Abstract
Secretory pore-forming proteins (PFPs) have been identified in organisms from all kingdoms of life. Our studies with the toad species Bombina maxima found an interaction network among aerolysin family PFPs (af-PFPs) and trefoil factors (TFFs). As a toad af-PFP, BmALP1 can be reversibly regulated between active and inactive forms, with its paralog BmALP3 acting as a negative regulator. BmALP1 interacts with BmTFF3 to form a cellular active complex called βγ-CAT. This PFP complex is characterized by acting on endocytic pathways and forming pores on endolysosomes, including stimulating cell macropinocytosis. In addition, cell exocytosis can be induced and/or modulated in the presence of βγ-CAT. Depending on cell contexts and surroundings, these effects can facilitate the toad in material uptake and vesicular transport, while maintaining mucosal barrier function as well as immune defense. Based on experimental evidence, we hereby propose a secretory endolysosome channel (SELC) pathway conducted by a secreted PFP in cell endocytic and exocytic systems, with βγ-CAT being the first example of a SELC protein. With essential roles in cell interactions and environmental adaptations, the proposed SELC protein pathway should be conserved in other living organisms.
Keywords: Pore-forming protein, Secretory endolysosome channel (SELC), Endocytosis, Exocytosis, Vesicular transport
INTRODUCTION
Endocytosis and exocytosis play fundamental roles in cell physiology
Endocytosis and exocytosis are fundamental in cell physiology for the exchange of information and material between cells and external environments as well as among cells. Macropinocytosis, phagocytosis, and receptor-mediated endocytosis are three major forms of endocytosis (Doherty & McMahon, 2009; Palm, 2019; Palm & Thompson, 2017; Scita & Di Fiore, 2010; Swanson & King, 2019; Wu et al., 2014). Different membrane organelles exist in distinctive endocytic pathways, including macropinosomes, phagosomes, and endosomes. Together with lysosomes, they are collectively referred to as endolysosomes. They are highly dynamic and play key roles in endocytosis and diverse cellular processes, including nutrient acquisition, membrane protein recycling, intracellular signaling, cell migration, metabolic adjustment, infection and immunity, development, and cell death (Antonescu et al., 2014; Cossart & Helenius, 2014; Cullen & Steinberg, 2018; Klumperman & Raposo, 2014; Moreno-Layseca et al., 2019; Palm & Thompson, 2017; Wang et al., 2018).
Cell exocytosis is a basic process in the homeostasis of cell physiology. The secretion of extracellular vesicles (EVs) comprised of exosomes and microvesicles, a heterogeneous group of cell-derived membranous structures, is an important aspect of cell exocytosis. The secretion of EVs can be viewed as the means of selective elimination of material from cells, as well as a new mode of intercellular communication (Russell et al., 2019; Van Niel et al., 2018). EVs are attributed numerous roles in regulating both physiological and pathological functions (Lindenbergh & Stoorvogel, 2018; Oggero et al., 2019; Yáñez-Mó et al., 2015).
Pore-forming proteins
Pore-forming proteins (PFPs) exist in a water-soluble monomeric form, and many are secreted out of cells (Dal Peraro & Van Der Goot, 2016). After undergoing extensive conformational change under specific conditions, these non-classic membrane proteins can form transmembrane pores (channels) of various size (1 to 30 nm). Based on the second structure of pores formed on the membrane, PFPs can be divided into two groups: i.e., α-helix and β-barrel PFPs. Pore-forming toxins (PFTs) are PFPs from microbes and are virulence factors for infection (Dal Peraro & Van Der Goot, 2016; Omersa et al., 2019). Knowledge on PFPs derived from plants and animals primarily includes their roles in cell death, including Bcl-2 proteins, MACPF/perforin proteins, and gasdermins (Banjara et al., 2020; Broz et al., 2020; Lukoyanova et al., 2016).
Aerolysin is a β-PFT produced by the bacterium Aeromonas hydrophila (Fivaz et al., 2001). The aerolysin domain is defined according to its structural similarity to the transmembrane domain of aerolysin (Szczesny et al., 2011). Proteins with an aerolysin fold can be found in all organisms. Particularly, a diverse array of aerolysin family PFPs (af-PFPs, previously referred to as aerolysin-like proteins, ALPs) harboring an aerolysin domain fused with other domains have been identified in various plant and animal species (Dang et al., 2017; Szczesny et al., 2011; Zhang, 2015).
Amphibian research model
Amphibians exhibit physiological traits in common with all vertebrates, including mammals. Our knowledge on many aspects of animal physiology has been gained from the study of amphibians. Historically, at least eight Nobel prizes have involved the use of amphibian models. Currently, amphibians are also used to answer fundamental questions on developmental biology, regeneration, genetics, and toxicology (Burggren & Warburton, 2007; Liu et al., 2016; Tandon et al., 2017; Yokoyama et al., 2018).
