Abstract
Several human coronaviruses (HCoVs) are distinguished by the ability to generate epidemics or pandemics, with their corresponding diseases characterized by severe respiratory illness, such as that which occurs in severe acute respiratory syndrome (SARS‐CoV), Middle East respiratory syndrome (MERS‐CoV), and, today, in SARS‐CoV‐2, an outbreak that has struck explosively and uncontrollably beginning in December 2019 and has claimed the lives of more than 1.9 M people worldwide as of January 2021. The development of vaccines has taken one year, which is why it is necessary to investigate whether some already‐existing alternatives that have been successfully developed in recent years can mitigate the pandemic's advance. Silver nanoparticles (AgNPs) have proved effective in antiviral action. Thus, in this review, several in vitro and in vivo studies of the effect of AgNPs on viruses that cause respiratory diseases are analyzed and discussed to promote an understanding of the possible interaction of AgNPs with SARS‐CoV‐2. The study focuses on several in vivo toxicological studies of AgNPs and a dose extrapolation to humans to determine the chief avenue of exposure. It can be concluded that the use of AgNPs as a possible treatment for SARS‐CoV‐2 could be viable, based on comparing the virus' behavior to that of similar viruses in in vivo studies, and that the suggested route of administration in terms of least degree of adverse effects is inhalation.
This article is categorized under:
Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
Therapeutic Approaches and Drug Discovery > Nanomedicine for Respiratory Disease
Toxicology and Regulatory Issues in Nanomedicine > Toxicology of Nanomaterials
Keywords: COVID‐19, in vitro studies, in vivo studies, SARS‐CoV‐2, Silver Nanoparticles
Bats are natural carriers of the new SARS‐CoV‐2 coronavirus, which can be transmitted to humans through certain intermediate hosts. AgNPs allow the internalization of the virus in the cells to be avoided by inhibiting the interaction between the spike (S) glycoprotein and its specific receptor. This study summarizes in vitro and in vivo studies in which AgNPs were used to counteract different viruses that cause respiratory diseases. In addition, the possible routes of AgNPs exposure and dose extrapolation to humans as a possible treatment for SARS‐CoV‐2 are analyzed.
1. INTRODUCTION
Viruses are pathogens that cause significant increasing morbidity and mortality around the world. For instance, viruses have been reported to cause 2 M human deaths annually worldwide (Colpitts, Verrier, & Baumert, 2015). Their potential negative health impacts stem from their highly contagious nature and a lack of effective control systems (Morens, Folkers, & Fauci, 2004). The limitation of current detection systems potentially increases the incidence and outbreak of viral infections (Gushulak & MacPherson, 2004).
In nature, viruses exist in different morphologies, ranging from 20 to 900 nm (Passi, Sharma, Dutta, Dudeja, & Sherma, 2015). Their infectious material usually comprises defined units of proteins and nucleic acids that assemble to form nanoparticle (NP) structures called virions. The difficulties involved in establishing a virus detection system stem from their nano size and simple structure compared to other specimens.
In recent years, a specific virus type has caught the attention of the scientific community. Human coronaviruses (HCoVs) have caused severe respiratory diseases, such as those caused by severe acute respiratory syndrome (SARS‐CoV), Middle East respiratory syndrome (MERS‐CoV), and, currently, SARS‐CoV‐2. The search for a drug against this last virus has become a race against time. At the end of January 2021, 92 M people had been reported as infected, and 1.9 M people have died (WHO, 2021).
Nanotechnology has shown promise in fighting viruses. Nanotechnology in biomedicine involves the development of individual components with nanoscale features (10−9 m) that allow for the creation of revolutionary biomolecular machines that can recognize certain types of cells, viruses, bacteria, and fungi. These systems are capable of transferring information from the nano level to the macromolecular level (Al‐Nemrawi, AbuAlSamen, & Alzoubi, 2020). NPs are often studied because of their unique properties, such as small size, improved solubility, surface adaptability, and multifunctionality, which can lead to the development of better and safer drugs, tissue‐targeted treatments, personalized nanomedicines, and early diagnosis and prevention of diseases (Soares, Sousa, Pais, & Vitorino, 2018). Many nano‐based formulations have been found to improve the target delivery and therapeutic efficacy of antiviral drugs (Lembo, Donalisio, Civra, Argenziano, & Cavalli, 2018). Thus, interest in nanotechnology has grown greatly since the beginning of the COVID‐19 pandemic, and various studies related to its potential contribution in different areas have been published (Abdul, Muhammad, Atta Ullah, Asmat, & Abdul, 2020; Aranda et al., 2020; Cardoso et al., 2020; Jindal & Gopinath, 2020; Jones et al., 2020; Talebian, Wallace, Schroeder, Stellacci, & Conde, 2020).
Countless NPs, and the different ways of using them, have been studied in recent years regarding their effectiveness against certain viruses that cause major respiratory diseases (Zhang, Salieb‐Beugelaar, Nigo, Weidmann, & Hunziker, 2015). NPs can be used as part of a vaccine or can deliver drugs to a specific organ or tissue (Karthik Pandiyan & Prabaharan, 2020). Some NPs have been used in vaccines for certain types of respiratory diseases caused by H1N1 influenza (g‐PGAa, chitosan, ferritin, Au), human parainfluenza virus type 3 (HPIV3) (PLGA), and respiratory syncytial virus (RSV) (polyanhydrides). NPs allow for the stabilizing and release of the active components of vaccines in the body; they can also act as transport vehicles (Lee & Wang, 2006). For the transport of vaccines through mucosa, NP size is important; it is very difficult for NPs with a diameter of over 100 nm to penetrate the mucosal barrier, unlike those of fewer than 50 nm.
A wide range of nanomaterials are used for virus detection, such as metal NPs (Cho & Glenn, 2020; Dougan, Karlsson, Smith, & Graham, 2007; Shan et al., 2020), polymeric NPs (Zhang et al., 2020), dendrimers (Kandeel, Al‐Taher, Park, Kwon, & Al‐Nazawi, 2020; Paull, Castellarnau, Luscombe, Fairley, & Heery, 2020), carbon nanotubes (Aasi, Aghaei, Moore, & Panchapakesan, 2020; Pinals et al., 2020; Sengupta & Hussain, 2021; Yang, 2020), graphene (Kumar Raghav & Mohanty, 2020; Srivastava et al., 2020), and virus‐like NPs (VLN) (Medhi, Srinoi, Ngo, Tran, & Lee, 2020), among others. These NPs are widely described as being suitable for numerous biosensing functions and applications that interact specifically with various biomolecules, like antibodies, DNA, and RNA, and represent the principal strategy of a large number of virus detection systems (Liao, Nehl, & Hafner, 2006).
Metallic NPs have been used in a myriad of ways. Some types of metallic NPs, like silver (Galdiero et al., 2011; Ghiuță & Cristea, 2020), copper (Jagaran & Singh, 2020), titanium (Khaiboullina, Uppal, Dhabarde, Subramanian, & Verma, 2020), zinc oxide, and iron oxide (Sarkar & Das Mukhopadhyay, 2021), have been incorporated into consumer products, including personal protective equipment (PPE) (masks and biosecurity suits) (Campos et al., 2020; Palmieri, De Maio, De Spirito, & Papi, 2021), disinfectants (Talebian et al., 2020), and cosmetics (Raj, Sumod, Jose, & Sabitha, 2012), among others (FDA, 2020). However, Talebian et al. (2020) proposed an alternative use of silver, copper, and titanium dioxide metallic NPs because of their broad‐spectrum antiviral activity, persistence, and ability to be effective in much lower doses. Regarding detecting SARS‐CoV‐2, the principal technique is the real‐time RT‐PCR (reverse transcription polymerase chain reaction) test, which consists of correctly and effectively extracting viral RNA; to expedite this process and analyze a larger number of samples, magnetic NPs have been used (Zhao et al., 2020).
In recent months, biosensors utilizing metallic NPs have been developed that are sensitive to detecting the sequence of the SARS‐CoV‐2 N gene, such as in the naked‐eye virus screening approach using gold NPs (AuNPs) capped by properly designed antisense oligonucleotides (Moitra, Alafeef, Dighe, Frieman, & Pan, 2020). In another example of NPs as electrochemical biosensors, ssDNA‐capped AuNPs were placed on graphene‐coated filter paper (Alafeef, Dighe, Moitra, & Pan, 2020). Further, used with porous nanomaterials, such as metal‐organic frameworks (MOF), which is a class of porous polymers consisting of clusters and metal ions coordinated with polytopic organic linkers, NPs can detect different viruses, including SARS‐CoV‐2 (Rabiee et al., 2020; Seo et al., 2020). Another biosensor using cobalt‐doped titanium dioxide nanotubes showed excellent results in detecting SARS‐CoV‐2 virus within 30 s in a wide range of concentrations (Vadlamani, Uppal, Verma, & Misra, 2020).
Although NPs' antiviral properties have been determined, the reaction of the human body and, therefore, the efficacy of NPs as drugs depends on the distribution of plasma proteins on their surface. NPs are designed to direct their action toward binding and penetration and in that way block the replication of viruses. They use mechanisms that either directly or indirectly inactivate the virus, which prevents the virus from binding to the host cells and blocks viral replication, as seen in Figure 1. Other mechanisms of action of NPs are related to their local action against receptors on the surface of the virus, where they alter the membrane potential and block virus penetration to a great extent (Abdul et al., 2020; Gurunathan et al., 2020).
FIGURE 1.
Possible mechanisms of the use of nanotechnology through nanobubbles, nanofibers, nanotraps, nanorobots, nanopolymers, nanodiamonds, and nanotransporters of biomolecules and drugs with antiviral action
Among the different types of metallic NPs, the antiviral activity of silver NPs (AgNPs) on SARS‐CoV‐2 is of particular interest (Argentiere, Cella, Cesaria, Milani, & Lenardi, 2016; Das et al., 2020; Durán, Silveira, Durán, & Martinez, 2015; Hossain, 2020; Teirumnieks, Balchev, Ghalot, & Lazov, 2021). AgNPs are known for their high antimicrobial activity, biocompatibility, and low toxicity in eukaryotic cells. There are few in vitro studies regarding the use of AgNPs against SARS‐CoV‐2 (Jeremiah, Miyakawa, Morita, Yamaoka, & Ryo, 2020), as in vivo studies are a more urgent need.
This study presents a review of the use of AgNPs against SARS‐CoV‐2. The properties of AgNPs and their possible interaction, in vitro and in vivo studies on viruses causing respiratory diseases, the toxicity of AgNPs induced by inhalation, dermal, and oral routes for AgNPs, and the dose extrapolation to humans, as a possible treatment for this virus.
2. CORONAVIRUS SARS‐CoV‐2
The Coronaviridae family of viruses has affected mankind since the beginning of the twentieth century, and these virus' sudden mutations in short periods have complicated the development of a specific treatment to limit their spread and lethality (Bonilla‐Aldana et al., 2020; de Wit, van Doremalen, Falzarano, & Munster, 2016; Lin, Hsu, & Lin, 2014). For example, SARS‐CoV crossed the species barrier in late 2002: the bat that carries the virus transferred it to an intermediate species, which then transferred it to humans, and its transmission was nosocomial and presented a lethality of 30% (Aguanno et al., 2018; Lin et al., 2018). Another HCoV disease, MERS‐CoV, was reported in the 1980s in camels; however, it did not begin to spread in humans until 2012, with a mortality of 36% (Chan et al., 2013). Two other HCoVs, HCoV‐NL63 and HCoV‐HKU1, appeared in 2004 and 2005, respectively; only minimal control efforts were needed, so their lethality is not fully known (Kasmi, Khataby, Souiri, & Ennaji, 2020).
