Abstract
The objective of the present study was to evaluate the circulating serum amino acid levels in children with attention deficit/hyperactivity disorder (ADHD). A total of 71 children with untreated ADHD and 31 neurotypical controls aged 7-14 years old were examined. Serum amino acid levels were evaluated using high-performance liquid chromatography (HPLC) with UV-detection. Laboratory quality control was performed with reference materials of human plasma amino acid levels. The obtained data demonstrated that children with ADHD were characterized by 29, 10 and 20% lower serum histidine (His), glutamine (Gln) and proline (Pro) levels compared with neurotypical children, respectively. In contrast, circulating aspartate (Asp), glutamate (Glu) and hydroxyproline (Hypro) levels exceeded the respective control values by 7, 7 and 42%. Correspondingly, the Gln-to-Glu and Pro-to-Hypro ratios were 28% and 49%, respectively, lower in ADHD cases compared with the controls. Total Gln/Glu levels were also significantly lower in ADHD patients. No significant group differences were observed between the groups in the other amino acids analyzed, including phenylalanine. Multiple linear regression analysis revealed significant associations between circulating serum Gln, lysine (Lys) (both negative) and Glu (positive) levels with total ADHD Rating Scale-IV scores. The observed alterations in Pro/Hypro and Gln/Glu levels and ratios are likely associated with the coexisting connective tissue pathology and alterations in glutamatergic neurotransmission in ADHD, respectively. Altered circulating levels of His, Lys and Asp may also be implicated in ADHD pathogenesis. However, further in vivo and in vitro studies are required in order to investigate the detailed mechanisms linking amino acid metabolism with ADHD pathogenesis.
Keywords: attention deficit/hyperactivity disorder, hydroxyproline, glutamine, lysine, neurodevelopmental disorder
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention and/or hyperactive-impulsivity that interferes with brain functioning or development. While contradictory, the existing data demonstrate that the prevalence of ADHD may be as high as 5.3% and 2.5% in children/adolescents and adults, respectively (1). The effects of ADHD have a significant impact on the social life of patients throughout the entirety of their lives, starting from disruptive behavior, resulting in poor performance in standardized tests and impacted social interactions, which may lead to criminal behavior, substance abuse, lack of motivation, school exclusion and subsequent effects on professional development in adulthood (2). In addition, ADHD has also been found to be associated with lower a health-related quality of life (3). Taken together, these impairments result in high ADHD-associated economic costs (4).
ADHD is characterized by complex alterations in the neurobiology and neurochemistry, with impaired dopamine and norepinephrine signaling being the most prominent (5). Previously, it was also demonstrated that altered glutamatergic neurotransmission is involved in ADHD pathogenesis (6). Therefore, unraveling the potential underlying mechanisms implicated in ADHD pathogenesis is essential for improving our understanding of the disorder and further development of management strategies (5).
Amino acids serve a significant role in brain development and functioning (7). In particular, certain amino acids or their precursors, including glutamine, glutamate and γ aminobutyric acid, are well-established to be involved in neuronal signaling as neurotransmitters (8). Correspondingly, disruption of amino acid metabolism results in significant neurological disorders, particularly in early ontogenesis (9).
Given the role of an altered neurochemistry in ADHD pathogenesis as well as the role of amino acids in neurodevelopment, it is posited that dysregulated amino acid metabolism may significantly interfere with ADHD. However, the existing data is rather contradictory. An earlier study by Bornstein et al (10) found significantly lower plasma levels of phenylalanine (Phe), tyrosine, tryptophan (Trp), His and isoleucine in patients with ADD compared with the healthy controls. Zavala et al (11) showed there was a significant decrease in plasma Phe and glutamine (Gln) levels, whereas plasma glycine (Gly) levels were found to be elevated in patients with ADHD. Improvement in ADHD symptoms was positively associated with tyrosine, Phe and Trp levels (12). At the same time, no significant alterations in blood and urinary levels of Trp, tyrosine and Phe levels were observed in children with ADHD (13). In view of these inconsistencies, as well as the use of amino acid supplementation in ADHD management, precise analyses of amino acid profiles in ADHD is required and may assist in reconciling these contrasting results. Thus, the objective of the present study was to evaluate the levels of circulating serum amino acid levels in children with ADHD.