Here, we review the discovery of the af-PFP and trefoil factor (TFF) complex (named βγ-CAT) from the Chinese red belly toad (Bombina maxima) as well as its cellular actions and biological functions. We then propose that this secreted PFP complex represents a hitherto unknown secretory endolysosome channel (SELC) pathway, with βγ-CAT as the first example of a SELC protein. Its regulatory assembly mode, cell action mode, and endocytosis and exocytosis regulation reflect interaction between cells and the environment, as well as new regulatory strategies and effectors in adapting to the environment. The putative conservation of the βγ-CAT-like SELC pathway in other living organisms is also discussed. Future directions and challenges in this newly emerging field are also addressed below.
SECRETORY PFP COMPLEX βγ-CAT AND ITS CELLULAR ACTIONS
Discovery of af-PFP and TFF complex βγ-CAT
Af-PFPs and TFFs are major components in B. maxima skin secretions: The toad species B. maxima was selected as our research model. In addition to the rich existence of well-known hormone-like and antimicrobial peptides, af-PFPs, TFFs, oxidoreductases, immunoglobulin G (IgG) fragment crystallizable (Fc)-binding protein, and albumin have also been identified in its skin secretions (Zhang, 2015; Zhao et al., 2014). We previously isolated a protein complex composed of an af-PFP (βγ-crystallin domain fused with an aerolysin domain, termed BmALP1 α-subunit) and a TFF (BmTFF3 β-subunit), which was subsequently named βγ-CAT to reflect its domain composition (Liu et al., 2008). This protein complex formed membrane pores with a functional diameter of about 1.5–2.0 nm (Gao et al., 2011b; Liu et al., 2008).
Regulated assembly of βγ-CAT complex relies on environmental cues: BmALP1 and BmTFF3 are products of two genes with distinct secretory pathways (Liu et al., 2008; Wang et al., 2020). For the assembly and dissociation of complexes in toads, positive and negative control methods are required. Indeed, as a novel af-PFP, BmALP3 is characterized by a lack of membrane pore formation capacity (Wang et al., 2020). BmALP3 senses environmental oxygen tension and acts as a negative regulator of the βγ-CAT complex. These two af-PFPs (i.e., BmALP1 and BmALP3) contain a conserved cysteine in their C-terminal regions. BmALP3 binds to a homodimer to oxidize BmALP1 through disulfide bond exchange, thereby forming a BmALP1 homodimer and polymer. This effect causes the βγ-CAT complex to dissociate and lose its biological activity (Wang et al., 2020).
Double receptor binding pattern for βγ-CAT endocytosis: βγ-CAT exerts biological actions intracellularly via its secretory components. Cell surface molecules mediate the binding and endocytosis of βγ-CAT. An interaction between βγ-CAT and acidic glycosphingolipids (AGSLs) was uncovered. Further detailed study revealed that the aerolysin-like domain of βγ-CAT, but not the βγ-crystallin domain, specifically binds to gangliosides, while the BmTFF3 subunit of the complex binds to sulfatides, thus revealing a double receptor binding pattern (Guo et al., 2019). Most AGSLs exist in membrane lipid raft microdomains (McGonigal et al., 2019). Accordingly, disruption of lipid rafts can impair the actions of βγ-CAT (Guo et al., 2019).
As βγ-CAT is an exogenous factor to mammals, who therefore lack relevant control or regulatory systems, βγ-CAT shows potent biological activities, including in vivo and in vitro toxicity to mammals and mammalian-derived cell lines (Gao et al., 2011a; He et al., 2008a, 2008b; Qian et al., 2008a, 2008b). However, growing experimental evidence suggests that this PFP complex is not a typical biological “toxin” or “weapon” for preventing potential attack by other living organisms, but is an important endogenous physiological element in B. maxima, in which it plays a fundamental physiological function (Li et al., 2017; Xiang et al., 2014).
Cellular actions of βγ-CAT and biological outcomes
Amphibian skin exhibits important physiological functions, like water economy, respiration, metabolite exchange, and immunity. Skin is constantly confronted by a complex mixture of potentially injurious factors and environmental interactions to ensure sufficient uptake of water, electrolytes, and oxygen (Haslam et al., 2014; Jørgensen, 2000; Varga et al., 2018). Obviously, mucosal barrier maintenance and immune defense are essential in the amphibian life cycle. Accordingly, we investigated and determined that the PFP complex βγ-CAT indeed plays an active role in these biological requirements.
