Abstract
Background
Current literature on COVID‐19 pandemic has identified diabetes as a common comorbidity in patients affected. However, the evidence that diabetes increases the risk of infection, effect of diabetes on outcomes and characteristics of patients at risk is not clear.
Objectives
To explore the prevalence of diabetes in COVID‐19 pandemic, effect of diabetes on clinical outcomes and to characterise the patients with diabetes affected by COVID‐19.
Methods
A literature review of articles published in English language and reported outcomes on prevalence and effect of diabetes on outcomes and patients’ characteristics.
Results
The prevalence of diabetes in COVID‐19 patients appears similar to that in the general population. The evidence of diabetes increasing the risk of severe infection and adverse outcomes is substantial. The progression of the disease into acute respiratory distress syndrome, the requirement for intensive care admission or mechanical ventilation and mortality all have been increased by the presence of diabetes. Patients with diabetes at risk of COVID‐19 appear to be obese, of older age, have uncontrolled glycaemia and have coexisting comorbidities especially cardiovascular disease and hypertension. Tight glycaemic control on admission to hospital using insulin infusion has shown some beneficial effects; however, the role of hypoglycaemic medications in the management of these patients is not yet clear.
Conclusion
High risk group should be identified and prioritised in future vaccination programmes. Future research is required to optimise management of patients with diabetes and develop new ways to manage them via technological developments such as telecare.
Key points.
Diabetes is a common comorbidity in patients with COVID‐19.
Diabetes is associated with severe illness and worse outcomes.
Uncontrolled diabetes and obesity appear to be the major characteristics of patients at risk.
Frailty may be a confounding factor that increases the risk of adverse outcomes.
Future research is required to optimise the management of patients with diabetes and COVID‐19.
1. INTRODUCTION
In December 2019, a new coronavirus—the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) —has emerged in Wuhan, China and was named COVID‐19 by the WHO. The virus spread rapidly across continents to cause a global pandemic. 1 Although the virus affected all age groups, the incidence was particularly shifted towards older age groups especially in those with underlying chronic health conditions. Among these chronic health conditions, diabetes mellitus in particular, appeared to be a major risk factor for both COVID‐19 infection and worse outcomes. Diabetes is already known to be associated with increased mortality from any acute and chronic illness including infection. 2 However, with this new pandemic, the characteristics of patients with diabetes infected with the COVID‐19 virus have not been clearly defined. Therefore, it is not yet known whether particular subgroups with diabetes are at increased risk of COVID‐19 infection. This manuscript aims to explore the prevalence of diabetes in COVID‐19 pandemic, characteristics of patients with diabetes affected by COVID‐19 and the effect of diabetes on clinical outcomes.
2. METHODS
We undertook a detailed literature search with full assessment of relevant articles by searching the following databases: Google Scholar, Medline and Embase. We used Medical Subject Heading (MeSH) terms: diabetes mellitus, COVID‐19, infection, comorbidity, patients’ characteristics, risk factors, adverse outcomes, prevalence, mortality, predictors, disease progression, disease severity and glycaemic control. We searched articles for three categories of data:
Articles that reported diabetes as comorbidity in patients with COVID‐19.
Articles that reported diabetes as a contributing factor to clinical outcomes.
Articles that specifically reported the characteristics of patients with diabetes affected by COVID‐19.
Articles were reviewed for relevance by abstract. A manual search of citations in retrieved articles was performed in addition to the electronic literature search. We limited our selection to studies published in English language and reported clear outcomes. For data extractions, all articles derived from the search enquiry were independently examined by the authors, and data were extracted in a standardised format, which included study design, population studied and main findings.
3. PREVALENCE
Diabetes is commonly reported as a premorbid condition for people infected with COVID‐19 although whether diabetes is a risk factor for this infection is still unclear. In a Chinese nationwide analysis of 1590 hospitalised patients with COVID‐19, at least one comorbidity was present in 25.1% of patients. The most prevalent comorbidity was hypertension (16.9%), followed by diabetes (8.2%). 3 In a data analysis of 12 studies that included 2108 Chinese patients with confirmed SARS‐ Cov‐2 infection, the prevalence of diabetes was 10.3%. 4 In another meta‐analysis of six studies including 1527 Chinese patients, the proportions of hypertension, cardio‐cerebrovascular disease and diabetes in patients with COVID‐19 were 17.1%, 16.4% and 9.7%, respectively. 5 A systematic review of eight studies including 46 248 Chinese patients found that diabetes was the second (8%) most prevalent comorbidity, after hypertension (17%), in people hospitalised with COVID‐19. 6 In a quantitative meta‐analysis of 19 studies (18 from China and one from Australia), 36.8% of CODID‐19 patients had underlying comorbidities (95% CI 24.7%‐48.9%), the most significant being hypertension (18.6%, 8.1‐29.0%), cardiovascular disease (14.4%, 5.7%‐23.1%), and diabetes (11.9%, 9.1%‐14.6%). 7 Data from a British report of 20 133 patients with severe COVID‐19 who were hospitalised showed a median age of 73 years (IQR 58, 82) and 77% had a documented comorbidity. The prevalence of diabetes was 21%, only second to chronic cardiac disease (31%). 8 In a US long‐term care facility report of 101 residents with COVID‐19, the median age was 83 years (range, 51‐100), the hospitalisation rate was 54.5% and the case fatality rate was 33.7%. Most (94.1% of 101) of the residents had chronic underlying health conditions, with hypertension (67.3%), cardiac disease (60.4%), renal disease (40.6%), diabetes mellitus (31.7%), pulmonary disease (31.7%), obesity (30.7%) and cancer (14.9%) being the most common. 9 The main international data reporting diabetes as a common comorbidity in patients with COVID‐19 are summarised in Table 1. 3 , 8 , 10 , 11
TABLE 1.