Materials and methods
The present study was performed in accordance with the ethical principles of the Declaration of Helsinki and its amendments (14). The protocol of the present study was considered and approved by the Local Ethics Committee of Yaroslavl State University (Yaroslavl, Russia). Parents or legal representatives signed an informed consent forms for participation of their children in the present study prior to investigation. Examination and blood sample collection was performed only in the presence of the parents/guardians.
A total of 71 children (54 boys, 17 girls) diagnosed with ADHD aged 7-14 years old (8.4±2.6 years old) were enrolled in the present study. The diagnosis of ADHD (ICD-10: F90.0) and other neuropsychiatric disorders (exclusion criteria) were based on the clinical records of the outpatient department. ADHD was defined according to ICD-10 criteria, including inattention, hyperactivity and impulsivity (≥3 symptoms of each) (15). Only patients that did not take any specific treatments for ADHD were included in the study, in order to avoid the confounding effects of any potential side effects of pharmacological treatment on amino acid metabolism.
In addition, 31 age (8.0±2.9 years old; age range 7-14 years old) and sex-matched neurotypical children (24 boys, 7 girls) were also examined, and used as the control group. The absence of neuropsychiatric disorders was confirmed during annual medical examinations. No significant group differences in age (P=0.183) or sex (P=0.885) were observed between the groups.
Children and their parents were invited to participate in the study during the annual medical examinations. A total of 35% of all contacted subjects refused to take part in the study (36 out of 102 children and parents).
The parents of all the examined children completed the ADHD Rating Scale-IV for additional verification of the ADHD diagnosis (16). Total ADHD Rating Scale-IV scores in the ADHD patients exceeded those in neurotypical children by a factor of >2 (14.9±9.4 vs. 7.0±5.4, P<0.001).
Whole blood samples were collected in the morning and after overnight fasting by cubital vein venipuncture using 9-ml Vacuette® tubes (Greiner Bio-One International AG). The samples were subsequently centrifuged for 10 min at 1,600 x g at room temperature to obtain blood serum that was stored in Eppendorf tubes at -18˚C until required for analysis.
Evaluation of serum levels of alanine, arginine (Arg), asparagine, Asp, citrulline, glutamine (Gln), glutamate (Glu), Gly, histidine (His), hydroxyproline (Hypro), isoleucine, leucine, lysine (Lys), methionine, ornithine, Phe, proline (Pro), serine, taurine, threonine, Trp, and Val was performed by high-performance liquid chromatography (HPLC) with UV-detection at PerkinElmer S200 (PerkinElmer, Inc.) using a reverse phase Pico Tag Column for Free Amino Acid Analysis (3.9x300 mm) C18 (EMD Millipore).
Precolumn derivatization with phenylisothiocyanate reagent containing 7:1:1:1 (v/v) methanol:triethylamine:water:phenylisothicyanate was performed prior to the analysis. Analysis was performed with aqueous sodium acetate and acetonitrile gradient mode with UV-detection at 240 nm. The commercially available ClinCal® Plasma Calibrator, lyophil., for Amino Acids (lot. no. 10213; ClinCheck) calibrators were used for HPLC-system calibration.
Laboratory quality control was routinely performed with reference materials of human plasma amino acid levels using ClinChek® Plasma Control, lyophil., for Amino Acids, Levels I (cat. no. 10280) and II (cat. no. 10281). The obtained values for all amino acids fitted the certified control range specified by the manufacturer (ClinCheck). The recovery rates for the studied amino acids varied from 94-109%.