Stimulating macropinocytosis: In nucleated cells, βγ-CAT α-subunit BmALP1 is rapidly endocytosed with large intracellular vacuole (>200 nm) formation, in which neutral red uptake of the cells increases in a βγ-CAT dose-dependent manner, indicating its capacity to stimulate cell macropinocytosis. Protein endocytosis is important for cellular functions, such as inducing cell detachment and migration (Liu et al., 2008). In addition, βγ-CAT stimulates macropinocytosis in dendritic cells (DCs) (Deng et al., 2020). The capacity of βγ-CAT to induce and participate in cell macropinocytosis has been also determined in cells from B. maxima, including peritoneal cells (Li et al., 2017) and epithelial cells from the toad (Zhao et al., unpublished observation). These results reveal the capacity of the protein in stimulating cell endocytosis in the form of macropinocytosis.
Enhancing antigen presentation via endolysosome modulation: βγ-CAT enhances the macropinocytosis of DCs, thereby increasing the internalization of ovalbumin (antigen) (Deng et al., 2020). At the same time, βγ-CAT and ovalbumin are rapidly endocytosed along the DC endocytic pathway. The acidification of the antigen-containing vesicles in DCs is neutralized, and the pores formed by the βγ-CAT α-subunit (BmALP1) serve as channels to deliver endocytic antigen peptides to the cytoplasm. Specifically, βγ-CAT stimulates robust EV release from DCs, which rapidly activates T lymphocytes. Finally, the action of βγ-CAT leads to both cellular and humoral immune responses in vitro and in vivo (Deng et al., 2020). Interestingly, the release of EVs promoted by βγ-CAT has also been observed in B. maxima peritoneal cells (Li et al., 2017).
Neutralizing endocytic organelle acidification to counteract intracellular pathogens: Upon intracellular infection with Listeria monocytogenes in B. maxima peritoneal cells and fibroblasts, the βγ-CAT α-subunit (BmALP1) co-localizes with the bacteria in endocytic vesicles. The presence of BmALP1 and its pore formation result in the acidification and neutralization of intracellular pathogen-containing vesicles. Furthermore, βγ-CAT stimulates cell exocytosis, and protein treatment leads to augmentation of non-lytic expulsion of pathogen-containing vesicles. Thus, in vivo, βγ-CAT effectively protects the toad against L. monocytogenes infection (Li et al., 2017).
Causing lysosome destabilization to activate inflammasomes: In the face of extracellular bacterial infection, the βγ-CAT α-subunit (BmALP1) is rapidly endocytosed in B. maxima peritoneal cells. It is oligomerized and forms pores along the endocytic pathway. Lysosomal destabilization and cathepsin B release occur. This action results in the activation of inflammasomes and subsequent interleukin-1β (IL-1β) maturation and release. Accordingly, in toad and mouse peritoneal infection models, βγ-CAT greatly accelerates bacterial clearance and increases animal survival (Xiang et al., 2014).
Promoting tissue repair: βγ-CAT exhibits a strong ability to promote tissue repair. For example, consistent with the above studies, inflammasomes are activated and IL-1β is rapidly released upon protein treatment in murine cutaneous injury models (Gao et al., 2019). The healing process is characterized by accelerated re-epithelialization, ameliorated dermal edema, and scar-less healing. Thus, βγ-CAT may fulfill the physiological requirement of rapid tissue repair and skin permeability for amphibian survival at the same time (Gao et al., 2019).
Taken together, the secreted PFP complex βγ-CAT assembles according to changes in environmental cues and acts on AGSLs in lipid rafts to induce endocytosis, especially macropinocytosis, in sensitive cells. Oligomerization and pore formation of the βγ-CAT α-subunit (BmALP1) along the endocytic pathway result in the formation of channels on the endolysosome membranes, which facilitate the exchange of substances between endocytic vesicles and cytoplasm. The content and biochemical properties of these intracellular vesicles can be modulated. Depending on various cellular and environmental conditions, βγ-CAT activity can lead to diverse cellular responses and biological outcomes (Li et al., 2017; Wang et al., 2020; Xiang et al., 2014).
βγ-CAT REPRESENTS A NOVEL STRATEGY AND EFFECTOR IN CELL PHYSIOLOGY
Secretory endolysosome channel (SELC) pathway in cell endocytic and exocytic systems
The experimental evidence associated with βγ-CAT combined with the necessity of cell physiology led us to propose the hypothesis of secretory endolysosome channels (SELCs) and related cellular pathways (SELC pathways), which function in cell physiology and homeostasis as well as in cell adaptations to environmental variations. βγ-CAT is the first example of a SELC protein.