| Country | Population | Main findings |
|---|---|---|
| China | 1590 patients |
A. Mean (SD) age 48.9 (16.3) y. B. 25.1% have ≥1 comorbidity. C. Diabetes was second common morbidity (8.2%) after hypertension (16.9%). |
| Italy | 1625 died patients |
Analysis of subsample of 355 patients: A. Mean (SD) age 79.5 y (8.1). B. Mean (SD) number of comorbidities 2.7 (1.6). C. 99.2% of patients had ≥1 comorbidity. D. Diabetes was the first common comorbidity (35.5%). |
| UK | 20 133 patients |
A. Median age 73 y (IQR 58, 82). B. 77% had documented comorbidity. C. Diabetes was second common comorbidity (21%) after cardiac disease (31%). |
| USA | 5700 patients |
A. Median age, 63 y (IQR 52‐75). B. Median (IQR) Charlson Comorbidity Index score was 4 (2‐6) points. C. Diabetes was third common comorbidity (33.8%) after hypertension (56.6%) and obesity (41.7%). |
Abbreviations: SD, standard deviation, IQR, inter quartile range.
4. PATIENT CHARACTERISTICS
A case series to describe the characteristics of 28 Chinese patients, mean (SD) age was 68.6 (9.0) years, 75% were males and 50% were admitted to Intensive care unit (ICU). The HbA1c level was similar between ICU (7.3%) and non‐ICU (7.5%) patients but random blood glucose concentrations were significantly higher in ICU patients (13.7 mmol/L vs 9.8 mmol/L, P = .03 respectively). Seventeen (60.7%) patients had coexisting chronic diseases, including hypertension, heart disease, cerebrovascular disease and chronic respiratory disease. 12 In another study of 193 Chinese patients with severe COVID‐19, 48 of them (24.9%) had diabetes. Compared with patients without diabetes, those with diabetes were older (70 years vs 60 years, P < .001), had more comorbidities (60.4% vs 44.8%, P = .06), were more likely to receive mechanical ventilation (81.3% vs 49.0%, P < .001) and had a higher mortality rate (81.3% vs 47.6%, P < .001). 13 In a Chinese study of 904 patients, 136 (15%) had diabetes, insulin usage was associated with poor prognosis while the use of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) was not associated with adverse clinical outcomes. 14 Data from the UK of a total of 3 154 300 patients with diabetes showed that uncontrolled diabetes (HbA1c > 86 mmol/mol vs 48‐53 mmol/mol) and obesity (BMI > 40 kg/m2 vs 25‐29.9 kg/m2) were independently associated with increased COVID‐19 mortality in both type 1 and type 2 diabetes. 15 In the univariate analysis of the French study that included 1317 patients with diabetes hospitalised for COVID‐19, characteristics significantly associated with the primary outcome (mechanical ventilation and/or death within 7 days of admission) were sex, BMI and previous treatment with renin angiotensin aldosterone system (RAAS) blockers, but not with age, type of diabetes, HbA1c, diabetic complications or glucose‐lowering therapies. However, in multivariable analyses, only BMI remained positively associated with the primary outcome. 16 Data from the US showed that mortality rate was 28.8% in patients with diabetes or uncontrolled hyperglycaemia compared with only 6.2% for patients without diabetes or hyperglycaemia. Also, hyperglycaemia‐related mortality was 41.7% compared with 14.8% for patients with diabetes but no hyperglycaemia suggesting that hyperglycaemia played a crucial role in adverse outcome. 17 Long duration of diabetes, uncontrolled glycaemia, obesity, increased prevalence of hypertension, chronic kidney disease and the common use of ACE inhibitors and ARBs were described in a British case series. 18 Characteristics of patients with diabetes and COVID‐19 infection are summarised in Table 2.
TABLE 2.
Main studies reporting characteristics of patients with diabetes and COVID‐19 12 , 13 , 14 , 15 , 16 , 17 , 18
| Study | Population | Main findings |
|---|---|---|
|
Wang F, et al Prospective observational, China. |
28 patients with COVID‐19 and diabetes. |
A. Mean (SD) age 68.6 (9.0) y, 75% males. B.17 (60.7%) had comorbidities: hypertension (53.6%), CVD (14.3%), cerebrovascular disease (14.3%), chronic pulmonary disease (14.3%). C. Common symptoms were fever (92.9%), dry cough (82.1%), fatigue (64.3%), dyspnoea (57.1%), anorexia (57.1%), diarrhoea (42.9%), expectoration (25.0%) and nausea (21.4%). C. 14 patients admitted to ICU, of whom 11 received non‐invasive and 7 received mechanical ventilation. D. 12 patients died in ICU, no mortality in non‐ICU patients, with ICU mortality rate 86% and overall mortality rate 43%. E. Mean (SD) HbA1c 7.5% (1.2) in non‐ICU and 7.3% (0.9) in ICU, P = .70. F. Mean (SD) RBG 13.7 (5.1) in ICU vs 9.8 (3.4) in ICU patients, P = .03. |
