresponding sequence of passenger strand of the best‐selected siRNA were considered for molecular docking by HNADOCK online server to analyze the potential of best siRNA for showing off‐target effect. All the other parameters were kept at default values of the docking tool. The binding energy scores were compared to evaluate the most probable off‐target for the best siRNA. 25
3. RESULT AND DISCUSSION
Targeting the gene expression by RNA interference has been widely employed to develop therapeutics for numerous diseases including viral diseases. siRNA, the essential component for RNAi, is a 21‐nucleotide duplex having 3′ overhang of two nucleotides. This small interfering RNA after entering the cell interacts with the RNA induced silencing complex (RISC). This complex arranges itself on one strand (guide strand) of the duplex and gets activated when the passenger strand is removed. 26 The activated complex consists of Argonaute core protein and single strand of siRNA which guides the complex to the target mRNA sequence. The 5′ terminal end of the guide strand anchors itself in binding pocket of middle domain while the 3′ terminal end anchors into the PAZ domain of Argonaute protein. Hence, 19 nucleotide bases of the siRNA guide strand that is 2‐20 nucleotides from 5′ terminus play a role in recognition of target RNA resulting in silencing of its expression by cleaving it. 27 Due to the dependence of this approach on sequence complementarity between siRNA and the target mRNA, silencing of similar non‐targeted sequences often limits the efficiency of the process. Such unintended silencing by siRNA of undesired sequences is called off‐target effect. Large‐scale studies based on knockdown experiments have made it evident that off‐target effect arises due to base pairing of the 2‐8 nucleotides at 5′ end or seed region of siRNA guide strand, with a nearly complementary sequence in the untranslated region of a non‐targeted mRNA. 15 Keeping the benchmark of 21.5°C for the seed duplex stability or T m value of the binding of seed region of siRNA to target can minimize the off‐target effect. The T m value, lower than the benchmark value, can significantly increase the specificity of the approach by alleviating off‐target binding. 14 Non‐seed region of siRNA guide strand has also been reported to contribute to off target effect along with seed region if found to have complementarity with off target sequence so analysis of whole siRNA guide sequence for off‐target effect would be beneficial in this regard. The presence of gene sequences in human host which have similarity or identicality with the leader sequence or the regions of leader sequence targeted by siRNA will increase complementarity to guide strand and thus the off‐target effect. So, considering the regions of leader sequence which do not have any similarity with the human gene sequences to design siRNA will further reduce the off‐target effect by non‐seed as well as seed region of guide siRNA strand. 15
The high specificity of siRNA becomes a major concern especially when have to be utilized for the development of gene therapy against the devastating pandemic like COVID‐19 where non‐specific silencing may lead to fatal circumstances. Although many researches have been conducted for the investigation of siRNA to study gene silencing in a highly related coronavirus strain of SARS‐CoV but studies related to SARS‐CoV‐2 are nearly absent. Wu et al, in their study on SARS‐CoV studied the effect of 7 siRNAs in a Vero E6 cell line culture and revealed promising siRNAs complementary to spike protein sequence and 3′ untranslated region capable of inhibiting the replication of virus. 28 Shi et al, targeted structural proteins like envelope, membrane, nucleocapsid of SARS‐CoV by siRNA therapy in Vero E6 cell line culture and obtained 3 siRNAs which can reduce the expression of proteins by 80%. 21 A recent computational analysis of SARS‐CoV‐2 sequences revealed potential siRNA target sequences which can be utilized to develop siRNA for effective silencing of potential proteins of SARS‐CoV‐2. 23 But, targeting the leader sequence of SARS‐CoV‐2 can give exceptional result by inhibiting the all‐round process of replication of virus in the host body. Past in‐vitro studies on cell lines cloned with leader sequence of SARS‐CoV and virus infected cell cultures provided evidence for immense potential of targeting the leader sequence by siRNA instead of structural proteins of the virus. 22 Hence the present study deals with the in silico prediction of high potential siRNA which can effectively target the leader sequence of the SARS‐CoV‐2 and can be utilized to develop efficient gene therapy against the highly victimizing pandemic disease of COVID‐19. Our siRNA prediction results by siDirect revealed 4 potent siRNAs having seed duplex T m values of less than 21.5°C with no off‐target sequence corresponding to the guide strands of siRNA (Tables 1 and 2).