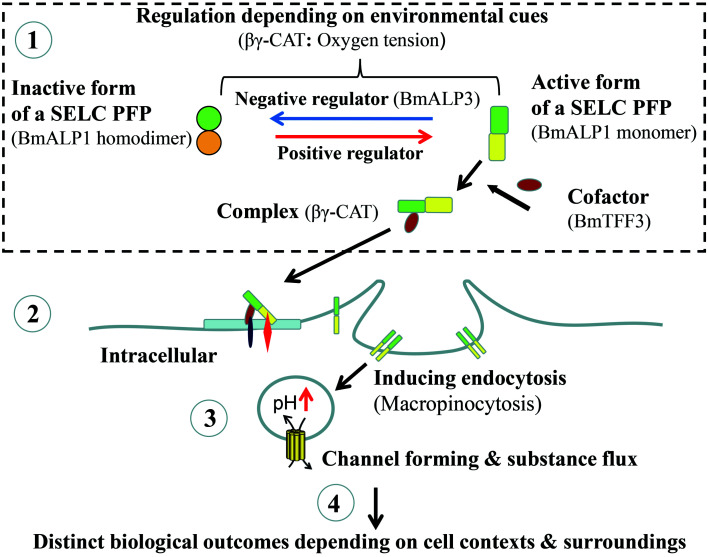
Major components in SELC pathway: The central element of the SELC pathway is a secretory SELC PFP that can form an endolysosome channel, and which belongs to af-PFPs, other PFP families, or unidentified proteins with pore forming capacity (Figure 1). In the extracellular environment, the SELC PFP can be reversibly converted between inactive and active forms. There should be specific sensors and regulators that can sense and integrate environmental conditions (like oxygen tension, water balance, pH, nutrients, metabolites, or pathogens) to regulate the conversion of the SELC PFP negatively or positively. In the case of βγ-CAT, BmALP3 senses environmental oxygen tension. Furthermore, active SELC PFPs may or may not need to interact with a cofactor chaperon to form an active PFP complex, with βγ-CAT being the former case (Figure 1).
Figure 1.
Proposed SELC pathway based on βγ-CAT evidence
There are four main steps in the proposed SELC pathway. Related elements of βγ-CAT pathway are bracketed. (1) In extracellular surroundings, a SELC PFP can be reversibly converted between inactive or active forms by specific negative or positive regulators in response to variations in environmental conditions (like oxygen tension, water balance, pH, nutrients, metabolites, pathogens). Active form of SELC PFP may or may not be necessary to interact with a cofactor to form a cellular active complex. βγ-CAT is the former case. (2) Active PFP or the complex binds membrane receptor(s) and stimulates endocytosis, especially macropinocytosis. (3) PFP then oligomerizes and forms channels on endolysosomes to facilitate material exchange. (4) Actions result in distinct biological outcomes depending on cell contexts and environment, see text.
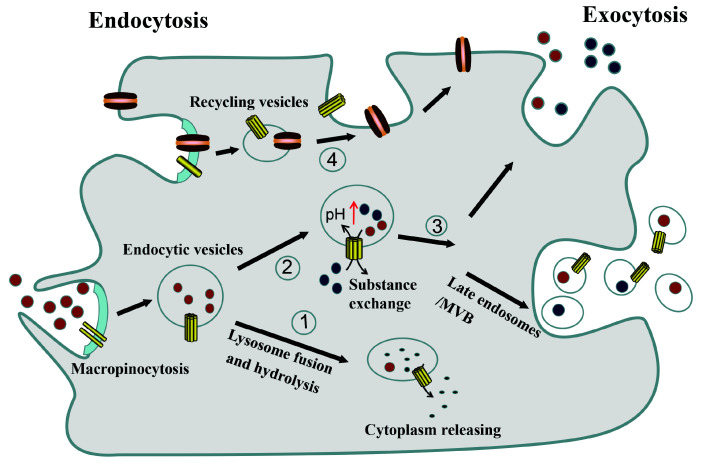
Manipulation of endocytic and exocytic systems by endolysosome channels: An active SELC protein or its complex (like βγ-CAT) stimulates and participates in endocytosis, especially in the form of macropinocytosis, which, in turn, facilitates the uptake of extracellular substances. SELC proteins then oligomerize and form pores on endolysosomes, which can be used as channels for material exchange between endocytic organelles and cytoplasm (Figures 1, 2).
Figure 2.