Serum amino acid concentrations are expressed as µmol/l. In addition, total glutamatergic metabolite concentration (Glx), defined as a sum of Glu and Gln levels, was calculated as described previously (17). The obtained values of serum Gln, Glu, Hypro and Pro were used for calculating the Gln/Glu and Pro/Hypro ratios.
Statistical analysis was performed using Statistica version 10.1 (Statsoft, Inc.). Evaluation of data distribution performed using a Shapiro-Wilk test revealed skewed distribution of data on amino acid levels in the study groups. Therefore, the median and the respective interquartile range (IQR) boundaries were used as descriptive statistics. Raw data were log-transformed for subsequent processing. Group comparisons were performed using analysis of covariance (ANCOVA) with adjustment for age and sex as covariates and subsequent Bonferroni adjustments. Multiple linear regression was performed in order to evaluate the relative association between serum amino acid levels (independent predictors) and ADHD (dependent variable) after adjustment for age and sex. Correlation analysis was performed using a Spearman's rank correlation coefficient. P<0.05 was considered to indicate a statistically significant difference, whereas 0.05<P<0.1 was considered nearly significant.
Results
The obtained data demonstrate that ADHD is associated with altered amino acid profiles in children. Evaluation of serum essential amino acids revealed 29% lower His levels in ADHD patients compared with the neurotypical controls (Table I). No significant group differences in other essential amino acids levels were observed.
Table I.
Essential amino acid levels in the serum of ADHD cases and neurotypical controls.
| Amino acid | Control, µMa | ADHD, µMb | F-value | P-value |
|---|---|---|---|---|
| Histidine | 85.0 (50-120.6) | 60.7 (45.2-94.6) | 3.140 | 0.081 |
| Isoleucine | 56.1 (47.6-63.9) | 54.1 (43.5-70.8) | 0.029 | 0.923 |
| Leucine | 118.6 (103.6-133.7) | 117.5 (96.2-138.5) | 0.105 | 0.862 |
| Lysine | 170.6 (147.5-198.4) | 161.5 (120.8-212.1) | 1.242 | 0.360 |
| Methionine | 54.2 (46.2-69.1) | 51.6 (44.1-60.2) | 0.416 | 0.396 |
| Phenylalanine | 55.8 (47.5-68.2) | 57.8 (48.4-66.4) | 0.009 | 0.923 |
| Threonine | 108.9 (90.5-129) | 111.4 (86.8-143.1) | 0.768 | 0.605 |
| Tryptophan | 56.5 (43.3-70.6) | 58.1 (44.6-71.5) | 0.011 | 0.794 |
| Valine | 167.2 (136.9-212.3) | 167.2 (128-223.4) | 0.060 | 0.907 |
aData are presented as the median (inter-quartile range).
bADHD, attention deficit/hyperactivity disorder.
Greater differences were observed in the levels of conditionally essential and non-essential amino acids (Table II). Particularly, serum Asp and Glu levels were 7% higher compared with the healthy controls, although the difference was significant only in the case of Glu levels. In contrast, serum Gln and Pro concentrations were 10% and 20% lower in ADHD cases compared with the neurotypical controls, respectively. In corroboration with the overall decrease in serum Gln levels, total Glx concentration (Glu+Gln) was also 12% lower in children with ADHD [425.5 (351.5-533.6) µM] compared with the control values [483.1 (406.0-577.4) µM] (F=4.007; P=0.048).
Table II.
Conditionally essential and non-essential amino acid levels the serum of children with ADHD and healthy controls.