SARS‐CoV‐2 was first detected in December 2019 in Wuhan City, Hubei Province (China); it has a percentage of lethality of 2% to 3% but is extremely contagious (Scavone et al., 2020; Weiss & Navas‐Martin, 2005). The virus produces symptoms similar to those of a common cold and more serious diseases such as severe pneumonia and respiratory failure. It particularly affects elderly people with preexisting conditions (Chen et al., 2020). The main route of infection is through the respiratory tract, although various sources of infection, such as tears and feces, have been reported (Li et al., 2020). The virus can survive as a particle suspended in the air for 3 h, and its incubation period is from 2 to 14 days (Dong, Du, & Gardner, 2020).
SARS‐CoV‐2 is the seventh type of coronavirus isolated in humans; it belongs to the genus β CoV, is round in shape with a crown appearance, and measures 60 to 140 nm in diameter under an electron microscope (Lu et al., 2020). It has a positive‐sense, single‐stranded genome made of RNA (+ssRNA), contains 29,891 nucleotides encoding 9880 amino acids (−29.8 kbp), and has a 5′ cap structure and 3′ poly‐A tail. The genomic RNA between ORF1a and ORF1b (open reading frames) is used for the direct production of two polypeptides: polyprotein 1a/1ab (pp1a/pp1ab), which encodes nonstructural proteins (nsps) (Guo et al., 2020).
The physiological binding of SARS‐CoV‐2 in the body is via epithelial cells containing surface sialic acids that bind with galactose α‐2,6. Sialic acid is an abundant molecule in all cells and defines the particular tropism of respiratory viruses, the epithelial cells that line the human trachea contain mainly carbohydrates and have an α‐2,6 bond (Allen, Tennant, & Franklin, 2019; Diefenbach, Gnafakis, & Shomrat, 2020; Kumazaki, Shimomura, Kiyono, Ochiya, & Yamamoto, 2020), which is why SARS‐CoV‐2 generally first enters the body here.
SARS‐CoV‐2 binds to its receptor in the host cell through the transmembrane spike protein (S), which forms homodimers that leave the viral surface. It is composed of two units, S1 and S2, which are responsible for binding with the host cell receptor and viral membrane fusion. The virus has been shown to bind to the angiotensin‐converting enzyme 2 (ACE2) receptor and is a significant determinant for the pathogenesis of CD209L infection (a C‐type lectin, also called L‐SIGN, and dipeptidyl peptidase 4 [DPP4], also known as CD26), which promotes transmission to humans and other species and their cellular infection (Li, Geng, Peng, Meng, & Lu, 2020). The envelope protein (E) is located in an intracellular traffic site, such as the Golgi complex, where it participates in its self‐assembly and is considered very important in the production and maturation of the viral particle (Schoeman & Fielding, 2019). The nucleocapsid protein (N) directs the genome to replicate, transcribe, and package. Additionally, the membrane protein (M) collaborates in the fixation of the nucleocapsid to the membrane of internal structures such as the Golgi complex and is responsible for the transmembrane transport of nutrients, the release of the virion, and the formation of the envelope (Cui, Li, & Shi, 2019). Once the viral genome is inside the cytoplasm and translates into two polyproteins and structural proteins, it begins to replicate (Huang et al., 2020). The envelope (E) glycoproteins are inserted into the Golgi endoplasmic reticulum membrane, and the nucleocapsid is formed by the combination of genomic RNA and the N protein. In these vesicles containing the virus particles, viral replication and transcription occur (Kasmi et al., 2020).
3. AgNPS: IN VITRO AND IN VIVO STUDIES
One of the most commonly used inorganic materials for fighting bacterial and viral diseases is silver (Sondi & Salopek‐Sondi, 2004). Silver is a noble metal that has been used since antiquity. In ionic form, it is used in concentrations higher than 40% to treat skin diseases such as warts and granulomas. As 1% silver sulfadiazine, it is used for burns and skin ulcers (Murthy, 2007; Ramírez, 2013). Currently, the use of ionic colloidal silver as an alternative treatment for SARS‐CoV‐2 has gained popularity through the Internet. However, the Food and Drug Administration (FDA) does not recognize it as a safe and effective agent (Morrill et al., 2013).
Like NPs, AgNPs have a wide range of biomedical applications (Prabhu & Poulose, 2012) because of their antibacterial capacity and selective toxicity against microorganisms (Wong & Liu, 2010). Various studies have indicated that AgNPs can adhere to the cellular membrane, altering cellular permeability, and the cell's respiratory functions. It is possible they not only interact with the membrane surface but also penetrate the bacteria. Further, silver ions can link with thiol groups of biomolecules and with phosphorus sulfide compounds, such as when DNA inactivates bacteria. Many studies concerning AgNPs' antimicrobial activity have been published (Alafeef, Moitra, & Pan, 2020; Devanesan, Ponmurugan, AlSalhi, & Al‐Dhabi, 2020; Elmehbad & Mohamed, 2020; Garibo et al., 2020; Rao, Saptami, Venkatesan, & Rekha, 2020; Sharma et al., 2020; Taghavizadeh Yazdi et al., 2020).
AgNPs have a highly reactive surface area in addition to unique optical and catalytic properties, unlike ionic colloidal silver (Marimuthu et al., 2020). Their surface chemistry controls AgNPs' properties and functionality. Various studies have demonstrated that silver nanoconjugates can easily enter living cells (Abdellah, Sliem, Bakr, & Amin, 2018). The use of organic compounds to stabilize AgNPs, whose function is to coat the surface and avoid coalescence phenomena that induce aggregation and, consequently, loss of characteristic properties. Stabilizing agents can be simple molecules or polymers, with or without surfactant characteristics, that interact with the surface of the AgNPs through their hydrophobic segments (Du et al., 2018; Randazzo, Fabra, Falcó, López‐Rubio, & Sánchez, 2018).
The size and shape of AgNPs play a very important role in antiviral activity. Several studies have shown that dimensions smaller than 10 nm produce much more reactive surfaces (Marimuthu et al., 2020). The shape can also vary—for example, triangular, bar, or spiral—which drastically affects the mechanism of viral action; those of the spherical and cylindrical type are more phagocytosed (Soiza, Donaldson, & Myint, 2018).
Many studies have pointed out the antiviral activity that AgNPs exert on different virus types that cause respiratory illness, such as influenza (Mehrbod et al., 2009), HPIV3 (Galdiero et al., 2013), human adenovirus serotype 3 (Ad3) (Nana Chen, Zheng, Yin, Li, & Zheng, 2013), respiratory syncytial virus (RSV) (Yang, Li, & Huang, 2016), and Rift Valley fever (RFV) (Borrego et al., 2016). In a recent in vitro study using polyvinylpyrrolidone‐coated AgNPs in Vero cells infected with SARS‐CoV‐2, the almost complete inhibition of the virus' replication was confirmed using NPs with diameters of 10 nm, as well as lower toxicity compared to NPs of smaller and larger sizes (Jeremiah et al., 2020). Other viruses for which AgNPs have been assayed, include the human immunodeficiency virus (HIV) (Elechiguerra et al., 2005; Lara, Ayala‐Nuñez, Ixtepan‐Turrent, & Rodriguez‐Padilla, 2010), herpes (HV) (Baram‐Pinto, Shukla, Perkas, Gedanken, & Sarid, 2009; Hu et al., 2014; Orlowski et al., 2014), hepatitis (HBV) (Lu et al., 2008), Ebola virus (Yen et al., 2015), Tacaribe virus (Speshock, Murdock, Braydich‐Stolle, Schrand, & Hussain, 2010), monkeypox virus (Rogers, Parkinson, Choi, Speshock, & Hussain, 2008), African swine fever virus (Thi Ngoc Dung et al., 2020), porcine epidemic diarrhea virus (PEDV) (Du et al., 2018), polio virus (Huy et al., 2017), and dengue virus (Suresh et al., 2015), among others.
Both in vitro and in vivo studies have been conducted with AgNPs and various viruses. When comparing studies on AgNPs' antiviral activity with that of other metallic NPs (e.g., iron oxides NPs, ZnONPs, AuNPs, TiO2NPs, CaONPs, and ZrONPs) for a specific virus like influenza A, we find a higher number of both in vitro and in vivo studies (Wieczorek, Szutkowska, & Kierzek, 2020). The first study related to the antiviral properties of AgNPs was conducted by Mehrbod et al. (2009), which demonstrated the 78% reduction of viral load after exposing the influenza virus to the treatment.
Finally, AgNPs have been tested in an influenza vaccine as an adjuvant to improve the immune response by increasing the production of immunoglobulin IgA, a predominant antibody in seromucosal secretions and an important defense against viral infections (Sanchez‐Guzman et al., 2019).
Table 1 summarizes the most important information of in vitro and in vivo studies of AgNPs antiviral activity on different viruses that cause respiratory diseases, such as SARS‐CoV‐2, influenza, HPIV3, Ad3, RSV, and RFV.
TABLE 1.