SELC proteins manipulate endocytosis and exocytosis
Depending on distinct cell contexts and environments, cellular action of SELC proteins is well adapted for material uptake and exchange as well as vesicular transport either within a cell or across cells. (1) Macropinocytosis induced by SELC protein results in uptake of external material, including water and solutes (nutrients or antigens). Solutes may be processed and/or hydrolyzed in endolysosomes, and channels formed by SELC protein can mediate the release of resulting products from vesicles to cytoplasm. (2) Channels formed by SELC protein on endolysosomes can mediate material exchange, which results in biochemical property modulation of vesicles (e.g., pH and/or content), leading to specific cellular responses. (3) Furthermore, exocytosis can be induced and modulated in the presence of a SELC protein, which plays a role in transcytosis of cell surrounding materials (like lipids with a carrier), secretion of intracellular materials, and waste expulsion. (4) SELC proteins may participate in the recycling and re-distribution of membrane components, like functional proteins and lipid components.
Depending on the various temporal and spatial parameters of distinct cell contexts as well as the conditions of cell surroundings, several cellular action modes can be proposed after SELC protein endocytosis (Figure 2). First, macropinocytosis induced by the SELC protein could result in the uptake of external materials (like antigens, water, and solutes). The channels formed in endolysosomes by the SELC protein could mediate the release of acquired solutes, which may have been hydrolyzed and processed into unfolded small molecular mass components in the organelles to cytoplasm, Second, SELC proteins could lead to the modification of the biochemical properties of SELC-containing vesicles (like pH and/or content) due to their channel formation on endolysosomes, resulting in specific cellular reactions. Third, exocytosis could be induced and modulated in the presence of SELC proteins, which function in the transcytosis of materials surrounding cells, such as lipids bound in a carrier (like albumin), as well as the secretion of intracellular materials. Fourth, SELC proteins may participate in the recycling and re-distribution of membrane-integrated components, like functional proteins and lipid components (Figure 2).
Necessity of SELC pathway in cell physiology
Characteristics of SELC pathway: SELC proteins have their own intrinsic advantages and characteristics in the communication between cells and their surroundings. (1) A SELC protein could be constitutively expressed in situ or could circulate in biological fluids, extending their actions remotely. (2) Depending on environmental cues, the related sensors and regulators of a SELC protein (Figure 1) could immediately manipulate the state of the protein to fulfill specific cell requirements with high efficiency, especially in emergency conditions. (3) An activated SELC protein could act in specific and distinct cell regions in a temporal and spatial manner to trigger proper cellular responses. (4) SELC proteins may undertake direct vesicular transportation of cargo, which can be released within cells or transported across cells in their native forms and/or processed products within endolysosomes (Figure 2).
Roles in cell physiology and homeostasis: The SELC pathway may play active and essential roles in cell interactions with and adaptations to the environment surrounding the cell. As exemplified by βγ-CAT and depending on cell contexts and environmental cues, the action of SELC proteins could lead to diverse cellular responses and biological outcomes. It can be reasonably predicted that the SELC pathway is necessary and functions in external material uptake (like oxygen, water, nutrients, antigen, and other necessary substances) and vesicular transport, immune surveillance and responses, metabolic adjustment, and exocytosis modulation (including expulsion of metabolites, poisons, and waste as well as unconventional secretion).
Collaboration with classic membrane-integrated proteins: Classic membrane proteins, like receptors, ion channels, and transporters, are responsible for communication between intracellular and extracellular environments. The SELC pathway conducted by a SELC protein and accessory components in the extracellular environment may function as the humoral regulatory network of cell endocytosis and exocytosis. Secreted PFPs like the SELC protein βγ-CAT may also be viewed as secretory vesicular transporters. Obviously, this humoral system could collaborate with classic membrane-integrated proteins in cell physiology and homeostasis. It is possible that the endocytosis stimulated by a SELC protein plays a role in the sorting of specific plasma membrane elements, such as functional integrated proteins or lipid components, which help to regulate cell responses to environmental variations (Figure 2).
It can be speculated that in undifferentiated cells, like oocytes, stem cells, progenitor cells, and end differentiated cells like keratinocytes in the epidermis, classic membrane-integrated proteins may be in short states, and the humoral system may be more significant and effective. For instance, the SELC pathway may play an essential role in the process of nutrient acquisition from egg white and yolk in proliferating embryonic cells of oviparous animals.
SELC protein candidates
In various living organisms, SELC protein candidates may come from secretory PFPs. These secretory PFPs may belong to different PFP families. In addition, new proteins with potential membrane pore-forming capacities are awaiting identification.
βγ-CAT is the pioneer SELC protein member. Interestingly, βγ-CAT-like PFPs, which are members of af-PFPs, are highly conserved within various species of the same class of plants and animals (Szczesny et al., 2011; Zhang, 2015). However, these proteins are characterized by low sequence similarity, especially in the aerolysin domain (<20% similarity), raising difficulties in resolution of their conservation by traditional methods (Szczesny et al., 2011; Zhang, 2015). Alternatively, many proteins are grouped into af-PFPs based on their 3D structures, though they are elusive at the sequence level (Cirauqui et al., 2017; De Colibus et al., 2012).