TABLE 1.
Predicted siRNAs for the leader sequence, corresponding target sequences and seed duplex stability parameters or T m values of respective strands
|
Leader sequence ACCUUCCCAGGUAACAAACCAACCAACUUUCGAUCUCUUGUAGAUCUGUUCUCUAAACGAAC | |||||
|---|---|---|---|---|---|
| S. no. | Target position | Target sequence |
RNA oligo sequences 21 nt guide (5′→3′) 21 nt passenger (5′→3′) along with 2‐nt 3′ overhangs |
Seed‐duplex stability (T m value °C) | |
| Guide | Passenger | ||||
| 1 | 13‐35 | AACAAACCAACCAACUUUCGAUC |
UCGAAAGUUGGUUGGUUUGUU CAAACCAACCAACUUUCGAUC |
19.7 | 18.8 |
| 2 | 25‐47 | AACUUUCGAUCUCUUGUAGAUCU |
AUCUACAAGAGAUCGAAAGUU CUUUCGAUCUCUUGUAGAUCU |
20.3 | 16.7 |
| 3 | 36‐58 | UCUUGUAGAUCUGUUCUCUAAAC |
UUAGAGAACAGAUCUACAAGA UUGUAGAUCUGUUCUCUAAAC |
20.2 | 20.3 |
| 4 | 38‐60 | UUGUAGAUCUGUUCUCUAAACGA |
GUUUAGAGAACAGAUCUACAA GUAGAUCUGUUCUCUAAACGA |
11.7 | 20.2 |
TABLE 2.
Details of off target sequences for the best siRNA‐4
| Strand (5′→3′) | No. of off targets | Complementary sequence (5′→3′) | Alignment of complementary sequence with off targets in humans a | Details of off target sequences (NCBI reference number or GenBank ID) |
|---|---|---|---|---|
| Guide GUUUAGAGAACAGAUCUACAA | 0 | TTGTAGATCTGTTCTCTAAAC | Nil | Nil |
|
Passenger GUAGAUCUGUUCUCUAAACGA |
7 | CGTTTAGAGAACAGATCTA |
CGTTTAGAGAACAGATCTA TATTTAGAAAACAGATCTA |
NM_022757.3 |
|
CGTTTAGAGAACAGATCTA TGTTTAGAAAACAGATCTCC |
AK054685 | |||
|
CGTTTAGAGAACAGATCTA AGTTTAGAGATCAGAGCTA |
NM_176814.3 | |||
|
CGTTTAGAGAACAGATCTA AGTTTAGAGACCAGATCAA |
NM_018451.2 | |||
|
CGTTTAGAGAACAGATCTA CTTTTTGAGAAAAGATCTA |
BX647535 | |||
|
CGTTTAGAGAACAGATCTA CATTTACAGAACAGCTCTA |
||||
|
CGTTTAGAGAACAGATCTA CATTTAGAGAACACATTTA |
NM_173645.1 |
Residues highlighted in red shows the mismatches with the passenger strand sequence
The 4th siRNA having the guide strand sequence 5′ GUUUAGAGAACAGAUCUACAA 3′ with no guide strand off‐targets in human, was predicted to have the lowest seed duplex stability with the T m value of 11.7°C and had the minimum number (7 only) of off‐target sequences for the passenger stand (Table 2). BLAST analysis of whole leader sequence by BLOCK iT RNAi designer with human gene database showed no similar or identical sequence to the leader sequence region targeted by the best considered 4th siRNA. BLAST analysis against the updated human gene and transcript database of NCBI gave no significantly similar sequences for the SARS‐CoV‐2 leader sequence. To enhance accuracy, BLAST analysis of the region of leader sequence targeted by best 4th siRNA with the human gene and transcript database with parameters adjusted for small sequence was performed and gave insignificant similarity matches with mostly predicted or uncharacterized sequences (sequences with very low chances to get translated in vivo) with very high E‐values of more than 44. Higher E‐value (>0.01) denotes similarity found by chance events. Nearly no similarity of the 4th (best) siRNA targeted region of leader sequence with human genetic and transcript database extremely reduces the chances of presence of complementary human gene sequences to the siRNA whole guide strand sequence and hence the off‐target effect.