| Amino acid | Control, µMb | ADHD, µMb | F-value | P-value |
|---|---|---|---|---|
| Alanine | 323.5 (248.3-395.5) | 342.7 (298.1-401.9) | 0.779 | 0.342 |
| Arginine | 68.9 (55.7-85.8) | 66.5 (55.9-81.6) | 0.008 | 0.988 |
| Asparagine | 77.8 (67.7-85.0) | 78.5 (64.8-91.6) | 0.032 | 0.872 |
| Aspartate | 13.9 (10.5-17.9) | 14.9 (11.1-20.3) | 2.335 | 0.103 |
| Glutamine | 433.4 (379.5-534.4) | 389 (312.8-474.7) | 5.935 | 0.024a |
| Glutamate | 39.3 (27.2-46.5) | 42.0 (34.2-53.5) | 4.130 | 0.039a |
| Glycine | 418.4 (374.7-455.2) | 396.7 (340.5-469.7) | 0.181 | 0.724 |
| Proline | 316.3 (219.0-418.1) | 254.6 (207.2-319.8) | 3.690 | 0.045a |
| Serine | 83.2 (68.7-92.8) | 82.5 (66.1-97.0) | 0.034 | 0.934 |
| Taurine | 64.5 (50.6-82.1) | 66.4 (55.6-82.7) | 0.709 | 0.416 |
| Tyrosine | 82.9 (72.6-98.3) | 85.5 (75.7-101.0) | 0.090 | 0.718 |
| Citrulline | 54.1 (42.1-70.1) | 50.9 (42.4-64.9) | 0.548 | 0.541 |
| Ornithine | 58.2 (46.5-71.1) | 60.4 (45.8-83.0) | 0.068 | 0.882 |
| Phosphoserine | 54.7 (26.9-89.8) | 50.3 (31.5-66.1) | 0.502 | 0.435 |
| Hydroxyproline | 11.4 (1.6-18.7) | 16.2 (11.3-20.8) | 4.752 | 0.018a |
aP<0.05;
bData are presented as the median (inter-quartile range). ADHD, attention deficit/hyperactivity disorder.
Amongst the amino acid derivatives analyzed, only serum Hypro concentrations were significantly elevated, being 42% higher in ADHD cases compared with the healthy controls.
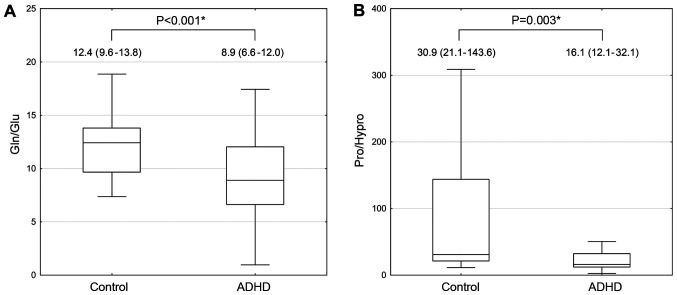
Given the observed group differences between serum Gln and Glu levels, as well as the levels of Pro and its derivative Hypro, Gln-to-Glu and Pro-to-Hypro ratios were evaluated (Fig. 1). The obtained data demonstrate that Gln/Glu values in ADHD cases were 28% lower compared with the healthy children (F=14.202). In turn, ADHD patients had almost 2-fold lower levels of Pro/Hypro levels compared with the neurotypical controls (F=8.936).
Figure 1.
Serum Gln/Glu and Pro/Hypro ratios in children with ADHD compared with the healthy controls. (A) Serum Gln/Glu ratio. (B) Serum Pro/Hypro ratio. Data are expressed as the median (line), inter-quartile ranges (box) and non-outlier ranges (whiskers). *P<0.05. Gln, glutamine; Glu, glutamine; Pro, proline; Hypro; hydroxyproline.
Correlation analysis demonstrated that serum Hypro and Glu concentrations were correlated significantly (r=0.270; P=0.006) and nearly significantly (r=0.194; P=0.051) with total ADHD-RS scores, respectively. Concomitantly, circulating Gln levels (r=-0.207; P=0.037) as well as Gln/Glu ratio (r=-0.376; P<0.001) were characterized by a significant direct correlation with the latter.