|
Yan Y, et al Retrospective observational, China. |
193 patients with severe COVID‐19, 48 with diabetes mellitus. |
Patients with compared with those without diabetes were: A. Significantly older, median (IQR) age 70 (62‐77) vs 64 (49‐73) y, P < .001. B. Have more comorbidities (60.4% vs 44.8%, P = .06), hypertension (50% vs 33.8%, P = .045), CVD (27.1% vs 12.4, P = .016), cerebrovascular disease (10.4% vs 2.1%, P = .036). C. Have significantly more ICU admission (66.7% vs 41.4%, P = .002). D. Required more mechanical ventilation (81.3% vs 49%, P < .001). E. Significantly higher mortality (81.3% vs 47.6%, P < .001). F. Median (IQR) HbA1c 7.2% (6.7‐8.3) vs 5.8% (5.5‐6.1), P < .001. |
|
Chen Y, et al Retrospective observational, China. |
Total 904 patients with COVID‐19, 136 with diabetes mellitus. |
A. Patients with were significantly older than those without diabetes (66 vs 56 y, P < .001), had more history of hypertension (61.2% vs 32.5%, P < .001) and higher HbA1c (7.3% vs 5.9%, P < .001). B. Risk factors for higher mortality of patients with diabetes were older age (adjusted OR 1.09, 95% CI 1.04‐1.15) per year increase (P = .001) and elevated CRP (1.12, 1.0‐1.24, P = .043). C. Insulin usage was associated with poor prognosis defined as progression to severe or critical illness (OR 3.58, 95% CI 1.37‐9.35, P = .009) but not with mortality. D. ACEI or ARBs use had no impact on outcomes. |
|
Holman N, et al National diabetes and mortality data, England |
265,090 people with type 1 and 2,889,210 with type 2 diabetes, all COVID‐19 positive. |
A. Mortality rate 0.16% in type 1 and 0.32% in type 2 diabetes. B. Adjusted mortality HR of HbA1c > 86 mmol/mol compared with HbA1c 48‐53 mmol/mol 2.19 (95% CI 1.46‐3.29) for type 1 and 1.62 (1.48‐1.79) for type 2 diabetes. C. U‐shaped relation between BMI and mortality, HRs for BMI > 40 kg/m2 compared with 25‐29.9 kg/m2 2.15 (95% 1.37‐3.36) and 1.46 (1.50‐1.79) for type 1 and type 2 respectively. |
|
Cariou B, et al nationwide 53 centres, observational, France |
1317 hospitalised patients with COVID‐19 and diabetes, primary outcome mechanical ventilation and/or death within 7 d of admission. |
A. Mean (SD) age 69.8 (13.0) y, 64.9% men, median BMI 28.4 (25th‐75th percentile 25.0‐32.7) kg/m2, type 2 diabetes (88.5%). B. Microvascular and macrovascular diabetic complications 46.8% and 40.8%, respectively. C. Primary outcome encountered in 29.0% (95% CI 26.6‐31.5), mortality 10.6% (9.0‐12.4), 18.0% (16.0‐20.2) discharged on day 7. D. In multivariable analyses, only BMI positively associated with primary outcome (OR 1.28, 95% CI 1.10‐1.47). E. Age (OR 2.48, 95% CI 1.74‐3.53), treated obstructive sleep apnoea (2.80, 1.46‐5.38), microvascular (2.14, 1.16‐3.94) and macrovascular complications (2.54, 1.44‐4.50) were independently associated with mortality risk on day 7. |
|
Bode B, et al retrospective observational US |
Total 1122 patients in 88 US hospitals, 451 with diabetes or uncontrolled hyperglycaemia. |
A. mortality rate 28.8% in 184 diabetes and/or uncontrolled hyperglycaemia patients, compared with 6.2% of 386 patients without diabetes or hyperglycaemia (P < .001). B. Mortality rate 41.7% in uncontrolled hyperglycaemia and 14.8% in diabetes patients, P < .001. C. LOS was longer in patients with uncontrolled hyperglycaemia or diabetes compared with those without (5.7 vs 4.3 d, P < .001). |
| Conway J, et al retrospective case series, UKA | Total 71 patients, 16 with diabetes. | Patients with compared with those without diabetes had more prevalence of hypertension (75% vs 36.4%), CKD (37.5 vs 5.5), obesity (56.2% vs 21.8%). Mean (SD) duration of diabetes 10 (2.8) y, mean (SD) HbA1c 60.3 (15.6) mmol/mol, use of ACE inhibitors, ARBs and NSAIDs was common (37.5%, 25% and 18.8% respectively). |
Abbreviations: ACEI, Angiotensin converting enzyme inhibitors; ARBs, Angiotensin receptor blockers; BMI, Body mass index; CI, Confidence interval; CRP, C‐reactive protein; CVD, Cardiovascular disease; HR, Hazard ratio; ICU, Intensive care unit; IQR, Inter quartile range; LOS, Length of stay; NSAIDs non‐steroidal anti‐inflammatory drugs; OR, Odds ratio; SD, Standard deviation.