Plant af-PFPs: In a single plant species, there are multiple gene copies encoding af-PFPs, which are composed of two N-terminal agglutinin domains and a C-terminal aerolysin-like domain and are well conserved in various species along plant evolutionary lineages. Sixteen genes encoding af-PFPs have been retrieved from the cucumber genome (Dang et al., 2017). Overexpression of a flower-specific af-PFP in Rumex acetosa alters flower development and induces male sterility in transgenic tobacco (Manzano et al., 2017). A gene encoding an af-PFP protein confers resistance to fusarium head blight, a devastating disease of wheat and barley (Rawat et al., 2016).
Animal af-PFPs: Lysenin and biomphalysin are af-PFPs found in the earthworm Eisenia fetida and snail Biomphalaria glabrata, respectively (Galinier et al., 2013; Sekizawa et al., 1997). Mutation of Lin-24, an af-PFP derived from Caenorhabditis elegans, causes abnormal vulva development, leading to a failure to lay eggs (Galvin et al., 2008). Ep37 proteins from the newt Cynops pyrrhogaster are homologous to BmALP1, which is proposed to be involved in epidermal development (Ogawa et al., 1997, 1998). Fish af-PFPs, including Danio rerio Dln1 (Jia et al., 2016), Thalassophryne nattereri natterins (Magalhães et al., 2005), and those from lampreys (Pang et al., 2017; Wu et al., 2017), are proposed as defense molecules in fish immune systems.
The regulatory C-terminal cysteine residue in the βγ-CAT α-subunit (BmALP1), which links protein machinery with B. maxima skin respiration, is highly conserved from fish to reptiles, suggesting that the SELC pathway may play a role in hypoxia adaptation (Wang et al., 2020). Interestingly, the conserved cysteine site is mutated to a serine residue in birds. Fish and amphibians live in water and sensing environmental oxygen tension and initiating proper cellular responses are essential in their life cycle. Accordingly, the regulatory site is a cysteine residue in these animals. Birds live on land with rich oxygen and a well-evolved lung respiration system, and actively sensing oxygen tension change may not be fatal. Serine substitution can convert the regulatory model into a new adaptation (Wang et al., 2020).
It should be noted that SELC proteins may not necessarily be af-PFP family members. In contrast, not all af-PFP members from eukaryotic organisms may function as a SELC protein. Obviously, in the evolutionary process, af-PFPs in different organisms have evolved to play distinct biological functions. Besides bacterial virulence factors, some af-PFPs have been recruited to animal venom glands as toxins (Zhang, 2015).
Do βγ-CAT-like af-PFPs exist in mammals?: Though af-PFPs are well conserved and easily identified from fish to birds, their existence in mammals remains elusive. Until now, no homologue to the aerolysin domain has been found in mammalian species following sequence-based search methods. In contrast, the domains that are often fused with the aerolysin domain to form af-PFPs in vertebrates, such as the βγ-crystallin, lectin, and Ig-like domains, are well conserved in mammals. Specifically, the β-subunit of βγ-CAT is a TFF, which are well conserved in mammals (Zhang et al., 2011). There are several possibilities to explain this phenomenon.
First, as mentioned above, af-PFPs are characterized by low sequence similarity with each other, which may lead to missing homologous sequences during searching (Cirauqui et al., 2017; Szczesny et al., 2011). Accordingly, the discovery of potential structural similarities shared by mammalian proteins with βγ-CAT-like PFPs depends on comparison of their 3D structures.
Second, for unknown reasons, other protein family members may have evolved to be SELC proteins in mammals along the evolutionary process. As mentioned above, SELC proteins may not necessarily be af-PFP family members. These alternative proteins may be responsible for cellular actions similar to those of βγ-CAT-like SELC proteins. It is worth noting that human RegIIIα (also known as HIP/PAP) possesses a previously unappreciated pore forming capacity for the C-type lectin family (Mukherjee et al., 2014). α-Synuclein, a presynaptic enriched protein, has been found to form pores in lipid membranes (Schmidt et al., 2012). Of course, other secretory proteins with pore forming capacities are awaiting future identification.
Third, the strategies and effectors involved in cellular interactions with and adaptations to the environment via modulation of cell endocytic and exocytic pathways by secretory elements, like SELC proteins, may not have been suitable and have been lost in mammals. As discussed above, the SELC pathway proposed here should represent one of the primary strategies and protein working networks in cell physiology because they interact with the surrounding environment as well as among cells themselves. Accordingly, the sudden and total disappearance of SELC proteins seems improbable. In our opinion, the first two possibilities are more likely the case in mammals.