Multiple regression analysis was also performed in order to determine if there was an independent association between the serum amino acid levels and total ADHD-RS scores (Table III). In a crude model incorporating all amino acids analyzed, serum Gln and Lys levels were found to be inversely associated with total ADHD-RS scores. The overall model trended towards statistically significant (P=0.072), accounting for only 12% of total ADHD-RS scores. In a model incorporating amino acids considered to be significantly and almost significantly associated with ADHD scores (Model 2), serum Gln and Lys remained significantly associated with ADHD, whereas circulating Glu levels appeared to be positively associated with total ADHD-RS scores. Serum Hypro levels were almost significantly associated with ADHD scores. Although the predictive value of Model 2 was significant, it accounted for only 17% of score variability. Neither age nor sex were associated significantly with ADHD in both regression models.
Table III.
Multivariate linear regression analysis of the association between serum amino acid levels (independent predictor) and attention deficit/hyperactivity disorder Rating Scale-IV scores (dependent variable).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Amino acid | β | P-value | β | P-value |
| Ala | 0.140 | 0.460 | 0.050 | 0.724 |
| Asp | -0.009 | 0.965 | -0.077 | 0.596 |
| Gln | -0.353 | 0.022a | -0.370 | 0.002b |
| Glu | 0.258 | 0.206 | 0.350 | 0.029a |
| His | -0.150 | 0.218 | -0.057 | 0.583 |
| Hypro | 0.227 | 0.111 | 0.211 | 0.065 |
| Lys | -0.527 | 0.027a | -0.339 | 0.021a |
| Orn | 0.281 | 0.087 | 0.161 | 0.248 |
| Pro | -0.051 | 0.643 | -0.082 | 0.391 |
| Thr | 0.327 | 0.081 | 0.169 | 0.214 |
| Multiple R | 0.576 | 0.518 | ||
| Multiple R2 | 0.332 | 0.268 | ||
| Adjusted R2 | 0.100 | 0.170 | ||
| P for the model | 0.116 | 0.004b | ||
aP<0.05,
bP<0.01. Data are expressed as the regression coefficient (β) and the respective P-value.
Discussion
The results of the present study demonstrated that children with ADHD were characterized by distinct amino acid profiles compared with the controls, indicative of predominant alteration in Glu, Pro and Lys metabolism. Significant group differences in Glu/Gln ratio may be indicative of altered neurotransmission in children with ADHD, whereas high Hypro levels and a high Hypro/Pro ratio may be considered as a marker of collagen catabolism and connective tissue pathology.
Existing data demonstrate that alterations in glutamatergic signaling may contribute significantly to ADHD pathogenesis (6). Specifically, it has been demonstrated that ADHD patients are characterized by significantly lower Gln plus Glu levels in basal ganglia (18), including in the striatum (19). An increase in anterior cingulate cortex Glu content was also associated with hyperactivity and impulsivity in adult ADHD patients (20). Pertinent genome-wide analyses for ADHD risk genes revealed altered expression profiles of genes associated with glutamatergic neurotransmission (21). Correspondingly, altered AMPAR-mediated transmission in pyramidal neurons of the prefrontal cortex was also found to be associated with ADHD in an experimental rat model (22).
In view of the role of Hypro as a marker of connective tissue pathology (23), the earlier proposed association between ADHD and joint hypermobility (24) may underlie the observed increase in plasma Hypro levels in children with ADHD. Particularly, joint hypermobility was found to be >2-fold more prevalent in ADHD subjects compared with the reference population (25). Moreover, connective tissue disorders are also closely associated with other neurodevelopmental disorders (26). These data also corroborate our earlier findings on increased Hypro levels in autism spectrum disorder (27) and cerebral palsy (28). In addition, in ADHD subjects, Pro levels were found to be reduced with a decreased Pro/Hypro ratio, and levels were found to be inversely associated with S100B and positively related to IL-10 levels (29), indicative of the potential contribution of dysregulated Pro metabolism in this neurodevelopmental disorder.