Furthermore, molecular docking results of the predicted siRNAs guide strands with the leader sequence revealed high binding energy scores ranging from −214.02 to −327.45 (Figure 2). The docking conformations revealed that due to low 5′ seed duplex stability the ends of the guide strand did not interact much with the target sequence but the high sequence complementarity between the siRNA guide strand and the target leader sequence regions promoted highly effective binding through the nucleotide bases present in the middle of the guide strand sequence. 15 In this molecular docking analysis, highest binding affinity was revealed to be associated with the 4th siRNA for the leader sequence with the docking or binding score of 327.45 for the siRNA guide strand‐leader complex thereby predicting high structural affinity of this siRNA for the leader sequence. In conclusion, the results of no guide strand off‐target sequences, considering both seed and non‐seed regions, and minimum number (only 7) of passenger strand off‐target sequences for the 4th siRNA along with high docking score of guide strand with the leader sequence proved it the best counterpart. Also, each off‐target corresponding to the 4th siRNA passenger strand had at least 3 mismatches which significantly reduces the probability of passenger strand to bind the undesired target, also most of the mismatches were present at the 5′ end of the passenger strand which in turn reduces the chances of off‐target binding. Eventually, the docking of the passenger strand with the off‐target sequences revealed significantly low binding scores compared to the binding score of the siRNA guide strand with the leader sequence (Figure 3).
FIGURE 2.
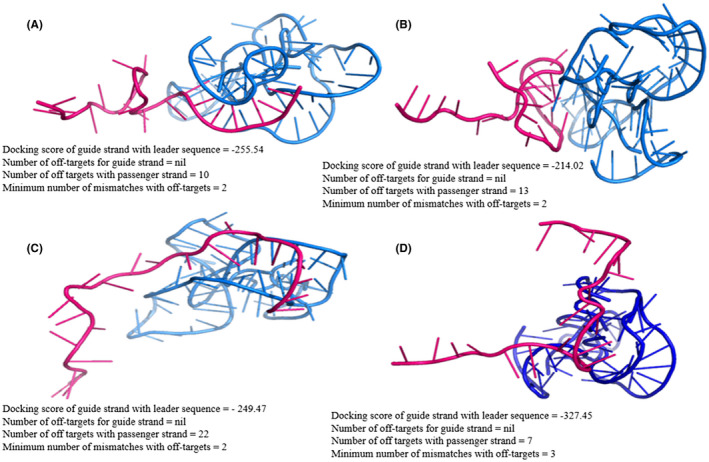
Docking pose and docking scores of leader sequence (blue) with the guide strands (A) UCGAAAGUUGGUUGGUUUGUU (B) AUCUACAAGAGAUCGAAAGUU (C) UUAGAGAACAGAUCUACAAGA (D) GUUUAGAGAACAGAUCUACAA of predicted four siRNAs (pink) by siDirect along with information related to off‐targets
FIGURE 3.