5. OUTCOMES
Diabetes was a common comorbidity in COVID‐19 patients who were either admitted to ICU or died. 19 , 20 , 21 In a meta‐analysis that included 1382 patients hospitalised for COVID‐19, diabetes was identified as a risk factor for both ICU admission (OR 2.79, 95% CI 1.85‐4.22, P < .0001) and mortality (3.21, 1.82‐5.64, P < .0001). 22 In another meta‐analysis of 1936 COVID‐19 patients, diabetes significantly correlated with COVID‐19 severity (OR 2.67, 95% CI 1.91‐3.74, P < .01). 23 Prevalence of diabetes was also double in patients with COVID‐19 who required ICU admission compared with those who did not. 24 Diabetes was the second most common morbidity in patients who reached a composite outcome of mechanical ventilation or death (26.9%) only second to hypertension (35.8%). 25 An outcome study has shown that in patients with COVID‐19 who developed acute respiratory distress syndrome (ARDS), more patients had diabetes (19.0%) compared with those who did not (5.1%), respectively (difference, 13.9%, 95% CI 3.6%‐24.2%). 26 It has also been reported that diabetes was the most common morbidity (22% in one study and 60% in another) of patients who died in hospital. 27 , 28 Compared with patients with non‐severe COVID‐19, diabetes was more common in severe cases although it did not reach statistical significance (OR 2.07, 95% CI 0.89‐4.82). 29 In a US retrospective chart review of all patients enrolled in a follow‐up telemedicine outpatient clinic, the rate of hospitalisation for patients with diabetes was double (10.2%) the rate of hospitalisation for all other patients (5.1%), suggesting that the risk of severe COVID‐19 is increased in individuals with diabetes. 30 Retrospective Chinese report has shown that compared with non‐diabetes subjects, patients with diabetes were more likely to develop severe COVID‐19 disease (P = .028) with more complications including acute respiratory distress (38.1% vs 19.5%, P = .001), acute cardiac injury (14.5% vs 5.1%, P = .016) and non‐invasive and invasive mechanical ventilation (P = .037). 31 Diabetes as well as fasting blood glucose were independent risk factors for COVID‐19 mortality (HR 3.641, 95% CI 1.086‐12.214, P = .036 and 1.187, 1.078‐1.306, P < .001, respectively) after adjustment for age, cardiovascular disease, chronic kidney disease and laboratory markers. 31 The recently reported British database analysis of 17 425 445 NHS registered adults showed that 5683 deaths were attributed to COVID‐19, and uncontrolled diabetes (HbA1c ≥ 7.5%) was independent risk factor for mortality (HR 2.36, 95% CI 2.18‐2.56). 32 In a systematic review that included 6452 patients with diabetes and COVID‐19, diabetes was associated with increased risk of mortality (RR 2.12, 95% CI 1.44‐3.11, P < .001), severe COVID‐19 (2.45, 1.79‐3.35, P < .001), ARDS (4.64, 1.86‐11.58, P = .001) and disease progression (3.31, 1.08‐10.14, P = .04). Meta‐regression showed that the association with composite poor outcome was influenced by age (P = .003) and hypertension (P < .001). However, the presence of older age and hypertension attenuated the association of diabetes with the composite poor outcome. 33 In another systematic review that included case reports and case series studies has concluded that diabetes increases the risk of poor ARDS prognosis, severe symptoms of COVID‐19 and mortality. 34 Main studies reporting outcomes of patients with diabetes and COVID‐19 are summarised in Table 3.
TABLE 3.
Main studies reporting diabetes as adverse outcome risk for COVID‐19 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32
| Study | Population | Main findings |
|---|---|---|
|
Wang D, et al Retrospective case series China |
138 hospitalised patients with COVID‐19 |
A. 102 (73.9%) patients admitted to isolation wards, and 36 (26.1%) admitted to ICU because of organ dysfunction. B. Patients admitted to ICU were significantly older, median (IQR) age 66 (57‐78) y vs 51 (37‐62) y, P < .001), more likely to have hypertension (58.3%) vs (21.6%), diabetes (22.2%) vs (5.9%), CVD (25.0%) vs (10.8%) and cerebrovascular disease (16.7%) vs (1.0%) compared with those who were not in ICU. |
|
Chinese CDC, Cross sectional analysis. |
44 672 confirmed cases of COVID‐19 |
A. Total 1023 deaths occurred, crude fatality rate 2.3%. B. Crude fatality rate of patients with no comorbidities 0.9%. C. Fatality rate of patients with comorbidities 10.5% for CVD, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension and 5.6% for cancer. |
|
Zhou F, et al Retrospective, multicentre, China |
191 patients hospitalised with COVID‐19 |
A. 54 (28%) patients died in hospital. B. In univariable analysis, odds of in‐hospital death was higher in patients with diabetes (OR 2.85, 95% CI 1.35‐6.05) or coronary heart disease (21.1, 4.64‐98.76). |
|
Roncon L, et al, Data base analysis Italy |
1382 patients hospitalised for COVID‐19 |
A. Mean age 51.5 y, 58% men. B. Patients with diabetes had a significantly higher risk for ICU admission (OR 2.79, 95% CI 1.85‐4.22, P < .0001) and mortality (3.21, 1.82‐5.64, P < .000). |
|