In vitro and in vivo studies of silver nanoparticles' (AgNPs) effects on viruses that cause respiratory diseases
| Virus | Antiviral NPs | Size (nm) | Concentration, (Time), Route of Administration | Type of Cells/Model | Brief Results | References |
|---|---|---|---|---|---|---|
| SARS‐CoV‐2 | AgNPs‐polyvinylpyrrolidone (PVP) | 2–100 | 0.1 to 10 ppm | Vero | Different sizes of AgNPs‐PVP were analyzed. At 10 nm, a larger percentage of viral replication inhibition was shown | Jeremiah et al. (2020) |
| Influenza H1N1 | AgNPs | 10 | 6.25, 12.5, 25, 50, 100, 200 μg/ml (24 and 48 h) | Madin‐Darby Canine Kidney (MDCK) | Binding of AgNPs with glycoproteins of the viral envelope, inhibiting viral entry into the host cell | Xiang, Chen, Pang, and Zheng (2011) |
| Influenza H1N1 | AgNPs | 5–20 | 4, 2, 1, 0.25, 0.125, 0.06, and 0.03 μg/ml (48 h) | MDCK | AgNPs form a disulfide bond that blocks virus binding receptors, increasing antiviral activity | Mehrbod et al. (2009) |
| Influenza H1N1 | AgNPs‐chitosan | 3–12 | Viral suspension in Phosphate‐buffered saline PBS (250 μl, titer ca. 1,000 TCID50) | MDCK | Increased antiviral activity by chitosan and inhibition of virus reproducibility | Mori et al. (2013) |
| Influenza H1N1 | AgNPs‐oseltamivir (OTV) | 2–3 | 0.3125, 0.625, 1.25, and 2.5 μg/ml (24 h) | MDCK | OTV reduces the toxicity of AgNPs and also inhibits neuraminidase and hemagglutinin, preventing binding with the virus | Li et al. (2016) |
| Influenza H1N1 | AgNPs‐peptides (FlulPed) | 10 | 0.008 to 0.2 nmol L−1 | MDCK | The peptide disables the formation of a viral surface in healthy cells, enhanced by the antiviral properties of AgNPs | Alghrair, Fernig, and Ebrahimi (2019) |
| Influenza H1N1 | AgNPs‐zanamivir | 2–3 | 1.25, 2.5, 5, and 10 μg/ml (2 h) | MDCK | Functionalized AgNPs inhibited neuraminidase activity; in addition, the cytopathic effect demonstrated that the nanomaterial withstands the attack of the virus and prevents the death of healthy cells | Lin et al. (2017) |
| Influenza H1N1 | AgNPs‐amantadine | 2–3 | 20 μl solution well MTT (5 h) | MDCK | The presence of functionalized AgNPs increased cell viability by 90% and inhibited virus proliferation by deactivating hemagglutinin and neuraminidase | Li et al. (2016) |
| Influenza H1N1 | AgNPs–SiO2 | 400 | 10 × 1010 particles/ml (1, 6, 12, and 24 h) | MDFK | AgNPs reduce virus infection due to their interaction with the viral components of the membrane | Park et al. (2018) |
| Influenza H7N3 | AgNPs–Cinnamomum cassia (cinnamon) | 42 | 15.6, 31.25, 62.5, 125, 250, and 500 μg/ml (24 h) | Vero | Cinnamon‐reduced AgNPs exhibited improved viricidal activity against the virus; the concentration used was nontoxic to cells | Fatima, Zaidi, Amraiz, and Afzal (2016)) |
| Influenza A subtype (H3N2) | AgNPs | 9.5 ± 0.8 |
6.25, 12.5, 25, and 50 μg/ml (48 h) 5 and 20 mg/kg (14 days), inhalation |
MDCK Mice BALB/c |
In vitro, at lower concentrations than 12.5 μg/ml, virus replication decreased due to the decrease in hemagglutinin levels Infected mice without inhalation of AgNPs died on day 7, while those exposed to the nanomaterial had a survival rate of 75% to 88%. However, they showed weight loss. Pulmonary analysis indicated that the virus spread was less with the use of AgNPs |
Xiang et al. (2013) |
| HPIV3 | AgNPs‐Alternaria AgNPs‐Phoma *AgNPs‐F. oxysporum *AgNPs‐Curvularia |
46 40 20 30 |
1, 5, 10, 50, and 100 μg/ml (1, 3, 10, 24, and 36 h) | Vero | Phenolic compounds extracted from rosemary Rosmarinus officinalis L prevented entry and inhibited viral DNA replication | Galdiero et al. (2013) |
| Ad3 | AgNPs | 11.4 ± 6.2 | 3.125, 6.25, 12.5, 25, 50, 100, 200, and 400 μg/ml (48 and 96 h) | Hela | The presence of AgNPs increased the viability of the cells and decreased the fluorescence intensity of the virus; this was potentially caused by the destruction of the viral structure | Chen et al. (2013)) |
| RSV | AgNPs‐Curcuma longa (ginger) | 11.95 ± 0.23 | 0.008, 0.015, 0.03, 0.06, 0.12, and 0.24 nmol/L (24 and 72 h) | CCK‐8 | Curcuma longa better stabilizes AgNPs, increasing antiviral properties that inhibit virus entry and replication | Yang et al. (2016) |
| RSV | AgNPS‐PVP | 10 |
10, 25, and 50 g/ml (24 and 48 h) 2 and 4 mg/kg, (16 days), inhalation |
A549 (type II) and HEp‐2 Mice BALB/c |
In vitro, the concentrations used did not generate cytotoxic effects after 24 h, but concentrations less than or equal to 10 g/ml increased virus replication In vivo, the concentrations used inhibited the replication of the virus between 45% and 55%; the mice did not lose weight or show adverse effects due to AgNPs |
Morris et al. (2019) |
| RVF | AgNPs‐PVP. Argovit | 35 ± 15 |
1/5000, 1/10,000 (24, 48, and 72 h) 1/1000, 1/100, 1/10 (10 to 15 days), Parenteral |
Vero Transgenic Mice 129Sv/Ev IFNAR−/− |
In vitro, the Argovit did not abolish viral production in its entirety; the reduction in virus production was 50% compared to the use of uncoated AgNPs. With 12 μg/ml of metallic silver, a reduction in infectivity of 98% could be achieved In vivo, mice with a lethal dose of the virus (1.2 mg/ml silver) showed disease and late clinical mortality; their survival was 60% |
Borrego et al. (2016) |
According to the studies carried out, AgNPs act directly with the viral genome through the proteins found on its surface (Mehrbod et al., 2009; Xiang et al., 2011). Infection with influenza viruses H1N1 and H7N3, HPIV3, Ad3, RSV, RVF, and SARS‐CoV‐2 starts by binding protein S to the specific host receptor (Borrego et al., 2016; Chen et al., 2013; Fatima et al., 2016; Galdiero et al., 2013; Huang et al., 2020; Park et al., 2018; Yang et al., 2016). This protein allows the virus to bind with the ACE2 receptor conversion enzyme (Jiang & He, 2012). According to its crystal structure, SARS‐CoV‐2 contains disulfide bonds Cys336‐Cys361, Cys379‐Cys432, and Cys391‐Cys525. Cys480‐Cys488 is key in the junction between the virus crest and the N‐terminal helix of ACE2 (Kang et al., 2020; Walls et al., 2020).
As Figure 2 shows, AgNPs bind to membrane of spike (S) glycoprotein through disulfide bonds, due to their chemical affinity for sulfur (Chung, Chen, & Chen, 2008; Yuan, He, Huang, & Su, 2019). This interaction hinders the internalization of the virus in the cells by inhibiting the interaction between the glycoprotein and its specific receptor. Cellular absorption of AgNPs occurs through the processes of endocytosis and macropinocytosis. Once inside the cell, the AgNPs inhibit the virus's replication capacity (Galdiero et al., 2011).
FIGURE 2.
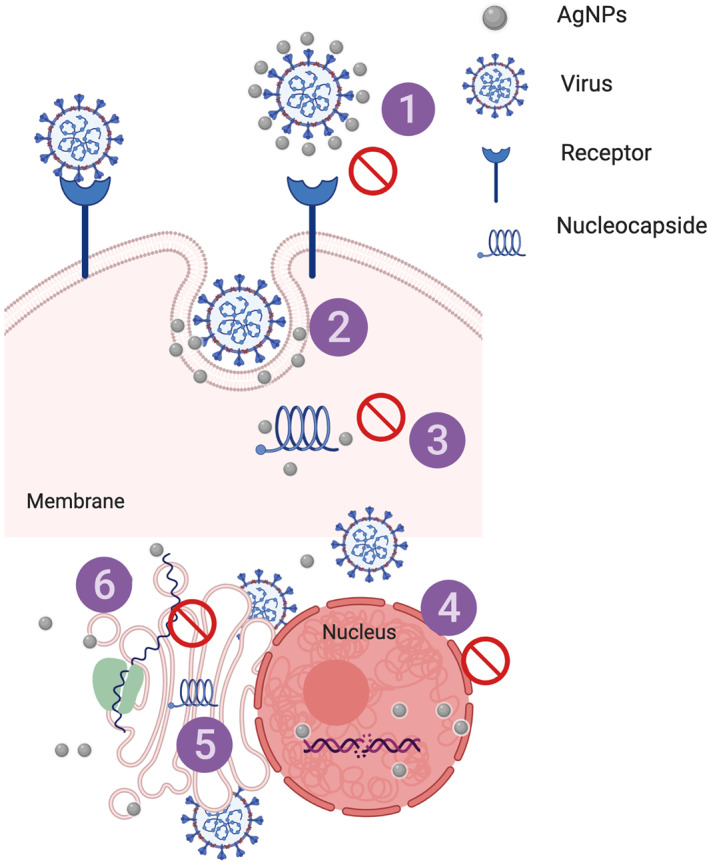
Mechanism of AgNPs' antiviral effect on different stages of virus replication: (1) interaction with viral surface, (2) interference with viral attachment, (3) inhibition of virus penetration into the cell, (4) interaction with viral genome, (5) inhibition of genome replication, (6) inhibition of protein synthesis
AgNPs also appear to exert an effect on H (hemagglutinin) and N (neuraminidase) proteins, which are the main determinants of pathogenicity. If AgNPs are also combined with oseltamivir (OTV), FlulPed, zanamivir, and amantadine (Alghrair et al., 2019; Li, Lin, Zhao, Guo, et al., 2016; Li, Lin, Zhao, Xu, et al., 2016) drugs, which possess antiviral properties and are currently used against SARS‐CoV‐2, reactive oxygen species (ROS) are produced, which minimize the destruction of MDFK cells and inhibit the neuraminidase and hemagglutinin activity of viruses with healthy cells (Li, Lin, Zhao, Xu, et al., 2016). Coatings also modify AgNPs' effects; for example, chitosan stabilizes AgNPs and allows them to increase their virucidal capacity, though their size modifies the mechanism of action (Mori et al., 2013). The use of other types of coatings, such as chitosan (Mori et al., 2013), SiO2 (Park et al., 2018), Cinnamomun cassia (Fatima et al., 2016), Curcuma longa (Yang et al., 2016), and polyvinylpyrrolidone (Borrego et al., 2016; Morris et al., 2019), markedly reduce cellular cytotoxicity compared to uncoated AgNPs. The antioxidant, anti‐inflammatory, and antibacterial properties from the plant from which the coating is derived.
4. TOXICITY OF AgNPs: IN VIVO STUDIES
Despite the marked antiviral effect of AgNPs, currently in Europe, there is no specific legislation for NPs, although they are classified as a “substance” in the European Regulation of Chemical Substances (REACH) (Regulation No. 1907/2006 of the Parliament, December 18, 2006). In the United States, the regulation of AgNPs by the FDA is limited by the impact that silver, as an element, can have on the environment (Sood & Chopra, 2018)
The use of AgNPs is in the preclinical phase, and a general assessment of public health risk is not yet possible, although several products are on the market. Due to their applications, especially in relation to pandemic control, we must assume that human exposure to AgNPs will increase substantially in the immediate future. The main factors that determine the toxic effects of AgNPs in organisms are classified according to the route of exposure (entry, concentration, and duration), factors that depend on the exposed organism, and those related to AgNPs' intrinsic toxicity (del Rocío Coutiño, Ávila, & Helguera, 2017), bioavailability, and accumulation in the body.
There are three main routes of exposure to AgNPs: inhalation, dermal/parenteral, and oral. Table 2 shows various published in vivo studies on toxicity that have used AgNPs. According to these studies, AgNPs' cytotoxic effect depends on their size, tissue distribution, penetration capacity, and cellular absorption (Khandelwal et al., 2014).
TABLE 2.