The results of serum amino acid profiling also demonstrated group differences in serum His, Asp and Lys levels between ADHD patients and neurotypical controls. Although an essential role of His in brain physiology has been demonstrated (30), data on the relationship between ADHD and His metabolism are insufficient. His supplementation was shown to reduce fatigue and improve mental performance in subjects with high fatigue and sleep disruption scores (31). In turn, experimental data demonstrated that a His-deficient diet resulted in formation of anxiety-like behaviors in mice through reduction of brain histamine levels (32). In turn, increased serum Asp levels in patients with ADHD generally correspond with an earlier observed higher Asp intake in ADHD patients (33).
It is noteworthy that the present study revealed a significant association between lower serum Lys levels and ADHD. Despite the role of Lys metabolism in brain physiology (34), earlier data on the involvement of Lys in ADHD pathophysiology are lacking. Nonetheless, Lys supplementation has been used in ADHD management (35), based on observations that Lys as well as Arg treatments reduce anxiety in stressed adults characterized by high cortisol levels (36).
In contrast to earlier reports (11,12), significant group differences in tyrosine, phenylalanine or Trp levels were not observed in the present study, in agreement with a study by Bergwerff et al (13) who did not reveal any significant group differences in serum and urinary levels of the studied amino acids (13).
The present study also has several limitations. First, this was a cross-sectional study involving a relatively small number of patients, and studying larger cohorts of both cases and controls may improve the statistical power of the results. Second, follow-up design of the study with evaluation of ADHD incidence and severity would be beneficial to provide an insight into the causal relationships between ADHD and the observed alterations in amino acid metabolism. Third, although the results demonstrate the potential alterations of amino acid-derived neurotransmitters in patients with ADHD, serum levels do not necessarily reflect brain levels of amino acids and their derivatives. Therefore, evaluation of cerebrospinal fluid amino acid levels or the use of techniques for direct brain metabolite assessment, such as proton magnetic resonance spectroscopy, would assist in highlighting ADHD-associated alterations in metabolism of brain amino acids and neurotransmitters.
In conclusion, the results of the present study demonstrated significant alterations in the serum amino acid profile of children with ADHD. The observed alterations of Pro/Hypro and Gln/Glu levels and their ratios may be associated with the coexisting connective tissue pathology and alterations of glutamatergic neurotransmission in ADHD, respectively. Altered circulating levels of His, Lys and Asp may also be implicated in the pathogenesis of ADHD. However, further in vitro and in vivo studies are required to investigate the specific underlying mechanisms linking amino acid metabolism with ADHD pathogenesis.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by funding from Russian Foundation for Basic Research (RFBR) (grant no. 19-013-00528).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
AVS, MGS and AAT conceived the study. AVS, AAT, AT, DAS and MA designed the study. AVS, AAT, AAS, ALM, IPZ, YNL and MGS performed the experiments. ALM, IPZ, AVS, AT and DAS analyzed the data. ALM, AAT, AAS, IPZ, YNL, AVS, MGS, AT, DAS and MA wrote and edited the manuscript. All authors have read and approved the final manuscript. AVS, MGS and AAT confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was performed in accordance with the ethical principles of the Declaration of Helsinki and its amendments (2013). The protocol of the present study was considered and approved by the Local Ethics Committee (Yaroslavl State University, Yaroslavl, Russia). Parents or legal representatives signed an informed consent form for participation of their children in the present study prior to investigation. Examination and blood sample collection was performed only in the presence of the parents/guardians.