Differences between physiological SELC protein βγ-CAT and bacterial aerolysin toxin
βγ-CAT is the first example of a SELC protein. Though the βγ-CAT α-subunit (BmALP1) is a bacterial PFP aerolysin family member, its nomenclature only reflects that the protein contains a membrane insertion domain similar to that of the aerolysin toxin. These two proteins are different in their molecular compositions, regulatory and activation patterns, cell targets, cellular acting pathways, and pathophysiological relevance.
Activation mechanism: Aerolysin is a bacterial single gene product that is activated via proteolytic cleavage of a C-terminal fragment by host proteases (Van Der Goot et al., 1994). Comparatively, the regulatory manner of βγ-CAT appears to be more meticulous. The initial action of the βγ-CAT α-subunit (BmALP1) needs a cofactor, i.e., BmTFF3, which is a distinct gene product secreted by a different pathway. Consistent with its physiological requirements, BmALP1 is reversibly regulated by its paralog BmALP3 depending on environmental redox states, like oxygen tension (Wang et al., 2020).
Cellular targets and actions: Glycosylphosphatidylinositol (GPI)-anchored proteins act as membrane receptors to mediate aerolysin action, whereas the SELC protein βγ-CAT interacts with membrane AGSLs in a double binding pattern (Guo et al., 2019). Unlike aerolysin, which forms pores on the host cell plasma membrane (Gurcel et al., 2006), βγ-CAT α-subunit (BmALP1) acts on cell endolysosomes. βγ-CAT is the first example of an endogenous PFP that stimulates the production and release of functional exosome-like EVs via the endosomal system, which differs from the plasma membrane-derived microvesicles stimulated by bacterial PFTs (Gurcel et al., 2006; Romero et al., 2017).
Biological functions and significance: The biological outcomes of these two PFPs are different. For example, aerolysin perturbs cell tight junction integrity and induces barrier defects in intestinal epithelial cells for the purpose of invasion (Bücker et al., 2011). In contrast, the SELC protein βγ-CAT maintains mucosal barrier integrity by accelerating re-epithelialization during tissue repair (Gao et al., 2019). The divergence of these two proteins is consistent with the fact that aerolysin is a virulence factor for bacterial invasion (Chang et al., 1997), whereas βγ-CAT is a physiological component of B. maxima (Gao et al., 2019; Li et al., 2017; Wang et al., 2020; Xiang et al., 2014).
FUTURE DIRECTION AND CHALLENGES
Evolutionary conservation of and variation in βγ-CAT-like SELC pathways
Investigations on evolutionary conservation of βγ-CAT-like SELC pathways in other vertebrates is an important future challenge. Predictably, variations with specific physiological relevance may have occurred in the SELC pathway in different vertebrates, which deserves further study. Many possibilities could be postulated for the physiological functions of the SELC pathway in vertebrates. Key points should focus on the fundamental processes related to animal interactions with and adaptations to distinct environments. These include, but are not limited to, external material uptake (e.g., water, oxygen, nutrients, antigens) and vesicular transport, as well as regulation of immune responses and maintenance of mucosal barrier function, metabolic flexibility, and exocytosis modulation.
Future study should help illustrate how the SELC pathway in vertebrates is linked to basic cellular processes (endocytosis and exocytosis) and to fundamental issues in animal interactions with and adaptations to various environments, especially the water-oxygen-metabolism axis in the vertebrate life cycle and conservation and variation within different animal classes along evolutionary processes.
SELC protein βγ-CAT in B. maxima skin water-oxygen balance
Water and oxygen are essential for life. Given that toads, such as B. maxima, live on land and in water, their skin is an important organ in water uptake and loss as well as respiration (gas exchange). While oxygen is abundant on land, dehydration is a real challenge. In contrast, low oxygen tension (potential hypoxia) can be problematic in water environments. Accordingly, water-oxygen balance is an essential issue for toads. As major protein components in skin secretions, af-PFPs and TFFs likely play direct and indirect roles in essential physiological processes, including the following.
Function in water uptake and maintenance: The SELC protein βγ-CAT in toads can induce cell macropinocytosis. Reasonably, this property of the protein machinery may function for water uptake to counteract dehydration under hypertonic irritation. Furthermore, internalization and recycling of aquaporins between the plasma membrane and endosomal compartments play roles in controlling water uptake and maintenance (Shibata et al., 2014; Suzuki et al., 2015). Potential endocytosis and re-distribution of aquaporins induced by βγ-CAT may also modulate water uptake and loss in toads. Thus, the potential actions of other PFP homologues of βγ-CAT should be considered in future study.