Chen Y, et al Data base analysis China |
1936 patients with COVID‐19. | There were significant correlations between COVID‐19 severity and hypertension (OR 2.3, 95% CI 1.76‐3.00, P < .01), diabetes (67, 1.91‐3.74, P < .01), coronary heart disease (2.85, 1.68‐4.84, P < .01). |
|
Bo L, et al Data analysis China |
1527 patients with COVID‐19. |
A. Proportions of hypertension, CCVD and diabetes were 17.1%, 16.4% and 9.7%, respectively. B. Incidences of hypertension, CCVD and diabetes were twofold, threefold and twofold, respectively higher in ICU/severe cases compared with non‐ICU/severe counterparts. C. Diabetes accounted for 11.7% of ICU/severe cases and 4.0% of non‐ICU/severe cases, RR 2.21, 95% CI 0.88‐5.57, P = .09 |
|
Guan WJ, et al Retrospective China |
1099 patients with COVID‐19. |
A. 81 (7.4%) of patients had diabetes. B. Primary composite end point of admission to ICU, mechanical ventilation or death occurred in 26.9% of patients with diabetes, only second to hypertension (35.8%). |
|
WU C, et al Retrospective China |
201 patients with COVID‐19. |
A. 41.8% of patients developed ARDS. B. Patients with compared with those without ARDS had comorbidities such as hypertension (27.4% vs 13.7%, difference 13.7%, 95% CI 1.3% to 26.1%) and diabetes (19.0% vs 5.1%, difference 13.9%, 95% CI 3.6% to 24.2%). |
|
Yang X, et al Retrospective China |
52 critically ill patients with COVID‐19. |
A. 98% had chronic illness. B. 61.5% died at 28 d. C. Top comorbidities among non‐survivors were diabetes (22%) and CVD (22%). |
|
Yuan M, et al Retrospective China |
27 patients with COVID‐19. |
A. 10 patients died in hospital. B. Patients who died were significantly older (median age 68 vs 55 y, P = .003), had more comorbidities (80% vs 29%, P = .02), diabetes (60% vs 0%, P = .001), hypertension (50% vs 0%, P = .003) and CVD (30% vs 0%, P = .04). |
|
Yang J, et al Data analysis China |
1576 patients with COVID‐19. | Significant comorbidity differences between severe and non‐severe group were hypertension (OR 2.36, 95% CI 1.46‐3.83), respiratory disease (2.46, 1.76‐3.44), CVD (3.42, 1.88‐6.22) but diabetes was not statistically significant (2.07, 0.89‐4.82). |
|
Shabto JM, et al Retrospective, US |
65 DM and COVID‐19 patients | Telemedicine outpatient follow up showed the rate of hospitalisation for patients with diabetes double (10.2%) that of other patients (5.1%). |
|
Zhang Y, et al Retrospective China |
258 (63 with diabetes) hospitalised COVID‐19 patients. |
A. Median age 64 y (range 23‐91). B. Patients with diabetes were more likely to develop severe disease, more complications, mechanical ventilation and death (11.1% vs 4.1%). C. Diabetes (aHR 3.64, 95% CI 1.09‐12.21) and fasting blood glucose (1.19, 1.08‐1.31) were associated with the fatality. |
|
Williamson E, et al Data base analysis, UK |
17,425,445 NHS registered adults. |
A.5683 died of COVID‐19. B. Uncontrolled diabetes (HbA1c > +7.5%) increased risk of death (HR 2.36, 95% CI 2.18‐2.56). |
Abbreviations: aHR, Adjusted hazard ratio; ARDS, Acute respiratory distress syndrome; CCVD, Cardio‐cerebrovascular disease; CDC, Centre for disease control; CI, Confidence interval; CVD, Cardiovascular disease; ICU, Intensive care unit; IQR, Inter quartile range, NHS, National Health Service; OR, Odds ratio; RR, Relative risk.
6. DISCUSSION
People with diabetes are at risk for greater susceptibility to COVID‐19 infection and worse outcomes. This review aimed to explore the prevalence of diabetes in COVID‐19 affected patients, whether it increases the risk of such infection, characteristics of patients at risk and their outcomes. (Figure 1).
FIGURE 1.
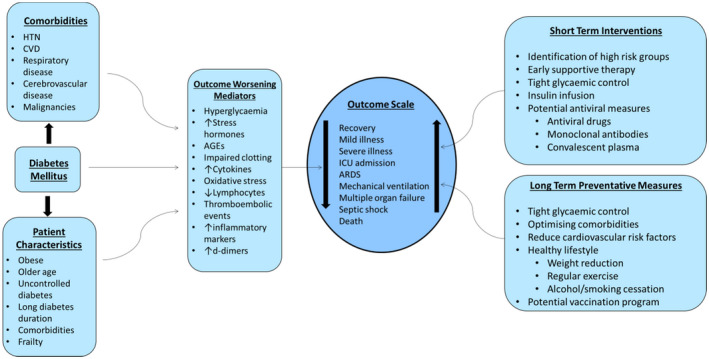
The effect of diabetes mellitus, patient characteristics, common comorbidities and the potential therapeutic interventions on the outcome of COVID‐19. CVD, cardiovascular disease; HTN, hypertension
6.1. Does diabetes increase the risk of COVID‐19?
Diabetes appears to be a common comorbidity in patients with COVID‐19 although a causal relationship is not clear. For example, a prevalence of diabetes among Chinese cohort, mean age 49.6 years, was reported to be 10.3% which is comparable to the prevalence of diabetes in the general population of China at 10.9% overall and 12.3% among people aged 40‐59 years. 4 , 35 Similarly, the prevalence of diabetes among British COVID‐19 patients, median age 73 years, of 21% was similar to the reported prevalence of 23.8% among those above the age of 75 years. 36 The US data showed slightly higher prevalence of 33.8% in COVID‐19 patients while the prevalence in the general population of a similar age is around 26.8%. 37 Therefore, the variable prevalence of diabetes reported in COVID‐19 studies may simply reflect the local demographics across different countries. Diabetes may increase the risk of infection because of immunologic defects, such as decreased neutrophilic migration, phagocytosis, intracellular killing and chemotaxis; however, the evidence remains controversial whether diabetes itself or the associated comorbidities is the main factor involved. 38