In vivo studies on toxicity of silver nanoparticles (AgNPs)
| NPs | Size (nm) | Dose, (Time), Route of Exposure | Model | Tissue Accumulation | Brief Results | References |
|---|---|---|---|---|---|---|
| AgNPs | 13–15 | 0.5, 3.5, 61 μg/m3 (28 days), inhalation |
Rats Sprague Dawley |
Nasal cavity and lungs | Exposure to AgNPs increased the neutral mucins of the animals, while the nasal cavity and lungs were not altered | Hyun et al. (2008) |
| 15 | 49, 117, 381 μg/m3, (90 days), inhalation |
Rats Sprague Dawley |
Lungs | Animals did not show a decrease in lung function during or after the exposure period | Song et al. (2013) | |
| 18 | 0.8 μg/ml (90 days), inhalation |
Rats Sprague Dawley |
Lungs and liver | Exposure to AgNPs of the indicated concentration did not induce genetic toxicity in animals during exposure period | Kim et al. (2011) | |
| Ultrafine elemental silver particles | 15 | 133 μg/m 3 (6 h), inhalation |
Rats Fischer 344 |
Liver, kidney, spleen, brain, heart, and lungs | The nasal cavities and lymph nodes related to the lungs showed silver accumulation. In the case of the brain and heart, accumulation was almost zero | Takenaka et al. (2001) |
| Nonagglomerated/aggregated AgNPs | 15 | 0.5, 3.5, 61.4 μg/m3 (28 days), inhalation |
Rats Sprague Dawley |
Bladder, testicles, ovaries, uterus, heart, esophagus, tongue, prostate, lungs, kidneys, liver, pancreas, and brain | Animals did not show significant changes in their body weight nor hematological changes; the concentrations used did not show any effects | Ji, Jung, Kim, et al. (2007)) |
| Inhalation of metallic AgNPs | 20–30 | 2.9 mg/m3 (6 h), inhalation |
Mice C57BL /6 |
Lungs, heart, spleen, and testicles | Inhalation of AgNPs caused pulmonary toxicity with spread to various organs. After 24 h exposure, toxicity decreased | Kwon et al. (2012) |
| Polyvinylpyrrolidone‐ (PVP) and citrate‐stabilized AgNPs |
20 110 |
0.1, 0.5, 1.0 mg/kg (24 h), parenteral |
Mice C57BL/6 |
Nasal cavity and lungs | Size and coating affect cell toxicity and enhance lung lesions | Wang et al. (2014)) |
| AgNPs‐ethylene glycol | 15–40 | 4, 10, 20, 40 mg/kg (32 days), parenteral |
Rats Wistar |
Liver | Animals showed hepatological changes with doses of 20 and 40 mg/kg, an increase in ROS in the blood was also reported | Tiwari et al. (2011) |
| AgNPs‐DMEM | 50–100 | 62.8 mg/kg (24 h), parenteral |
Rats Wistar |
Kidney, liver, spleen, brain, and lungs | AgNPs induced destruction of blood vessels and inflammation of astrocytes, causing long‐term neuronal degeneration | Tang et al. (2009) |
| AgNPs‐deionized water | 25 | 100, 500, 1000 mg/kg (24 h), parenteral |
Mice C57BL/6N |
Brain (caudate nucleus, frontal cortex, and hippocampus) | AgNPs can cause neurotoxicity due to the generation of oxidative stress that kills brain cells | Rahman et al. (2009) |
| Citrate‐capped AgNPs‐deionized water | 7.9 ± 0.95 | 1.58 ± 0.25 μg/ml (24 h), oral |
Rats Sprague Dawley |
Liver, kidneys, and lungs | Silver distribution occurred to a greater extent with colloidal silver than with AgNPs; there were no weight changes; an increase in the platelet count occurred with AgNPs. Treatment with AgNPs (2 or 20 mg/kg) also raised AST | Park (2013) |
| AgNPs | 60 | 30, 300, 1000 mg/kg (28 days), oral |
Rats Sprague Dawley |
Bladder, testicles, ovaries, uterus, tongue, lungs, and kidneys | Both female and male rats showed no changes in body weight. However, the values of alkaline phosphatase and cholesterol were altered. Exposures greater than 300 mg/kg can cause liver problems | Kim et al. (2008) |
| AgNPs‐hydrazine‐PVP | 14 ± 4 | 9 mg/kg (28 days), oral |
Rats Wistar |
Liver, kidneys, lungs, and brain | The highest concentration of AgNPs was found in the liver and kidneys. PVP used as core‐shell does not intervene in any of the conditions, since it was only used as a vehicle and stabilizer | Loeschner et al. (2011) |
| AgNPs‐distilled water | 10–20 | 0.25, 2.5, 25 mg/L (28 days), oral |
Mice NMRI |
Spleen | All analyzed doses had a significant, albeit different, effect on splenocyte activity. With the lowest dose, a decrease in T cells was observed; the intermediate dose stimulated the proliferation of B cells, and the highest dose generated adverse effects | Małaczewska (2014) |
| AgNPs‐tannic acid‐PVP | 8–20 | 100, 1000, 5000 mg/kg (7,14, and 21 days), oral |
Mice Wistar |
Kidney, liver, testicles, and brain | AgNPs increased the concentration of malondialdehyde and superoxide dismutase, although they also decreased glutathione, S‐transferase, and catalase levels; this indicates that AgNPs may be agents of oxidative stress | Adeyemi and Faniyan (2014) |
| AgNPs‐sodium hydroxide‐hydrazine | 3–20 | 5, 10, 15, 20 mg/kg (21 days), oral |
Mice Swiss‐albino |
Kidney, liver, spleen, brain, and lung | The animals presented a significant reduction in their weight; maximum weight losses were observed at doses higher than 10 mg/kg | Shahare and Yashpal (2013) |
After entering the body, the largest AgNPs can be exhaled, while the smallest AgNPs can be deposited in the lungs and can reach other organs through the bloodstream. With average sizes of 15 to 30 nm and in concentrations of 0.5 to 381 μg/m3, according to histopathological analysis, no significant changes regarding AgNPs presence after exposure were found in the nasal cavities (Hyun et al., 2008), lungs (Song et al., 2013), or liver (Kim et al., 2011), among other organs (Ji et al., 2007). However, at high concentrations above 2.9 mg/m3, AgNPs can produce brain injuries after being inhaled (Kwon et al., 2012). Further, the existence of an electric charge on NPs' surface can affect their adhesiveness in biological tissues (Sharma, Mukkur, Benson, & Chen, 2009). Negatively charged NPs allow for efficient DNA encapsulation, although their stability is very low. In contrast, positively charged NPs form complexes with DNA plasmids through electrostatic interactions, promoting their encapsulation and increasing their stability. Furthermore, it has been shown that AgNPs improve immunogenicity when they are administered through the mucosa. However, they also have the ability to form electrostatic interactions with blood proteins, ions, and others (Kumar et al., 2002).
In parenteral injections, AgNPs cytotoxicity increases with a size of 20 to 100 nm and concentrations of 0.1 to 1000 mg/kg, causing lung (Wang et al., 2014), liver (Tiwari, Jin, & Behari, 2011), renal (Tang et al., 2009), and cerebral lesions (Rahman et al., 2009).
The level of toxicity from oral ingestion is intermediate. AgNPs ranging from 3 to 60 nm were used (Gaillet & Rouanet, 2015), but NPs size were not as decisive as dose for oral toxicity. The dosage range was from 0.5 μg to 500 mg/L. Doses of 10 mg/kg caused weight loss (Shahare & Yashpal, 2013), doses higher than 300 mg/kg led to liver disorders (Kim et al., 2008), and doses higher than 1000 mg/kg generated oxidative stress (Adeyemi & Faniyan, 2014). In terms of the spread of ionic colloidal silver and AgNPs, less distribution was observed with AgNPs in organs such as the liver, spleen, and kidneys, among others (Loeschner et al., 2011; Park, 2013). However, accumulation is minimal compared to the subcutaneous or parenteral injection route; this is because many of the applied doses are largely expelled in feces (Gaillet & Rouanet, 2015).
Regarding dosage of AgNPs, few studies have involved human participants, so extrapolation of in vivo toxicological information from rats to humans is of great importance. Certain dosimetric concepts can be considered, adapted to the parameters for each species because of their organic structural differences (Kim et al., 2011). Extrapolation to estimate human exposure through AgNPs inhalation has already been performed (Oller & Oberdörster, 2010). Specifically, rats have an alveolar surface area of 0.409 m2, while that of humans is 62.7 m2. Correlation is done through multipath particle dosimetry modeling, using exposure concentration in relation to the deposited mass per alveolar surface area. Using this model, through aerosol tests and measuring the elimination of AgNPs 90 days after exposure, it was determined that rats exposed to 100 μg/m3 is equivalent to 19 μg/m3 in humans (Ji & Yu, 2012).
Nevertheless, it is difficult to determine AgNPs toxicity for humans because studies on exposure in humans are still scarce (del Rocío Coutiño et al., 2017). A study carried out on workers exposed to AgNPs showed that exposure time is not the most important factor for the generation of toxicity; in certain cases, the exposure exceeded five years with concentrations of 0.35 and 1.35 mg/m3, and in none of these cases were there hematological or blood changes (Lee, Mun, Park, & Yu, 2012). Further, exposure levels depend on the activity. A person who recovers silver in close proximity to soluble compounds has concentrations of 1.3 to 20 mg/L, while jewelry manufacturers present concentrations of 0.2 to 2.8 mg/L, showing that concentrations for the manufacturers of AgNPs are much lower than the limit. Therefore, long exposure time does not affect workers' health (Armitage, White, & Wilson, 1996). With medium‐level exposure, blood levels of AgNPs should range between 0.1 and 23 mg/L, while for sporadic exposure, levels should be around ≤0.1 mg/L (Armitage et al., 1996; Lee et al., 2012). However, studies of NPs conditions should be expanded, especially those focusing on levels in the lungs, as highest exposure is by inhalation.
5. CONCLUSION
AgNPs' inhibitory effects on SARS‐CoV‐2 may be a new clinical strategy for the prevention of infection during its early dissemination stage. This review analyzed the in vitro and in vivo effects of AgNPs on some viruses that produce respiratory diseases. To initiate animal studies, according to the in vivo toxicological analysis, the routes of exposure to AgNPs by inhalation present fewer adverse effects, unlike oral and parenteral administration. With an average AgNPs size of 10 nm and in concentrations of 0.5 to 381 μg/m3, there were no significant changes in the nasal cavities when AgNPs were inhaled. If extrapolated from rats to humans, 100 μg/m3 of AgNPs is equivalent to 19 μg/m3, respectively. Available studies on the toxicity of AgNPs are scarce and sometimes contradictory. Further, evidence for coating AgNPs to reduce their toxicity and enhance their specificity is still emerging. Therefore, it is necessary to carry out a greater number of studies on the effective dose and possible toxic effects of the use of AgNPs to establish safe conditions for humans against highly virulent viruses such as SARS‐CoV‐2.
AUTHOR CONTRIBUTIONS
Fernanda Pilaquinga: Writing‐original draft. Jeroni Morey: Writing‐review and editing. Marbel Torres: Writing‐original draft. Rachid Seqqat: Writing‐original draft. María de las Nieves Piña: Writing‐review and editing.
RELATED WIREs ARTICLES
Advances in the synthesis and application of nanoparticles for drug delivery
ACKNOWLEDGMENTS
Thanks to Miguel Vivas‐Rodríguez for assisting in the bibliographic information search.
Pilaquinga F, Morey J, Torres M, Seqqat R, Piña MdlN. Silver nanoparticles as a potential treatment against SARS‐CoV‐2: A review. WIREs Nanomed Nanobiotechnol. 2021;13:e1707. 10.1002/wnan.1707
Edited by: Dipanjan Pan, Associate Editor and Gregory Lanza, Co‐Editor‐in‐Chief
DATA AVAILABILITY STATEMENT
Research data are not shared.