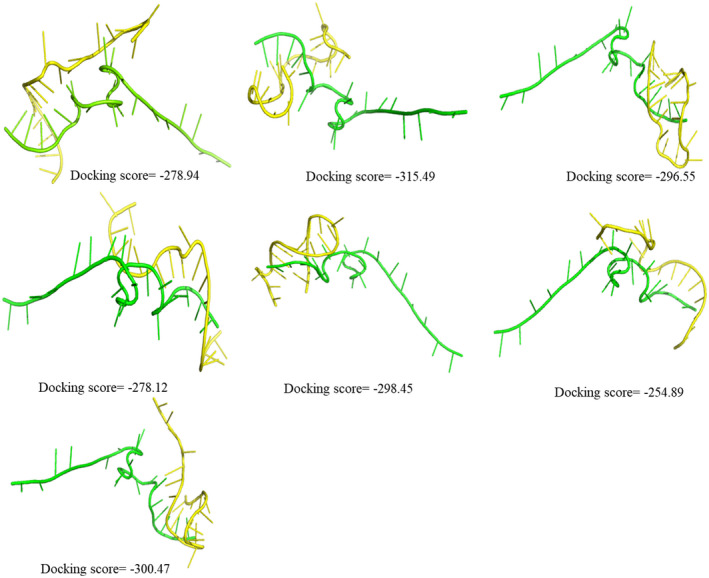
Docking poses and docking scores of the passenger strand (green) of predicted siRNA‐4 with the corresponding predicted off target sequences (yellow) present in human genome
The most interesting fact revealed by the docking conformations of passenger strand with the off‐target sequences was that in none of the docking conformations, the 5′ end of the passenger interacted with the off‐target sequences, this might be due to low seed duplex stability or T m value (Table 1) and due to the presence of mismatches at the 5′ end (Table 2). 13 , 15 Hence, the present analysis revealed a highly potent siRNA sequence which can efficiently interact with the leader sequence of SARS‐CoV‐2 and can hamper its replication in the host human body with least effect on the gene expression levels of the host. Thus, siRNA sequence (5′GUUUAGAGAACAGAUCUACAA3′) seems to be highly promising for the development of an effective RNAi‐based gene therapy against the deadly disease of COVID‐19 and stands in urgent demand of further in vitro and in vivo investigations.
4. CONCLUSION
The increasing victimization rate of the current COVID‐19 pandemic and scarcity of effective treatments are posing a great challenge to the scientific society. RNA interference‐based approaches have shown highly promising results in the treatment of a wide range of diseases by silencing the expression of virulent genes. SARS‐CoV‐2 the causative agent of COVID‐19 contains a leader sequence at the 5′ end of its genome which is incorporated in the majority of subgenomic RNAs by process of discontinuous transcription and mediates their translation and hence the replication of the virus. This fact makes the leader sequence an effective target to develop therapies against SARS‐CoV‐2. The present analysis deals with the investigation of highly specific siRNA to target the leader sequence for the development of RNAi based therapy against the virus. Our analysis revealed 5′GUUUAGAGAACAGAUCUACAA3′ as the most potent siRNA with high binding affinity to the target leader sequence along with least seed duplex stability with T m value of 11.7°C and no off‐target sequences for the guide stand. The 7 predicted off‐targets for the passenger strands had at least 3 mismatches majority of them lying at the 5′ end of the sequence. Also, the binding scores of the off‐target‐passenger complexes were significantly low thus depicting very low chances for off‐target effect. Henceforth, the predicted potent siRNA shows great scope for the development of RNAi based therapy against the highly infectious SARS‐CoV‐2; therefore, further research in this concern is urgently demanded.
CLINICAL IMPLICATION
As no effective drug or treatment has yet been reported against the deadly virus of SARS‐CoV‐2 for clinical applications. This study will provide significant contribution in the development of an effective siRNA‐based gene therapy against the current pandemic of COVID‐19.
CONFLICT OF INTEREST
There is no conflict of interest among authors.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is an in silico prediction study.