6.2. Who is at risk?
The most common characteristics of patients with diabetes who are affected by COVID‐19 in addition to older age and multiple morbidities are uncontrolled glycaemia and obesity. 15 , 16 , 17 Diabetes and uncontrolled glycaemia have been previously reported as significant predictors of severity and mortality in patients with viral pneumonias. 39 , 40 , 41 Infection with COVID‐19 in people with diabetes may increase stress that leads to increased secretion of hyperglycaemic hormones such as glucocorticoids and adrenaline thereby causing hyperglycaemia and fluctuating blood glucose levels. 42 The use of glucocorticoids in the treatment of severe COVID‐19 may exacerbate the hyperglycaemia and the worse outcome observed could be as a result of the disease severity, manifested by hyperglycaemia, rather than hyperglycaemia per se. However, hyperglycaemia prior to the use of steroids was a determinant factor for short‐term adverse outcomes. It has been shown that patients with admission glucose >11 mmol/L were more likely to develop ARDS (23.2% vs 9.2%), acute cardiac injury (12.5% vs 1.3%), to require ICU admission (21.4% vs 9.2%) and had a higher mortality (19.6% vs 5.3%) compared with those with admission glucose ≤11 mmol/L. 43 Another study has also demonstrated that patients with impaired fasting glycaemia or diabetes had more composite end‐point adverse events when compared with those with normal fasting glycaemia (18%, 31% and 5% respectively). 44 Poor glycaemic control before hospital admission has been shown to be associated with increased in‐hospital mortality in a British cohort of 5500 patients with COVID‐19. In a model analysis adjusted for sociodemographic factors and comorbidities has found that COVID‐19 mortality is higher in patients with diabetes and HbA1c > 86 mmol/mol (10%) compared with those with HbA1c < 48 mmol/mol (6.5%). 45 Other studies have shown hyperglycaemia to be associated with worst chest radiographic findings, higher risk of composite adverse outcomes or mortality. 46 , 47 , 48 These findings suggest that hyperglycaemia, independent of glucocorticoid use, is a detrimental factor in adverse outcomes.
Also, the occurrence of hypoglycaemia because of variability of blood glucose levels may stimulate pro‐inflammatory monocytes and increase platelet reactivity, contributing to a higher cardiovascular mortality in patients with diabetes. 49 , 50 A recent study has shown that insulin infusion to tighten blood glucose control in patients with hyperglycaemia and hospitalised with COVID‐19 has a protective effect. The study demonstrated that optimal glucose control in the immediate post‐admission period for almost 18 days was associated with a significant reduction of inflammatory cytokines and pro‐coagulant state which may reduce the risk of disease progression. 51 Globally, obesity has emerged as a significant risk factor for severe COVID‐19 infection. 52 , 53 , 54 In a small study of 49 outpatients with diabetes and COVID‐19, the reported median BMI was 33.9 with a range that reaches as high as 63.9 kg/m2. 30 Obesity is known to be associated with the severity and the longer duration of viral infections. 55 Central obesity, which is the predominant type in individuals with diabetes, is particularly associated with higher risk resulting from the increased secretion of cytokines and chronic low‐grade inflammation that may induce an impaired immune response. 56 Other factors may be related to the fact that obesity may reduce lung compliance, has maladaptive effects on ventilation and perfusion, mechanically impair ventilation with reduced aeration of the lung bases therefore leading to accumulation of secretions and increased risk of infection. 57 , 58 , 59
6.3. What causes worse outcomes?
The evidence for the increased adverse outcomes of COVID‐19 because of pre‐existing diabetes is consistent. For example, diabetes prevalence among COVID‐19‐deceased Italian cohort was 35.5% and only 20.3% in the general Italian citizens with the same age and gender distribution. 4 One explanation is the fact that diabetes usually coexists with other cardiovascular disease especially hypertension which has been shown to be associated with worse outcomes in COVID‐19. 5 , 6 , 7 , 8 Poorly controlled diabetes is also associated with increased oxidative stress and increased inflammatory cytokines such as tumour necrosis factor and interleukin‐6, as a result of increased insulin resistance, which may increase the risk of progression to severe disease. 38 In addition, endothelial dysfunction and enhanced platelet aggregation lead to the development of a hypercoagulable, pro‐thrombotic state that may contribute to poor control of the virus replication, prolonged pro‐inflammatory response and eventually poor outcome. 21 Therefore, increased coagulation activity, chronic inflammation, impaired immune response and possible direct pancreatic injury by the SARS‐CoV‐2 may be among the underlying mechanisms of the association between diabetes and COVID‐19 infection and adverse outcomes in individuals with diabetes. 60 Diabetes may also be associated with decreased forced expiratory volume and forced vital capacity compromising optimal pulmonary function. 61 Diabetes‐related complications may also be a factor. Microangiopathic changes that occur in the respiratory tract may impair gas exchange and lung compliance, increasing susceptibility to lower respiratory tract infections by atypical microorganisms and severe pneumonias. 38 Other mechanisms may be related to the fact that the COVID‐19 virus gains entry to pulmonary cells through binding to the membrane ACE2 receptor. The ACE inhibitors, ARBs and non‐steroidal anti‐inflammatory drugs that appear to increase ACE2 receptors expression, and therefore their use in people with diabetes may increase COVID‐19 infectivity and illness severity. 62 On the contrary to this, these medications may reduce the pulmonary and systemic inflammatory response by decreasing cytokines and therefore may be beneficial. 63 Therefore, it is still unclear what is the balance between the benefits and the risks might be in continuing or stopping RAAS inhibitors. So far, no conclusive evidence exists to support the discontinuation of these agents in people with diabetes and COVID‐19. 60 Other drugs used in the treatment of COVID‐19 may have a deleterious effect on blood glucose level regulation such as the hyperglycaemic effect of glucocorticoids and the hypoglycaemic effect of hydroxychloroquine although this has not been clearly reported in the literature.