References
FURTHER READING
- Ji, J. H. , Jung, J. H. , Yu, I. J. , & Kim, S. S. (2007). Long‐term stability characteristics of metal nanoparticle generator using small ceramic heater for inhalation toxicity studies. Inhalation Toxicology, 19(9), 745–751. 10.1080/08958370701399828 [DOI] [PubMed] [Google Scholar]
REFERENCES
- Aasi, A. , Aghaei, S. , Moore, M. , & Panchapakesan, B. (2020). Pt‐, Rh‐, Ru‐, and Cu‐single‐wall carbon nanotubes are exceptional candidates for design of anti‐viral surfaces: A theoretical study. International Journal of Molecular Sciences, 21(15), 5211. 10.3390/ijms21155211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdellah, A. M. , Sliem, M. A. , Bakr, M. , & Amin, R. M. (2018). Green synthesis and biological activity of silver–curcumin nanoconjugates. Future Medicinal Chemistry, 10(22), 2577–2588. 10.4155/fmc-2018-0152 [DOI] [PubMed] [Google Scholar]
- Abdul, W. , Muhammad, A. , Atta Ullah, K. , Asmat, A. , & Abdul, B. (2020). Role of nanotechnology in diagnosing and treating COVID‐19 during the Pandemi. International Journal of Clinical Virology, 4(1), 65–70. 10.29328/journal.ijcv.1001017 [DOI] [Google Scholar]
- Adeyemi, O. S. , & Faniyan, T. O. (2014). Antioxidant status of rats administered silver nanoparticles orally. Journal of Taibah University Medical Sciences, 9(3), 182–186. 10.1016/j.jtumed.2014.03.002 [DOI] [Google Scholar]
- Aguanno, R. , ElIdrissi, A. , Elkholy, A. A. , Ben Embarek, P. , Gardner, E. , Grant, R. , Mahrous, H. , Malik, M. R. , Pavade, G. , VonDobschuetz, S. , Wiersma, L. , & Van Kerkhove, M. D. (2018). MERS: Progress on the global response, remaining challenges and the way forward. Antiviral Research, 159, 35–44. 10.1016/j.antiviral.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafeef, M. , Dighe, K. , Moitra, P. , & Pan, D. (2020). Rapid, ultrasensitive, and quantitative detection of SARS‐CoV‐2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano, 14(12), 17028–17045. 10.1021/acsnano.0c06392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafeef, M. , Moitra, P. , & Pan, D. (2020). Nano‐enabled sensing approaches for pathogenic bacterial detection. Biosensors and Bioelectronics, 165, 112276 10.1016/j.bios.2020.112276 [DOI] [PubMed] [Google Scholar]
- Alghrair, Z. K. , Fernig, D. G. , & Ebrahimi, B. (2019). Enhanced inhibition of influenza virus infection by peptide‐noble‐metal nanoparticle conjugates. Beilstein Journal of Nanotechnology, 10(1), 1038–1047. 10.3762/BJNANO.10.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, K. J. , Tennant, K. V. , & Franklin, S. H. (2019). Effect of inclusion or exclusion of epithelial cells in equine respiratory cytology analysis. The Veterinary Journal, 254, 105405 10.1016/j.tvjl.2019.105405 [DOI] [PubMed] [Google Scholar]
- Al‐Nemrawi, N. K. , AbuAlSamen, M. M. , & Alzoubi, K. H. (2020). Awareness about nanotechnology and its applications in drug industry among pharmacy students. Currents in Pharmacy Teaching and Learning, 12(3), 274–280. 10.1016/j.cptl.2019.12.003 [DOI] [PubMed] [Google Scholar]
- Aranda, P. , Wicklein, B. , Ruiz‐Garcia, C. , Martín‐Sampedro, R. , Darder, M. , del Real, G. , & Ruiz‐Hitzky, E. (2020). Research and patents on coronavirus and COVID‐19: A review. Recent Patents on Nanotechnology, 14(4), 328–350. 10.2174/1872210514666201021145735 [DOI] [PubMed] [Google Scholar]
- Argentiere, S. , Cella, C. , Cesaria, M. , Milani, P. , & Lenardi, C. (2016). Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. Journal of Nanoparticle Research, 18(8), 253. 10.1007/s11051-016-3560-5 [DOI] [Google Scholar]
- Armitage, S. , White, M. , & Wilson, H. (1996). The determination of silver in whole blood and its application to biological monitoring of occupationally exposed groups. The Annals of Occupational Hygiene, 40(3), 331–338. 10.1016/0003-4878(95)00076-3 [DOI] [PubMed] [Google Scholar]
- Baram‐Pinto, D. , Shukla, S. , Perkas, N. , Gedanken, A. , & Sarid, R. (2009). Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chemistry, 20(8), 1497–1502. 10.1021/bc900215b [DOI] [PubMed] [Google Scholar]
- Bonilla‐Aldana, D. K. , Holguin‐Rivera, Y. , Cortes‐Bonilla, I. , Cardona‐Trujillo, M. C. , García‐Barco, A. , Bedoya‐Arias, H. A. , Rabaan, A. A. , Sah, R. , & Rodriguez‐Morales, A. J. (2020). Coronavirus infections reported by ProMED, February 2000–January 2020. Travel Medicine and Infectious Disease, 35, 101575 10.1016/j.tmaid.2020.101575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego, B. , Lorenzo, G. , Mota‐Morales, J. D. , Almanza‐Reyes, H. , Mateos, F. , López‐Gil, E. , de la Losa, N. , Burmistrov, V. A. , Pestryakov, A. N. , Brun, A. , & Bogdanchikova, N. (2016). Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine: Nanotechnology, Biology and Medicine, 12(5), 1185–1192. 10.1016/j.nano.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Campos, E. V. R. , Pereira, A. E. S. , de Oliveira, J. L. , Carvalho, L. B. , Guilger‐Casagrande, M. , de Lima, R. , & Fraceto, L. F. (2020). How can nanotechnology help to combat COVID‐19? Opportunities and urgent need. Journal of Nanobiotechnology, 18(1), 125. 10.1186/s12951-020-00685-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, V. M. d. O. , Moreira, B. J. , Comparetti, E. J. , Sampaio, I. , Ferreira, L. M. B. , Lins, P. M. P. , & Zucolotto, V. (2020). Is nanotechnology helping in the fight against COVID‐19? Frontiers in Nanotechnology, 2, 588915. 10.3389/fnano.2020.588915 [DOI] [Google Scholar]
- Chan, J. F.‐W. , To, K. K.‐W , Tse, H. , Jin, D.‐Y. , & Yuen, K.‐Y. (2013). Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends in Microbiology, 21(10), 544–555. 10.1016/j.tim.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zheng, Y. , Yin, J. , Li, X. , & Zheng, C. (2013). Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. Journal of Virological Methods, 193(2), 470–477. 10.1016/j.jviromet.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10223), 507–513 10.1016/S0140-6736(20)30211‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, N. J. , & Glenn, J. S. (2020). Materials science approaches in the development of broad‐spectrum antiviral therapies. Nature Materials, 19(8), 813–816. 10.1038/s41563-020-0698-4 [DOI] [PubMed] [Google Scholar]
- Chung, Y. C. , Chen, I. H. , & Chen, C. J. (2008). The surface modification of silver nanoparticles by phosphoryl disulfides for improved biocompatibility and intracellular uptake. Biomaterials, 29(12), 1807–1816. 10.1016/j.biomaterials.2007.12.032 [DOI] [PubMed] [Google Scholar]
- Colpitts, C. C. , Verrier, E. R. , & Baumert, T. F. (2015). Targeting viral entry for treatment of hepatitis B and C virus infections. ACS Infectious Diseases, 1(9), 420–427. 10.1021/acsinfecdis.5b00039 [DOI] [PubMed] [Google Scholar]
- Cui, J. , Li, F. , & Shi, Z.‐L. (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology, 17(3), 181–192. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, C. , Paul, S. S. , Saha, A. , Singh, T. , Saha, A. , Im, J. , & Biswas, G. (2020). Silver‐based nanomaterials as therapeutic agents against coronaviruses: A review. International Journal of Nanomedicine, 15, 9301–9315. 10.2147/IJN.S280976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, E. , van Doremalen, N. , Falzarano, D. , & Munster, V. J. (2016). SARS and MERS: Recent insights into emerging coronaviruses. Nature Reviews Microbiology, 14(8), 523–534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rocío Coutiño, E. , Ávila, L. , & Helguera, O. A. (2017). Nanopartículas de plata: Mecanismos de entrada, toxicidad y estrés oxidativo. Revista de Educación Bioquímica, 36(2), 39–54. [Google Scholar]
- Devanesan, S. , Ponmurugan, K. , AlSalhi, M. S. , & Al‐Dhabi, N. A. (2020). Cytotoxic and antimicrobial efficacy of silver nanoparticles synthesized using a traditional phytoproduct, asafoetida gum. International Journal of Nanomedicine, 15, 4351–4362. 10.2147/IJN.S258319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach, A. , Gnafakis, S. , & Shomrat, O. (2020). Innate lymphoid cell‐epithelial cell modules sustain intestinal homeostasis. Immunity, 52(3), 452–463. 10.1016/j.immuni.2020.02.016 [DOI] [PubMed] [Google Scholar]
- Dong, E. , Du, H. , & Gardner, L. (2020). An interactive web‐based dashboard to track COVID‐19 in real time. The Lancet Infectious Diseases, 20(5), 533–534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan, J. A. , Karlsson, C. , Smith, W. E. , & Graham, D. (2007). Enhanced oligonucleotide‐nanoparticle conjugate stability using thioctic acid modified oligonucleotides. Nucleic Acids Research, 35(11), 3668–3675. 10.1093/nar/gkm237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, T. , Liang, J. , Dong, N. , Lu, J. , Fu, Y. , Fang, L. , Xiao, S. , & Han, H. (2018). Glutathione‐capped Ag 2 s nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Applied Materials & Interfaces, 10(5), 4369–4378. 10.1021/acsami.7b13811 [DOI] [PubMed] [Google Scholar]
- Du, T. , Lu, J. , Liu, L. , Dong, N. , Fang, L. , Xiao, S. , & Han, H. (2018). Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Applied Bio Materials, 1(5), 1286–1293. 10.1021/acsabm.8b00154 [DOI] [PubMed] [Google Scholar]
- Durán, N. , Silveira, C. P. , Durán, M. , & Martinez, D. S. T. (2015). Silver nanoparticle protein corona and toxicity: A mini‐review. Journal of Nanobiotechnology, 13(1), 55. 10.1186/s12951-015-0114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elechiguerra, J. L. , Burt, J. L. , Morones, J. R. , Camacho‐Bragado, A. , Gao, X. , Lara, H. H. , & Yacaman, M. J. (2005). Interaction of silver nanoparticles with HIV‐1. Journal of Nanobiotechnology, 3(6), 1–10. 10.1186/1477-3155-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmehbad, N. Y. , & Mohamed, N. A. (2020). Designing, preparation and evaluation of the antimicrobial activity of biomaterials based on chitosan modified with silver nanoparticles. International Journal of Biological Macromolecules, 151, 92–103. 10.1016/j.ijbiomac.2020.01.298 [DOI] [PubMed] [Google Scholar]
- Fatima, M. , Zaidi, N.‐S. S. , Amraiz, D. , & Afzal, F. (2016). In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza A virus. Journal of Microbiology and Biotechnology, 26(1), 151–159. 10.4014/jmb.1508.08024 [DOI] [PubMed] [Google Scholar]