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395:450–462. doi: 10.1016/S0140-6736(19)33004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harpin VA. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child. 2005;90 (Suppl 1):2–7. doi: 10.1136/adc.2004.059006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peasgood T, Bhardwaj A, Biggs K, Brazier JE, Coghill D, Cooper CL, Daley D, De Silva C, Harpin V, Hodgkins P, et al. The impact of ADHD on the health and well-being of ADHD children and their siblings. Eur Child Adolesc Psychiatry. 2016;25:1217–1231. doi: 10.1007/s00787-016-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkley RA. The high economic costs associated with ADHD. ADHD Rep. 2020;28:10–12. [Google Scholar]
- 5.Mehta TR, Monegro A, Nene Y, Fayyaz M, Bollu PC. Neurobiology of ADHD: A review. Curr Dev Disord Rep. 2019;6:235–240. [Google Scholar]
- 6.Levy F, de Leon J. Dopamine ADHD/OCD theories: Is glutamine part of the story? Neurotransmitter (Houst) 2015;2(e891) [Google Scholar]
- 7.Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1:31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenzuela CF, Puglia MP, Zucca S. Focus on: Neurotransmitter systems. Alcohol Res Health. 2011;34:106–120. [PMC free article] [PubMed] [Google Scholar]
- 9.Saudubray JM, Garcia-Cazorla A. An overview of inborn errors of metabolism affecting the brain: From neurodevelopment to neurodegenerative disorders. Dialogues Clin Neurosci. 2018;20:301–325. doi: 10.31887/DCNS.2018.20.4/jmsaudubray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornstein RA, Baker GB, Carroll A, King G, Wong JT, Douglass AB. Plasma amino acids in attention deficit disorder. Psychiatry Res. 1990;33:301–306. doi: 10.1016/0165-1781(90)90046-8. [DOI] [PubMed] [Google Scholar]
- 11.Zavala M, Castejón HV, Ortega PA, Castejón OJ, Marcano de Hidalgo A, Montiel N. Imbalance of plasma amino acids in patients with autism and subjects with attention deficit/hyperactivity disorder. Rev Neurol. 2001;33:401–408. [PubMed] [Google Scholar]
- 12.Stern M, Perez L, Johnstone J, Gracious B, Leung B, Tost G, Arnold E, Hatsu I, Kopec R. Using Metabolomics to classify the underlying effects of multi-nutrient supplementation in ADHD youth. Curr Dev Nutr. 2020;4 (Suppl 2)(1351) [Google Scholar]
- 13.Bergwerff CE, Luman M, Blom HJ, Oosterlaan J. No tryptophan, tyrosine and phenylalanine abnormalities in children with attention-deficit/hyperactivity disorder. PLoS One. 2016;11(e0151100) doi: 10.1371/journal.pone.0151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–18. General Assembly of the World Medical Association. [PubMed] [Google Scholar]
- 15. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10): Chapter V Mental and behavioural disorders (F00-F99). Behavioural and emotional disorders with onset usually occurring in childhood and adolescence (F90-F98). https://icd.who.int/browse10/2019/en#/F90-F98. Accessed December 8, 2020. [Google Scholar]
- 16.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. Guilford Press, 1998. [Google Scholar]
- 17.Goff DC, Hennen J, Lyoo IK, Tsai G, Wald LL, Evins AE, Yurgelun-Todd DA, Renshaw PF. Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biol Psychiatry. 2002;51:493–497. doi: 10.1016/s0006-3223(01)01321-x. [DOI] [PubMed] [Google Scholar]
- 18.Maltezos S, Horder J, Coghlan S, Skirrow C, O'Gorman R, Lavender TJ, Mendez MA, Mehta M, Daly E, Xenitidis K, et al. Glutamate/glutamine and neuronal integrity in adults with ADHD: A proton MRS study. Transl Psychiatry. 2014;4(e373) doi: 10.1038/tp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrey NJ, MacMaster FP, Gaudet L, Schmidt MH. Striatal creatine and glutamate/glutamine in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:11–17. doi: 10.1089/cap.2006.0008. [DOI] [PubMed] [Google Scholar]