6.4. Any role of frailty?
Frailty has not been formally assessed as a risk factor for COVID‐19 infection in people with diabetes. However, because old age and multiple comorbidities are being associated with worse outcome in a proportional fashion, it is likely that frailty is a confounding unmeasured factor. 64 Diabetes is known to increase the risk of frailty. 65 Frailty is a syndrome that is characterised by multisystem, including immune system dysregulation which leads to reduced physiologic reserve and increased risk of adverse health outcomes including increased susceptibility to severe infections. Frailty has been shown to be associated with poor post‐vaccination immune response, increased rates of influenza such as illness and laboratory‐confirmed influenza infection. 66 In a prospective cohort study in a tertiary hospital investigating older patients (aged ≥ 65 years) admitted with community acquired pneumonia, nursing home residency (a proxy for frailty) was an independent predictor of viral pneumonia (relative risk {RR} 3.06, P = .01), which highlights the role of frailty in institutionalised populations for the increased risk of viral illness. 67 In the British cohort study of patients with diabetes mellitus and COVID‐19, the relationship of BMI association with the risk of COVID‐19‐related mortality was U‐shaped. The risk was greatest for those with very high BMI and the nadir of risk being in those with a BMI 25‐29.9 kg/m2. The authors suggested that the higher risks in people with lower BMI could be linked to the effect of confounding by factors that are associated with weight loss which have either not been considered in their analysis. 15 Therefore, frailty may be an unmeasured factor for adverse outcome in people with diabetes and COVID‐19. Recently, in a multicentre European cohort study, frailty assessed by the clinical frailty score (CFS), proportionately predicted COVID‐19 mortality. Compared with CFS 1‐2, the adjusted hazard ratios for time from hospital admission to death were 1.55 (95% CI 1.00‐2.41) for CFS 3‐4, 1.83 (1.15‐2.91) for CFS 5‐6 and 2.39 (1.50‐3.81) for CFS 7‐9, and adjusted odds ratios for day‐7 mortality were 1.22 (95% CI 0.63‐2.38) for CFS 3‐4, 1.62 (0.81‐3.26) for CFS 5‐6, and 3.12 (1.56‐6.24) for CFS 7‐9. 68
7. FUTURE PERSPECTIVES
The appropriate management of patients with diabetes and COVID‐19 is not yet clear. Patients with diabetes should be declared as risk group for severe disease and worse outcomes. Future research may help develop a scoring system to identify vulnerable patients who may benefit from aggressive treatment or intensive support from outset to limit deterioration. Although a specific anti‐viral drug is urgently required, a multi‐target agent is also required to help regulate the dysregulated neuro–endocrine–immune system that is common in metabolic disease, including diabetes, cardiovascular disease, atherosclerosis, insulin resistance, hypertension, dyslipidaemia and obesity. The role of hypoglycaemic medications such as dipeptidyl peptidase‐4 (DPP‐4) inhibitors, which may act as a receptor for COVID‐19 and the glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), that may reduce inflammation, will need further investigation. 69 , 70 Similarly, the role of RAAS inhibitors with COVID‐19 infection need exploration. It is not yet clear why insulin users had worse prognosis compared with other hypoglycaemic medications. 14 Whilst insulin users tend to have advanced disease with long duration of diabetes, prevalent complications and co‐existing comorbidities to explain their worse outcome, further clarification is required. The role of hyperglycaemia, hyperinsulinaemia and hypoglycaemic agents in the pathogenesis of COVID‐19 and how diabetes affects the efficacy of future vaccines and antiviral agents currently in trials are warranted. COVID‐19‐related mortality was reported to be higher in the Black and Asian ethnic groups with diabetes compared with the White population. 15 Therefore, future research is needed to provide a better understanding of the potential differences in genetic predispositions across populations and the underlying pathophysiological mechanisms. Finally, novel ways to deliver care to patients with diabetes using telehealth, remote patient monitoring and wearable technologies are required.
8. CONCLUSION
Diabetes appears to be a common morbidity in patients affected by COVID‐19; however, the evidence that it increases the risk of infection is not yet clear. On the other hand, the evidence that diabetes increases the risk of severe disease and risk of adverse outcomes is substantial. Patients with diabetes who are at risk of COVID‐19 tend to be obese, of older age, have uncontrolled diabetes and comorbidities, in particular cardiovascular disease and hypertension. Further research is still required to investigate appropriate management of people with diabetes and COVID‐19 and develop novel ways of management including telecare.
CONFLICTS OF INTEREST
The authors have declared no conflicts of interest.
Abdelhafiz AH, Emmerton D, Sinclair AJ. Diabetes in COVID‐19 pandemic‐prevalence, patient characteristics and adverse outcomes. Int J Clin Pract. 2021;75:e14112. 10.1111/ijcp.14112
REFERENCES
- 1. Hui DS, Azhar EI, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health ‐ The latest, , et al. novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2019;2020:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zoppini G, Fedeli U, Schievano E, et al. Mortality from infectious diseases in diabetes. Nutr Metab Cardiovasc Dis. 2018;28:444‐450. [DOI] [PubMed] [Google Scholar]
- 3. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest. 2020;43:867‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109:531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang J, Zhen Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid‐19 in a long‐term care facility in King County, Washington. NEJM. 2020;382:2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323:1775‐1776. [DOI] [PubMed] [Google Scholar]
- 11. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang F, Yang Y, Dong K, et al. Clinical characteristics of 28 patients with diabetes and COVID‐19 in Wuhan, China. Endocr Pract. 2020;26:668‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid‐19 with diabetes. BMJ Open Diab Res Care. 2020;8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43:1399‐1407. [DOI] [PubMed] [Google Scholar]
- 15. Holman N, Knighton P, Kar P, et al. Type 1 and Type 2 diabetes and COVID‐19 related mortality in England: a cohort study in people with diabetes. WWW.england.nhs.uk/publication/type‐1‐and‐type‐1diabetes‐and‐covid‐19‐related‐mortality‐in‐england. Accessed October 20, 2020.