- FDA . (2020). CLIA (clinical laboratory improvement amendments) testing . Coronavirus Disease 2019 Testing Basics | FDA. https://www.fda.gov/consumers/consumer-updates/coronavirus-disease-2019-testing-basics
- Gaillet, S. , & Rouanet, J.‐M. (2015). Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms – A review. Food and Chemical Toxicology, 77, 58–63. 10.1016/j.fct.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Galdiero, S. , Falanga, A. , Vitiello, M. , Cantisani, M. , Marra, V. , & Galdiero, M. (2011). Silver nanoparticles as potential antiviral agents. Molecules, 16(10), 8894–8918. 10.3390/molecules16108894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero, S. , Rai, M. , Gade, A. , Falanga, A. , Incoronato, N. , Russo, L. , Galdiero, M. , Gaikwad, S. , & Ingle, A. (2013). Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. International Journal of Nanomedicine, 8(1), 4303–4314. 10.2147/IJN.S50070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibo, D. , Borbón‐Nuñez, H. A. , de León, J. N. D. , García Mendoza, E. , Estrada, I. , Toledano‐Magaña, Y. , Tiznado, H. , Ovalle‐Marroquin, M. , Soto‐Ramos, A. G. , Blanco, A. , Rodríguez, J. A. , Romo, O. A. , Chávez‐Almazán, L. A. , & Susarrey‐Arce, A. (2020). Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high‐antimicrobial activity. Scientific Reports, 10(1), 12805. 10.1038/s41598-020-69606-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiuță, I. , & Cristea, D. (2020). Silver nanoparticles for delivery purposes. In Nanoengineered biomaterials for advanced drug delivery (pp. 347–371). Elsevier; 10.1016/B978-0-08-102985-5.00015-2 [DOI] [Google Scholar]
- Guo, Y.‐R. , Cao, Q.‐D. , Hong, Z.‐S. , Tan, Y.‐Y. , Chen, S.‐D. , Jin, H.‐J. , Tan, K.‐S. , Wang, D.‐Y. , & Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – An update on the status. Military Medical Research, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan, S. , Qasim, M. , Choi, Y. , Do, J. T. , Park, C. , Hong, K. , Kim, J.‐H. , & Song, H. (2020). Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials, 10(9), 1645. 10.3390/nano10091645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushulak, B. D. , & MacPherson, D. W. (2004). Globalization of infectious diseases: the impact of migration. Clinical Infectious Diseases, 38(12), 1742–1748. 10.1086/421268 [DOI] [PubMed] [Google Scholar]
- Hossain, S. (2020). A study on understanding potential gold and silver nanoparticle: An overview. International Journal of Nanoscience, 20, 2150009. 10.1142/S0219581X21500095 [DOI] [Google Scholar]
- Hu, R. L. , Li, S. R. , Kong, F. J. , Hou, R. J. , Guan, X. L. , & Guo, F. (2014). Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genetics and Molecular Research, 13(3), 7022–7028. 10.4238/2014.March.19.2 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506 10.1016/S0140-6736(20)30183‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huy, T. Q. , Hien Thanh, N. T. , Thuy, N. T. , Van Chung, P. , Hung, P. N. , Le, A.‐T. , & Hong Hanh, N. T. (2017). Cytotoxicity and antiviral activity of electrochemical – Synthesized silver nanoparticles against poliovirus. Journal of Virological Methods, 241, 52–57. 10.1016/j.jviromet.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Hyun, J. , Lee, B. , Ryu, H. , Sung, J. , Chung, K. , & Yu, I. (2008). Effects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in rats. Toxicology Letters, 182(1–3), 24–28. 10.1016/j.toxlet.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Jagaran, K. , & Singh, M. (2020). Nanomedicine for COVID‐19: Potential of copper nanoparticles. Biointerface Research in Applied Chemistry, 11(3), 10716–10728. 10.33263/BRIAC113.1071610728 [DOI] [Google Scholar]
- Jeremiah, S. S. , Miyakawa, K. , Morita, T. , Yamaoka, Y. , & Ryo, A. (2020). Potent antiviral effect of silver nanoparticles on SARS‐CoV‐2. Biochemical and Biophysical Research Communications, 533(1), 195–200. 10.1016/j.bbrc.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, J. H. , Jung, J. H. , Kim, S. S. , Yoon, J.‐U. , Park, J. D. , Choi, B. S. , Chung, Y. H. , Kwon, I. H. , Jeong, J. , Han, B. S. , Shin, J. H. , Sung, J. H. , Song, K. S. , & Yu, I. J. (2007). Twenty‐eight‐day inhalation toxicity study of silver nanoparticles in Sprague‐Dawley rats. Inhalation Toxicology, 19(10), 857–871. 10.1080/08958370701432108 [DOI] [PubMed] [Google Scholar]
- Ji, J. H. , & Yu, I. J. (2012). Estimation of human equivalent exposure from rat inhalation toxicity study of silver nanoparticles using multi‐path particle dosimetry model. Toxicology Research, 1(3), 206. 10.1039/c2tx20029e [DOI] [Google Scholar]
- Jiang, S. , & He, Y. (2012). Anticuerpos monoclonales neutralizantes contra el coronavirus asociado al síndrome respiratorio agudo severo (Patent No. 2 384 497). In TRADUCCIÓN DE PATENTE EUROPEA (2 384 497). OFICINA ESPAÑOLA DE PATENTES Y MARCAS. https://patentimages.storage.googleapis.com/06/28/92/2fe0d65c3acad4/ES2384497T3.pdf
- Jindal, S. , & Gopinath, P. (2020). Nanotechnology based approaches for combatting COVID‐19 viral infection. Nano Express, 1(2), 022003. 10.1088/2632-959X/abb714 [DOI] [Google Scholar]
- Jones, G. W. , Monopoli, M. P. , Campagnolo, L. , Pietroiusti, A. , Tran, L. , & Fadeel, B. (2020). No small matter: A perspective on nanotechnology‐enabled solutions to fight COVID‐19. Nanomedicine, 15(24), 2411–2427. 10.2217/nnm-2020-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel, M. , Al‐Taher, A. , Park, B. K. , Kwon, H. , & Al‐Nazawi, M. (2020). A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. Journal of Medical Virology, 92(9), 1665–1670. 10.1002/jmv.25928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. , Yang, M. , Hong, Z. , Zhang, L. , Huang, Z. , Chen, X. , He, S. , Zhou, Z. , Zhou, Z. , Chen, Q. , Yan, Y. , Zhang, C. , Shan, H. , & Chen, S. (2020). Crystal structure of SARS‐CoV‐2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharmaceutica Sinica B, 10(7), 1228–1238. 10.1016/j.apsb.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik Pandiyan, G. , & Prabaharan, T. (2020). Implementation of nanotechnology in fuel cells. Materials Today: Proceedings, 33, 2681–2685. 10.1016/j.matpr.2020.01.368 [DOI] [Google Scholar]
- Kasmi, Y. , Khataby, K. , Souiri, A. , & Ennaji, M. M. (2020). Coronaviridae: 100,000 years of emergence and reemergence. In Emerging and reemerging viral pathogens (pp. 127–149). Elsevier; 10.1016/B978-0-12-819400-3.00007-7 [DOI] [Google Scholar]
- Khaiboullina, S. , Uppal, T. , Dhabarde, N. , Subramanian, V. R. , & Verma, S. C. (2020). Inactivation of human coronavirus by titania nanoparticle coatings and UVC radiation: Throwing light on SARS‐CoV‐2. Viruses, 13(1), 19. 10.3390/v13010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal, N. , Kaur, G. , Kumar, N. , Tiwari, A. , Gandhi, R. , & Vishwavidyalaya, P. (2014). Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Digest Journal of Nanomaterials and Biostructures, 9(1), 175–186. [Google Scholar]
- Kim, J. S. , Sung, J. H. , Ji, J. H. , Song, K. S. , Lee, J. H. , Kang, C. S. , & Yu, I. J. (2011). In vivo genotoxicity of silver nanoparticles after 90‐day silver nanoparticle inhalation exposure. Safety and Health at Work, 2(1), 34–38. 10.5491/SHAW.2011.2.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. S. , Kim, J. S. , Cho, H. S. , Rha, D. S. , Kim, J. M. , Park, J. D. , Choi, B. S. , Lim, R. , Chang, H. K. , Chung, Y. H. , Kwon, I. H. , Jeong, J. , Han, B. S. , & Yu, I. J. (2008). Twenty‐eight‐day oral toxicity, genotoxicity, and gender‐related tissue distribution of silver nanoparticles in Sprague‐Dawley rats. Inhalation Toxicology, 20(6), 575–583. 10.1080/08958370701874663 [DOI] [PubMed] [Google Scholar]
- Kumar, M. , Behera, A. K. , Lockey, R. F. , Zhang, J. , Bhullar, G. , de la Cruz, C. P. , Chen, L.‐C. , Leong, K. W. , Huang, S.‐K. , & Mohapatra, S. S. (2002). Intranasal Gene Transfer by Chitosan–DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Human Gene Therapy, 13(12), 1415–1425. 10.1089/10430340260185058 [DOI] [PubMed] [Google Scholar]
- Kumar Raghav, P. , & Mohanty, S. (2020). Are graphene and graphene‐derived products capable of preventing COVID‐19 infection? Medical Hypotheses, 144, 110031 10.1016/j.mehy.2020.110031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki, M. , Shimomura, I. , Kiyono, T. , Ochiya, T. , & Yamamoto, Y. (2020). Cell‐type specific tumorigenesis with Ras oncogenes in human lung epithelial cells. Biochemical and Biophysical Research Communications, 525(2), 483–490. 10.1016/j.bbrc.2020.02.113 [DOI] [PubMed] [Google Scholar]
- Kwon, J.‐T. , Minai‐Tehrani, A. , Hwang, S.‐K. , Kim, J.‐E. , Shin, J.‐Y. , Yu, K.‐N. , Chang, S.‐H. , Kim, D.‐S. , Kwon, Y.‐T. , Choi, I.‐J. , Cheong, Y.‐H. , Kim, J.‐S. , & Cho, M.‐H. (2012). Acute pulmonary toxicity and body distribution of inhaled metallic silver nanoparticles. Toxicological Research, 28(1), 25–31. 10.5487/TR.2012.28.1.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara, H. H. , Ayala‐Nuñez, N. V. , Ixtepan‐Turrent, L. , & Rodriguez‐Padilla, C. (2010). Mode of antiviral action of silver nanoparticles against HIV‐1. Journal of Nanobiotechnology, 8(1), 1. 10.1186/1477-3155-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Mun, J. , Park, J. D. , & Yu, I. J. (2012). A health surveillance case study on workers who manufacture silver nanomaterials. Nanotoxicology, 6(6), 667–669. 10.3109/17435390.2011.600840 [DOI] [PubMed] [Google Scholar]
- Lee, L. , & Wang, Q. (2006). Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine: Nanotechnology, Biology and Medicine, 2(3), 137–149. 10.1016/j.nano.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Lembo, D. , Donalisio, M. , Civra, A. , Argenziano, M. , & Cavalli, R. (2018). Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opinion on Drug Delivery, 15(1), 93–114. 10.1080/17425247.2017.1360863 [DOI] [PubMed] [Google Scholar]
- Li, P. , Fu, J.‐B. , Li, K.‐F. , Liu, J.‐N. , Wang, H.‐L. , Liu, L.‐J. , Chen, Y. , Zhang, Y.‐L. , Liu, S.‐L. , Tang, A. , Tong, Z.‐D. , & Yan, J.‐B. (2020). Transmission of COVID‐19 in the terminal stages of the incubation period: A familial cluster. International Journal of Infectious Diseases, 96, 452–453. 10.1016/j.ijid.2020.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Geng, M. , Peng, Y. , Meng, L. , & Lu, S. (2020). Molecular immune pathogenesis and diagnosis of COVID‐19. Journal of Pharmaceutical Analysis, 10(2), 102–108. 10.1016/j.jpha.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Lin, Z. , Zhao, M. , Guo, M. , Xu, T. , Wang, C. , Xia, H. , & Zhu, B. (2016). Reversal of H1N1 influenza virus‐induced apoptosis by silver nanoparticles functionalized with amantadine. RSC Advances, 6(92), 89679–89686. 10.1039/C6RA18493F [DOI] [Google Scholar]