Pandey AK, Verma S. An in silico analysis of effective siRNAs against COVID‐19 by targeting the leader sequence of SARS‐CoV‐2. Adv Cell Gene Ther. 2021;4:e107. 10.1002/acg2.107
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2): an update. Cureus. 2020;12:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan S, Tombuloglu H, Hassanein SE, et al. Coronavirus diseases 2019: current biological situation and potential therapeutic perspective. Eur J Pharmacol. 2020;886:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ullrich S, Nitsche C. The SARS‐CoV‐2 main protease as drug target. Bioorg Med Chem Lett. 2020;30:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hillen HS, Kokic G, Farnung L, Dienemann C, Tegunov D, Cramer P. Structure of replicating SARS‐CoV‐2 polymerase. Nature. 2020;584:154‐156. [DOI] [PubMed] [Google Scholar]
- 5. Romano M, Ruggiero A, Squeglia F, Maga G, Berisio R. A structural view of SARS‐CoV‐2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finkel Y, Mizrahi O, Nachshon A, et al. The coding capacity of SARS‐CoV‐2. Nature. 2021;589:125‐130. [DOI] [PubMed] [Google Scholar]
- 7. Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181(4):914‐921.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taiaroa G, Rawlinson D, Featherstone L, et al. Direct RNA sequencing and early evolution of SARS‐CoV‐2. bioRxiv 2020.03.05.976167.
- 9. Watts JK, Corey DR. Silencing disease genes in the laboratory and the clinic. J Pathol. 2012;226:365‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Tar Ther. 2020;5:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartoszewski R, Sikorski AF. Editorial focus: understanding off‐target effects as the key to successful RNAi therapy. Cell Mol Biol Lett. 2019;24:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez‐Berestein G. Preclinical and clinical development of siRNA‐based therapeutics. Adv Drug Deliv Rev. 2015;87:108‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ui‐Tei K. Optimal choice of functional and off‐target effect‐reduced siRNAs for RNAi therapeutics. Front Genet. 2013;4:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naito Y, Yoshimura J, Morishita S, Ui‐Tei K. siDirect 2.0: updated software for designing functional siRNA with reduced seed‐dependent off‐target effect. BMC Bioinformatics. 2009;10:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamola PJ, Nakano Y, Takahashi T, Wilson PA, Ui‐Tei K. The siRNA non‐seed region and its target sequences are auxiliary determinants of off‐target effects. PLoS Comput Biol. 2015;11:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gitlin L, Stone JK, Andino R. Poliovirus escape from RNA interference: short interfering RNA‐target recognition and implications for therapeutic approaches. J Virol. 2005;79:1027‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Devi GR. siRNA‐based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819‐829. [DOI] [PubMed] [Google Scholar]
- 18. Bobbin ML, Burnett JC, Rossi JJ. RNA interference approaches for treatment of HIV‐1 infection. Genome Med. 2015;7:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandra PK, Kundu AK, Hazari S, et al. Inhibition of hepatitis C virus replication by intracellular delivery of multiple siRNAs by nanosomes. Mol Ther. 2012;20:1724‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Chen Y, Cheng G, Mahato RI. RNAi for treating hepatitis B viral infection. Pharm Res. 2008;25:72‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y, Yang DH, Xiong J, Jia J, Huang B, Jin YX. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. 2005;15:193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li T, Zhang Y, Fu L, et al. siRNA targeting the leader sequence of SARS‐CoV inhibits virus replication. Gene Ther. 2005;12:751‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Feng P, Liu K, Wu M, Lin H. Computational identification of small interfering RNA targets in SARS‐CoV‐2. Virologica Sinica. 2020;35:359‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID‐19 and related human coronavirus diseases. ACS Central Sci. 2020;6:315‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He J, Wang J, Tao H, Xiao Y, Huang SY. HNADOCK: a nucleic acid docking server for modeling RNA/DNA‐RNA/DNA 3D complex structures. Nucleic Acids Res. 2019;47:W35‐W42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu W, Jiang X, Huang L. RNA interference technology. Comprehensive Biotechnol. 2019;5:560‐575. [Google Scholar]
- 27. Sheu‐Gruttadauria J, MacRae IJ. Structural foundations of RNA silencing by argonaute. J Mol Biol. 2017;429:2619‐2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu CJ, Huang HW, Liu CY, Hong CF, Chan YL. Inhibition of SARS‐CoV replication by siRNA. Antiviral Res. 2005;65:45‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