- 16. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia .2020;63:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conway J, Gould A, Westley R, et al. Characteristics of patients with diabetes hospitalised for COVID‐19 infection‐a brief case series report. Diabetes Res Clin Pract. 2020;169:108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China . JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Epidemiological analysis of new coronavirus pneumonia . Epidemiology group of new coronavirus pneumonia emergency response mechanism of Chinese centre for disease control and prevention. Chin J Epidemiol. 2020;41:145‐151. [Google Scholar]
- 21. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roncon L, Zuin M, Rigatelli G, et al. Diabetic patients with COVID‐19 infection are at higher risk of ICU admission and poor short‐term outcome. J Clin Virol. 2020;127:104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Gong X, Wang L, et al. Effects of hypertension, diabetes and coronary heart disease on COVID‐19 diseases severity: a systematic review and meta‐analysis.
- 24. Bo L, Yang J, Zaho F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109:531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu C, Chen X, Cai Y, et al. Pneumonia in Wuhan, China. JAMA Internal Med. 2020;180:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan M, Yin W, Tao Z, et al. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE. 2020;15:e0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shabto JM, Loerinc L, O’Keefe GA, et al. Characteristics and outcomes of COVID‐19 positive patients with diabetes managed as outpatients. Diabetes Res Clin Pract. 2020;22:108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Cui Y, Shen M, et al. Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID‐19: a retrospective cohort study. Medrxiv. 2020. 10.1101/2020.03.24.20042358. [DOI] [Google Scholar]
- 32. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14:395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdi A, Jalilian M, Sarbarzeh PA, Vlaisavljevic Z. Diabetes and COVID‐19: a systematic review on the current evidences. Diabetes Res Clin Pract. 2020;166:108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. 8 million people in England now have diabetes‐Gov.uk. Accessed May 28, 2020.
- 37. Statistics about diabetes‐American diabetes association. Accessed May 29, 2020.
- 38. Knapp S. Diabetes and infection: is there a link? ‐ a mini‐review. Gerontology. 2013;59:99‐104. [DOI] [PubMed] [Google Scholar]
- 39. Schoen K, Horvat N, Guerreiro NFC, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623‐628. [DOI] [PubMed] [Google Scholar]
- 41. Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS‐CoV: analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31:81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID‐19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short‐term outcomes in 132 COVID‐19 patients with diabetes in Wuhan China: a retrospective study. Diabetes Res Clin Pract. 2020;166:108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang J, Kong W, Xia P, et al. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol (Lausanne). 2020;11:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iacobellis G, Penaherrera CA, Bermudez LE, Bernal Mizrachi E. Admission hyperglycemia and radiological findings of SARS‐COV‐2 in patients with and without diabetes. Diabetes Res Clin Pract. 2020;164:108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Li H, Zhang J, et al. The clinical characteristics and outcomes of patients with diabetes mellitus and secondary hyperglycaemia with coronavirus disease 2019: a single‐center, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22:1443‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID‐19. Diabetes Obes Metab. 2020;22:1897‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou J, Tan J. Diabetes patients with COVID‐19 need better care. Metabolism. 2020;107:154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iqbal A, Prince LR, Novodvorsky P, et al. Effect of hypoglycemia on inflammatory responses and the response to low‐dose endotoxemia in humans. J Clin Endocrinol Metab. 2019;104:1187‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by Covid‐19: can we do more on glycemic control? Diabetes Care. 2020;43:1408‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3:e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020;71:896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Honce R, Schultz‐Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37:333‐340. [DOI] [PubMed] [Google Scholar]
- 57. Petrakis D, Margina D, Tsarouhas K, et al. Obesity a risk factor for increased COVID19 prevalence, severity and lethality (Review). Mol Med Rep. 2020;22:9‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL‐6 and CRP predict the need for mechanical ventilElevated levels of IL‐6 and CRP predict the need for mechanical ventilation in COVID‐19ation in COVID‐19. J Allergy Clin Immunol. 2020;146:128‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hussain A, Bhowmik B, Moreira NC. COVID‐19 and diabetes: KNOWLEDGE in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klein OL, Aviles‐Santa L, Cai J, et al. Hispanics/Latinos with type 2 diabetes have functional and symptomatic pulmonary impairment Mirroring kidney microangiopathy: findings from the Hispanic community health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2016;39:2051‐2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Esler M, Esler D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J Hypertension. 2020;38:781‐782. [DOI] [PubMed] [Google Scholar]
- 63. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. 2020;81:537‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sinclair AJ, Abdelhafiz AH. Age, frailty and diabetes – triple jeopardy for vulnerability to COVID‐19 infection. E Clin Med. 2020;22:100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sinclair AJ, Abdelhafiz AH, Rodríguez‐Mañas L. Frailty and sarcopenia ‐ newly emerging and high impact complications of diabetes. J Diabetes Complication. 2017;31:1465‐1473. [DOI] [PubMed] [Google Scholar]
- 66. Yao X, Hamilton RG, Weng NP, et al. Frailty is associated with impairment of vaccine‐induced antibody response and increase in post‐vaccination influenza infection in community‐dwelling older adults. Vaccine. 2011;29:5015‐5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma HM, Lee KP, Woo J. Predictors of viral pneumonia: the need for viral testing in all patients hospitalized for nursing home‐acquired pneumonia. Geriatr Gerontol Int. 2013;13:949‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hewitt J, Carter B, Vilches‐Moraga A, et al. The effect of frailty on survival in patients with COVID‐19 (COPE): a multicentre, European, observational cohort study. Lancet. 2020;5:e444‐e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS‐CoV infection. JCI Insight. 2019;4:131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41:457‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]