- Li, Y. , Lin, Z. , Zhao, M. , Xu, T. , Wang, C. , Hua, L. , Wang, H. , Xia, H. , & Zhu, B. (2016). Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS‐mediated signaling pathways. ACS Applied Materials & Interfaces, 8(37), 24385–24393. 10.1021/acsami.6b06613 [DOI] [PubMed] [Google Scholar]
- Liao, H. , Nehl, C. L. , & Hafner, J. H. (2006). Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine, 1(2), 201–208. 10.2217/17435889.1.2.201 [DOI] [PubMed] [Google Scholar]
- Lin, L.‐T. , Hsu, W.‐C. , & Lin, C.‐C. (2014). Antiviral natural products and herbal medicines. Journal of Traditional and Complementary Medicine, 4(1), 24–35. 10.4103/2225-4110.124335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M.‐H. , Moses, D. C. , Hsieh, C.‐H. , Cheng, S.‐C. , Chen, Y.‐H. , Sun, C.‐Y. , & Chou, C.‐Y. (2018). Disulfiram can inhibit MERS and SARS coronavirus papain‐like proteases via different modes. Antiviral Research, 150, 155–163. 10.1016/j.antiviral.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Z. , Li, Y. , Guo, M. , Xu, T. , Wang, C. , Zhao, M. , Wang, H. , Chen, T. , & Zhu, B. (2017). The inhibition of H1N1 influenza virus‐induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Advances, 7(2), 742–750. 10.1039/C6RA25010F [DOI] [Google Scholar]
- Loeschner, K. , Hadrup, N. , Qvortrup, K. , Larsen, A. , Gao, X. , Vogel, U. , Mortensen, A. , Lam, H. , & Larsen, E. H. (2011). Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Particle and Fibre Toxicology, 8(1), 18. 10.1186/1743-8977-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L. , Sun, R. W. Y. , Chen, R. , Hui, C. K. , Ho, C. M. , Luk, J. M. , Lau, G. K. K. , & Che, C. M. (2008). Silver nanoparticles inhibit hepatitis B virus replication. Antiviral Therapy, 13(2), 252–262. [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , Wang, W. , Song, H. , Huang, B. , Zhu, N. , Bi, Y. , Ma, X. , Zhan, F. , Wang, L. , Hu, T. , Zhou, H. , Hu, Z. , Zhou, W. , Zhao, L. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395(10224), 565–574 10.1016/S0140-6736(20)30251‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małaczewska, J. (2014). The splenocyte proliferative response and cytokine secretion in mice after 28‐day oral administration of silver nanocolloid. Polish Journal of Veterinary Sciences, 17(1), 27–35. 10.2478/pjvs-2014-0004 [DOI] [PubMed] [Google Scholar]
- Marimuthu, S. , Antonisamy, A. J. , Malayandi, S. , Rajendran, K. , Tsai, P.‐C. , Pugazhendhi, A. , & Ponnusamy, V. K. (2020). Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. Journal of Photochemistry and Photobiology B: Biology, 205, 111823 10.1016/j.jphotobiol.2020.111823 [DOI] [PubMed] [Google Scholar]
- Medhi, R. , Srinoi, P. , Ngo, N. , Tran, H.‐V. , & Lee, T. R. (2020). Nanoparticle‐based strategies to combat COVID‐19. ACS Applied Nano Materials, 3(9), 8557–8580. 10.1021/acsanm.0c01978 [DOI] [PubMed] [Google Scholar]
- Mehrbod, P. , Motamed, N. , Tabatabaian, M. , Soleimani Estyar, R. , Amini, E. , Shahidi, M. , & Kheiri, M. T. (2009). Vitro antiviral effect of “nanosilver” on influenza virus. DARU Journal of Pharmaceutical Sciences, 17(2), 88–93. [Google Scholar]
- Moitra, P. , Alafeef, M. , Dighe, K. , Frieman, M. B. , & Pan, D. (2020). Selective naked‐eye detection of SARS‐CoV‐2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano, 14(6), 7617–7627. 10.1021/acsnano.0c03822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens, D. M. , Folkers, G. K. , & Fauci, A. S. (2004). The challenge of emerging and re‐emerging infectious diseases. Nature, 430(6996), 242–249. 10.1038/nature02759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, Y. , Ono, T. , Miyahira, Y. , Nguyen, V. Q. , Matsui, T. , & Ishihara, M. (2013). Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Research Letters, 8(1), 93. 10.1186/1556-276X-8-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill, K. , May, K. , Leek, D. , Langland, N. , Jeane, L. D. , Ventura, J. , Skubisz, C. , Scherer, S. , Lopez, E. , Crocker, E. , Peters, R. , Oertle, J. , Nguyen, K. , Just, S. , Orian, M. , Humphrey, M. , Payne, D. , Jacobs, B. , Waters, R. , & Langland, J. (2013). Spectrum of antimicrobial activity associated with ionic colloidal silver. The Journal of Alternative and Complementary Medicine, 19(3), 224–231. 10.1089/acm.2011.0681 [DOI] [PubMed] [Google Scholar]
- Morris, D. , Ansar, M. , Speshock, J. , Ivanciuc, T. , Qu, Y. , Casola, A. , & Garofalo, R. (2019). Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses, 11(8), 732. 10.3390/v11080732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy, S. K. (2007). Nanoparticles in modern medicine: State of the art and future challenges. International Journal of Nanomedicine, 2(2), 129–141. http://www.ncbi.nlm.nih.gov/pubmed/17722542 [PMC free article] [PubMed] [Google Scholar]
- Oller, A. R. , & Oberdörster, G. (2010). Incorporation of particle size differences between animal studies and human workplace aerosols for deriving exposure limit values. Regulatory Toxicology and Pharmacology, 57(2–3), 181–194. 10.1016/j.yrtph.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Orlowski, P. , Tomaszewska, E. , Gniadek, M. , Baska, P. , Nowakowska, J. , Sokolowska, J. , Nowak, Z. , Donten, M. , Celichowski, G. , Grobelny, J. , & Krzyzowska, M. (2014). Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE, 9(8), e104113. 10.1371/journal.pone.0104113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, V. , De Maio, F. , De Spirito, M. , & Papi, M. (2021). Face masks and nanotechnology: Keep the blue side up. Nano Today, 37, 101077 10.1016/j.nantod.2021.101077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, K. (2013). Toxicokinetic differences and toxicities of silver nanoparticles and silver ions in rats after single oral administration. Journal of Toxicology and Environmental Health, Part A, 76(22), 1246–1260. 10.1080/15287394.2013.849635 [DOI] [PubMed] [Google Scholar]
- Park, S. , Ko, Y.‐S. , Lee, S. J. , Lee, C. , Woo, K. , & Ko, G. (2018). Inactivation of influenza A virus via exposure to silver nanoparticle‐decorated silica hybrid composites. Environmental Science and Pollution Research, 25(27), 27021–27030. 10.1007/s11356-018-2620-z [DOI] [PubMed] [Google Scholar]
- Passi, D. , Sharma, S. , Dutta, S. R. , Dudeja, P. , & Sherma, V. (2015). Ebola virus disease (the killer virus): Another threat to humans and bioterrorism: Brief review and recent updates. Journal of Clinical and Diagnostic Research, 9(6), LE01–LE08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, J. R. A. , Castellarnau, A. , Luscombe, C. A. , Fairley, J. K. , & Heery, G. P. (2020). Astodrimer sodium, dendrimer antiviral, inhibits replication of SARS‐CoV‐2 in vitro. Biorxiv. 10.1101/2020.08.20.260190 [DOI] [Google Scholar]
- Pinals, R. L. , Ledesma, F. , Yang, D. , Navarro, N. , Jeong, S. , Pak, J. E. , Kuo, L. , Chuang, Y.‐C. , Cheng, Y.‐W. , Sun, H.‐Y. , & Landry, M. P. (2020). Rapid SARS‐CoV‐2 detection by carbon nanotube‐based near‐infrared nanosensors. Medrxiv. 10.1101/2020.11.02.20223404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu, S. , & Poulose, E. K. (2012). Silver nanoparticles: Mechanism of antimicrobial. International Nano Letters, 2, 32–41. http://www.inl-journal.com/content/pdf/2228-5326-2-32.pdf [Google Scholar]
- Rabiee, N. , Bagherzadeh, M. , Ghasemi, A. , Zare, H. , Ahmadi, S. , Fatahi, Y. , Dinarvand, R. , Rabiee, M. , Ramakrishna, S. , Shokouhimehr, M. , & Varma, R. S. (2020). Point‐of‐use rapid detection of SARS‐CoV‐2: Nanotechnology‐enabled solutions for the COVID‐19 pandemic. International Journal of Molecular Sciences, 21(14), 5126. 10.3390/ijms21145126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, M. F. , Wang, J. , Patterson, T. A. , Saini, U. T. , Robinson, B. L. , Newport, G. D. , Murdock, R. C. , Schlager, J. J. , Hussain, S. M. , & Ali, S. F. (2009). Expression of genes related to oxidative stress in the mouse brain after exposure to silver‐25 nanoparticles. Toxicology Letters, 187(1), 15–21. 10.1016/j.toxlet.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Raj, S. , Sumod, U. , Jose, S. , & Sabitha, M. (2012). Nanotechnology in cosmetics: Opportunities and challenges. Journal of Pharmacy and Bioallied Sciences, 4(3), 186. 10.4103/0975-7406.99016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez, C. D. (2013). Los virus y la nanomedicina. Revista Del Instituto Nacional de Higiene Rafael Rangel, 44(2), 73–80. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-04772013000200012 [Google Scholar]
- Randazzo, W. , Fabra, M. J. , Falcó, I. , López‐Rubio, A. , & Sánchez, G. (2018). Polymers and biopolymers with antiviral activity: Potential applications for improving food safety. Comprehensive Reviews in Food Science and Food Safety, 17(3), 754–768. 10.1111/1541-4337.12349 [DOI] [PubMed] [Google Scholar]
- Rao, S. S. , Saptami, K. , Venkatesan, J. , & Rekha, P. D. (2020). Microwave‐assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. International Journal of Biological Macromolecules, 163, 745–755. 10.1016/j.ijbiomac.2020.06.230 [DOI] [PubMed] [Google Scholar]
- Rogers, J. V. , Parkinson, C. V. , Choi, Y. W. , Speshock, J. L. , & Hussain, S. M. (2008). A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Research Letters, 3(4), 129–133. 10.1007/s11671-008-9128-2 [DOI] [Google Scholar]
- Sanchez‐Guzman, D. , Le Guen, P. , Villeret, B. , Sola, N. , Le Borgne, R. , Guyard, A. , Kemmel, A. , Crestani, B. , Sallenave, J.‐M. , & Garcia‐Verdugo, I. (2019). Silver nanoparticle‐adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA‐mediated mucosal immunity. Biomaterials, 217, 119308. 10.1016/j.biomaterials.2019.119308 [DOI] [PubMed] [Google Scholar]