- 20.Bauer J, Werner A, Kohl W, Kugel H, Shushakova A, Pedersen A, Ohrmann P. Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. World J Biol Psychiatry. 2018;19:538–546. doi: 10.1080/15622975.2016.1262060. [DOI] [PubMed] [Google Scholar]
- 21.Lesch KP, Merker S, Reif A, Novak M. Dances with black widow spiders: Dysregulation of glutamate signalling enters centre stage in ADHD. Eur Neuropsychopharmacol. 2013;23:479–491. doi: 10.1016/j.euroneuro.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Cheng J, Liu A, Shi MY, Yan Z. Disrupted glutamatergic transmission in prefrontal cortex contributes to behavioral abnormality in an animal model of ADHD. Neuropsychopharmacology. 2017;42:2096–2104. doi: 10.1038/npp.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava AK, Khare P, Nagar HK, Raghuwanshi N, Srivastava R. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases. Curr Protein Pept Sci. 2016;17:596–602. doi: 10.2174/1389203717666151201192247. [DOI] [PubMed] [Google Scholar]
- 24.Baeza-Velasco C, Sinibaldi L, Castori M. Attention-deficit/hyperactivity disorder, joint hypermobility-related disorders and pain: Expanding body-mind connections to the developmental age. Atten Defic Hyperact Disord. 2018;10:163–175. doi: 10.1007/s12402-018-0252-2. [DOI] [PubMed] [Google Scholar]
- 25.Doğan ŞK, Taner Y, Evcik D. Benign joint hypermobility syndrome in patients with attention deficit/hyperactivity disorders. Arch Rheumatol. 2011;26:187–192. [Google Scholar]
- 26.Baeza-Velasco C, Grahame R, Bravo JF. A connective tissue disorder may underlie ESSENCE problems in childhood. Res Dev Disabil. 2017;60:232–242. doi: 10.1016/j.ridd.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Skalny AV, Skalny AA, Lobanova YN, Korobeinikova TV, Ajsuvakova OP, Notova SV, Burtseva TI, Skalnaya MG, Tinkov AA. Serum amino acid spectrum in children with autism spectrum disorder (ASD) Res Autism Spectr Disord. 2020;77(101605) [Google Scholar]
- 28.Tinkov AA, Skalnaya MG, Skalny AV. Serum trace element and amino acid profile in children with cerebral palsy. J Trace Elem Med Biol. 2021;64(126685) doi: 10.1016/j.jtemb.2020.126685. [DOI] [PubMed] [Google Scholar]
- 29.Oades RD, Dauvermann MR, Schimmelmann BG, Schwarz MJ, Myint AM. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism - effects of medication. Behav Brain Funct. 2010;6(29) doi: 10.1186/1744-9081-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holeček M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients. 2020;12(848) doi: 10.3390/nu12030848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasahara I, Fujimura N, Nozawa Y, Furuhata Y, Sato H. The effect of histidine on mental fatigue and cognitive performance in subjects with high fatigue and sleep disruption scores. Physiol Behav. 2015;147:238–244. doi: 10.1016/j.physbeh.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa T, Nakamura T, Shibakusa T, Sugita M, Naganuma F, Iida T, Miura Y, Mohsen A, Harada R, Yanai K. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J Nutr. 2014;144:1637–1641. doi: 10.3945/jn.114.196105. [DOI] [PubMed] [Google Scholar]
- 33.Holton KF, Johnstone JM, Brandley ET, Nigg JT. Evaluation of dietary intake in children and college students with and without attention-deficit/hyperactivity disorder. Nutr Neurosci. 2019;22:664–677. doi: 10.1080/1028415X.2018.1427661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallen A, Jamie JF, Cooper AJ. Lysine metabolism in mammalian brain: An update on the importance of recent discoveries. Amino Acids. 2013;45:1249–1272. doi: 10.1007/s00726-013-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikirova NA, Rogers AM, Taylor PR, Hunninghake RE, Miranda-Massari JR, Gonzalez MJ. Metabolic correction for attention deficit/hyperactivity disorder: A biochemical-physiological therapeutic approach. Funct Food Health Dis. 2013;3:1–20. [Google Scholar]
- 36.Smriga M, Ando T, Akutsu M, Furukawa Y, Miwa K, Morinaga Y. Oral treatment with L-lysine and L-arginine reduces anxiety and basal cortisol levels in healthy humans. Biomed Res. 2007;28:85–90. doi: 10.2220/biomedres.28.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.