- Sarkar, P. K. , & Das Mukhopadhyay, C. (2021). Ayurvedic metal nanoparticles could be novel antiviral agents against SARS‐CoV‐2. International Nano Letters. 10.1007/s40089-020-00323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone, C. , Brusco, S. , Bertini, M. , Sportiello, L. , Rafaniello, C. , Zoccoli, A. , Berrino, L. , Racagni, G. , Rossi, F. , & Capuano, A. (2020). Current pharmacological treatments for COVID‐19: What's next? British Journal of Pharmacology, 177(21), 4813–4824. 10.1111/bph.15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: Current knowledge. Virology Journal, 16(1), 69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta, J. , & Hussain, C. M. (2021). Carbon nanomaterials to combat virus: A perspective in view of COVID‐19. Carbon Trends, 2, 100019 10.1016/j.cartre.2020.100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, G. , Lee, G. , Kim, M. J. , Baek, S.‐H. , Choi, M. , Ku, K. B. , Lee, C.‐S. , Jun, S. , Park, D. , Kim, H. G. , Kim, S.‐J. , Lee, J.‐O. , Kim, B. T. , Park, E. C. , & Kim, S. I. (2020). Rapid detection of COVID‐19 causative virus (SARS‐CoV‐2) in human nasopharyngeal swab specimens using field‐effect transistor‐based biosensor. ACS Nano, 14(4), 5135–5142. 10.1021/acsnano.0c02823 [DOI] [PubMed] [Google Scholar]
- Shahare, B. , & Yashpal, M. (2013). Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicology Mechanisms and Methods, 23(3), 161–167. 10.3109/15376516.2013.764950 [DOI] [PubMed] [Google Scholar]
- Shan, B. , Broza, Y. Y. , Li, W. , Wang, Y. , Wu, S. , Liu, Z. , Wang, J. , Gui, S. , Wang, L. , Zhang, Z. , Liu, W. , Zhou, S. , Jin, W. , Zhang, Q. , Hu, D. , Lin, L. , Zhang, Q. , Li, W. , Wang, J. , … Haick, H. (2020). Multiplexed nanomaterial‐based sensor array for detection of COVID‐19 in exhaled breath. ACS Nano, 14(9), 12125–12132. 10.1021/acsnano.0c05657 [DOI] [PubMed] [Google Scholar]
- Sharma, M. , Monika, T. P. , Saini, R. V. , Kumar, R. , & Torino, E. (2020). Unveiling antimicrobial and anticancerous behavior of AuNPs and AgNPs moderated by rhizome extracts of Curcuma longa from diverse altitudes of Himalaya. Scientific Reports, 10(1), 10934. 10.1038/s41598-020-67673-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Mukkur, T. K. S. , Benson, H. A. E. , & Chen, Y. (2009). Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. Journal of Pharmaceutical Sciences, 98(3), 812–843. 10.1002/jps.21493 [DOI] [PubMed] [Google Scholar]
- Soares, S. , Sousa, J. , Pais, A. , & Vitorino, C. (2018). Nanomedicine: Principles, properties, and regulatory issues. Frontiers in Chemistry, 6, 360. 10.3389/fchem.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza, R. L. , Donaldson, A. I. C. , & Myint, P. K. (2018). Vaccine against arteriosclerosis: An update. Therapeutic Advances in Vaccines, 9(6), 259–261. 10.1177/https [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondi, I. , & Salopek‐Sondi, B. (2004). Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram‐negative bacteria. Journal of Colloid and Interface Science, 275(1), 177–182. 10.1016/j.jcis.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Song, K. S. , Sung, J. H. , Ji, J. H. , Lee, J. H. , Lee, J. S. , Ryu, H. R. , Lee, J. K. , Chung, Y. H. , Park, H. M. , Shin, B. S. , Chang, H. K. , Kelman, B. , & Yu, I. J. (2013). Recovery from silver‐nanoparticle‐exposure‐induced lung inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology, 7(2), 169–180. 10.3109/17435390.2011.648223 [DOI] [PubMed] [Google Scholar]
- Sood, R. , & Chopra, D. S. (2018). Regulatory approval of silver nanoparticles. Applied Clinical Research, Clinical Trials and Regulatory Affairs, 5(2), 74–79 10.2174/2213476X05666180614121601 [DOI] [Google Scholar]
- Speshock, J. L. , Murdock, R. C. , Braydich‐Stolle, L. K. , Schrand, A. M. , & Hussain, S. M. (2010). Interaction of silver nanoparticles with Tacaribe virus. Journal of Nanobiotechnology, 8(1), 19. 10.1186/1477-3155-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A. K. , Dwivedi, N. , Dhand, C. , Khan, R. , Sathish, N. , Gupta, M. K. , Kumar, R. , & Kumar, S. (2020). Potential of graphene‐based materials to combat COVID‐19: Properties, perspectives, and prospects. Materials Today Chemistry, 18, 100385 10.1016/j.mtchem.2020.100385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh, U. , Murugan, K. , Benelli, G. , Nicoletti, M. , Barnard, D. R. , Panneerselvam, C. , Kumar, P. M. , Subramaniam, J. , Dinesh, D. , & Chandramohan, B. (2015). Tackling the growing threat of dengue: Phyllanthus niruri‐mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitology Research, 114(4), 1551–1562. 10.1007/s00436-015-4339-9 [DOI] [PubMed] [Google Scholar]
- Taghavizadeh Yazdi, M. E. , Darroudi, M. , Amiri, M. S. , Hosseini, H. A. , Nourbakhsh, F. , Mashreghi, M. , Farjadi, M. , Mousavi Kouhi, S. M. , & Mousavi, S. H. (2020). Anticancer, antimicrobial, and dye degradation activity of biosynthesised silver nanoparticle using Artemisia kopetdaghensis . Micro & Nano Letters, 15(14), 1046–1050. 10.1049/mnl.2020.0387 [DOI] [Google Scholar]
- Takenaka, S. , Karg, E. , Roth, C. , Schulz, H. , Ziesenis, A. , Heinzmann, U. , Schramel, P. , & Heyder, J. (2001). Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environmental Health Perspectives, 109(suppl 4), 547–551. 10.1289/ehp.01109s4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebian, S. , Wallace, G. G. , Schroeder, A. , Stellacci, F. , & Conde, J. (2020). Nanotechnology‐based disinfectants and sensors for SARS‐CoV‐2. Nature Nanotechnology, 15(8), 618–621. 10.1038/s41565-020-0751-0 [DOI] [PubMed] [Google Scholar]
- Tang, J. , Xiong, L. , Wang, S. , Wang, J. , Liu, L. , Li, J. , Yuan, F. , & Xi, T. (2009). Distribution, translocation and accumulation of silver nanoparticles in rats. Journal of Nanoscience and Nanotechnology, 9(8), 4924–4932. 10.1166/jnn.2009.1269 [DOI] [PubMed] [Google Scholar]
- Teirumnieks, E. , Balchev, I. , Ghalot, R. S. , & Lazov, L. (2021). Antibacterial and anti‐viral effects of silver nanoparticles medicine against COVID‐19 ‐ A review. Laser Physics, 31(1), 013001. [Google Scholar]
- Thi Ngoc Dung, T. , Nang Nam, V. , Thi Nhan, T. , Ngoc, T. T. B. , Minh, L. Q. , Nga, B. T. T. , Phan Le, V. , & Viet Quang, D. (2020). Silver nanoparticles as potential antiviral agents against African swine fever virus. Materials Research Express, 6(12), 1250g9. 10.1088/2053-1591/ab6ad8 [DOI] [Google Scholar]
- Tiwari, D. K. , Jin, T. , & Behari, J. (2011). Dose‐dependent in‐vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicology Mechanisms and Methods, 21(1), 13–24. 10.3109/15376516.2010.529184 [DOI] [PubMed] [Google Scholar]
- Vadlamani, B. S. , Uppal, T. , Verma, S. C. , & Misra, M. (2020). Functionalized TiO2 nanotube‐based electrochemical biosensor for rapid detection of SARS‐CoV‐2. Sensors, 20(20), 5871. 10.3390/s20205871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y.‐J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Ji, Z. , Chang, C. H. , Zhang, H. , Wang, M. , Liao, Y.‐P. , Lin, S. , Meng, H. , Li, R. , Sun, B. , Van Winkle, L. , Pinkerton, K. E. , Zink, J. I. , Xia, T. , & Nel, A. E. (2014). Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small, 10(2), 385–398. 10.1002/smll.201301597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S. R. , & Navas‐Martin, S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Reviews, 69(4), 635–664. 10.1128/MMBR.69.4.635-664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2021). Coronavirus disease (COVID‐19) situation reports: Weekly epidemiological update and weekly operational update . https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- Wieczorek, K. , Szutkowska, B. , & Kierzek, E. (2020). Anti‐influenza strategies based on nanoparticle applications. Pathogens, 9(12), 1020. 10.3390/pathogens9121020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K. K. Y. , & Liu, X. (2010). Silver nanoparticles—The real “silver bullet” in clinical medicine? MedChemComm, 1(2), 125. 10.1039/c0md00069h [DOI] [Google Scholar]
- Xiang, D.‐x. , Chen, Q. , Pang, L. , & Zheng, C. (2011). Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. Journal of Virological Methods, 178(1–2), 137–142. 10.1016/j.jviromet.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Xiang, D. , Zheng, C. , Zheng, Y. , Li, X. , Yin, J. , O'Conner, M. , Marappan, M. , Miao, Y. , Xiang, B. , Duan, W. , Shigdar, S. , & Zhao, X. (2013). Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. International Journal of Nanomedicine, 8(1), 4103–4114. 10.2147/IJN.S53622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. (2020). Inhibition of SARS‐CoV‐2 replication by acidizing and RNA lyase‐modified carbon nanotubes combined with photodynamic thermal effect. Journal of Exploratory Research in Pharmacology, 000(000), 1–6. 10.14218/JERP.2020.00005 [DOI] [Google Scholar]
- Yang, X. X. , Li, C. M. , & Huang, C. Z. (2016). Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale, 8(5), 3040–3048. 10.1039/C5NR07918G [DOI] [PubMed] [Google Scholar]
- Yen, C.‐W. , de Puig, H. , Tam, J. O. , Gómez‐Márquez, J. , Bosch, I. , Hamad‐Schifferli, K. , & Gehrke, L. (2015). Multicolored silver nanoparticles for multiplexed disease diagnostics: Distinguishing dengue, yellow fever, and Ebola viruses. Lab on a Chip, 15(7), 1638–1641. 10.1039/C5LC00055F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, R. , He, H. , Huang, J. , & Su, C. (2019). Characterization of interfacial behaviour of ortho‐positioned diaryl disulfide on silver substrates by surface‐enhanced Raman scattering and electroreduction. Materials Chemistry and Physics, 235, 121726 10.1016/j.matchemphys.2019.121726 [DOI] [Google Scholar]
- Zhang, B. , Salieb‐Beugelaar, G. B. , Nigo, M. M. , Weidmann, M. , & Hunziker, P. (2015). Diagnosing dengue virus infection: Rapid tests and the role of micro/nanotechnologies. Nanomedicine: Nanotechnology, Biology and Medicine, 11(7), 1745–1761. 10.1016/j.nano.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Honko, A. , Zhou, J. , Gong, H. , Downs, S. N. , Vasquez, J. H. , Fang, R. H. , Gao, W. , Griffiths, A. , & Zhang, L. (2020). Cellular nanosponges inhibit SARS‐CoV‐2 infectivity. Nano Letters, 20(7), 5570–5574. 10.1021/acs.nanolett.0c02278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Cui, H. , Song, W. , Ru, X. , Zhou, W. , & Yu, X. (2020). A simple magnetic nanoparticles‐based viral RNA extraction method for efficient detection of SARS‐CoV‐2. Biorxiv. 10.1101/2020.02.22.961268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