In many species of amphibian, the skin surface is covered with a lipid layer, which prevents evaporative water loss (EWL) on land (Barbeau & Lillywhite, 2005; Centeno et al., 2015; Haslam et al., 2014; Sadowski-Fugitt et al., 2012). The SELC protein βγ-CAT and/or its PFP homologues may modulate the lipid barrier in a temporal and spatial manner via endocytosis and vesicular trafficking to facilitate water balance. In B. maxima, albumin is expressed in the skin and is widely distributed around the membranes of epithelial layer cells and within the stratum spongiosum of the dermis (Zhang et al., 2005). Albumin may participate in the modulation of skin lipids by acting as a lipid carrier and could be transported via βγ-CAT- and/or PFP homologue-induced macropinocytosis.
Function in respiration: The oxygen tension-dependent control of the SELC protein βγ-CAT by its paralog BmALP3 links protein machinery with skin respiration (Wang et al., 2020). Albumin expressed in B. maxima skin is distinct from that in serum by binding to a haem b cofactor (Zhang et al., 2005). One of possible functions of the haem b cofactor in toad skin albumin may be in cutaneous gas exchange. Whether albumin transport mediated by βγ-CAT and/or PFP homologues from external environments to intercellular spaces can facilitate gas exchange in toad skin is an important question.
Hypoxia and inflammation are intertwined at the molecular, cellular, and clinical levels (Eltzschig & Carmeliet, 2011; Medzhitov, 2008). In some cell contexts under specific environmental conditions, βγ-CAT can activate inflammasomes to induce inflammatory responses (Xiang et al., 2014), which should dilate blood vessels and increase blood flow to facilitate gas exchange, especially under acute hypoxic conditions. The possible link between βγ-CAT and the activation and translocation of hypoxia-inducible factor 1 (HIF-1) deserves further investigation.
Membrane trafficking and recycling of endosomes play active roles in epithelial remodeling, during which cells change shape and position while maintaining cell to cell contact (Jouette et al., 2019; Le Droguen et al., 2015). It is speculated that the endocytosis and re-distribution of cell adhesion proteins and cytoskeleton elements induced by βγ-CAT and/or its PFP homologues may lead to cell shape changes and remodeling of epidermal cell layers to modulate water-oxygen balance in toad skin.
Roles in material acquisition and vesicular transport
In cell metabolism: Endocytosis in the form of macropinocytosis can uptake extracellular material (solutes in fluid phase) (Lim & Gleeson, 2011; Palm, 2019). As mentioned, the SELC protein βγ-CAT may be viewed as a novel type of secretory vesicular transporter. It is highly possible that this af-PFP and its homologues participate in the uptake and vesicular transport of external material and/or membrane components (Figure 2). Furthermore, βγ-CAT and its PFP homologues may facilitate cells to acquire macromolecules as nutrients as well as other necessary solutes in the cell life cycle, and the related significance in cell metabolism and biomass building deserves further study. On the other hand, due to the molecular pathway shown in Figure 2, these PFPs may play a role in vesicular transport and output of cell metabolites, which also requires further investigation.
In cell migration: Macropinocytosis is involved in cell motility (Donaldson et al., 2009; Gu et al., 2011; Llanses Martinez & Rainero, 2019). βγ-CAT promotes cell migration rapidly in wound healing (Gao et al., 2019). The potential regulation of cell adhesion and cytoskeleton components via endocytic and exocytic recycling triggered by βγ-CAT could modulate cell shape and cytoskeleton network to facilitate cell migration.
In transcytosis: Transcytosis is a strategy used by multicellular organisms to move material between two environments while maintaining cellular barrier functions (Conner & Schmid, 2003; Tuma & Hubbard, 2003). The neutralization of the acidification of endolysosomes along the endocytic pathway by βγ-CAT could prevent lysosome degradation of material in the βγ-CAT-containing vesicles, which may result in material transcytosis via EV release (Figure 2).
SELC protein βγ-CAT directly acts on EVs
EVs circulate in all biological fluids and can trigger biological responses at a distance. To deliver their cargo into recipient cells, the cells engulf EVs via receptor-mediated endocytosis, macropinocytosis, or membrane fusion (Meldolesi, 2018; Record et al., 2018; Van Niel et al., 2018). However, many molecules in EVs, such as cytokines, growth factors, and metabolites, act on their targets on the cell surface or exhibit biological effects in the extracellular environments. Conceivably, there may be alternative ways in vivo for the release of specific EV cargo into extracellular fluids, which could be mediated by secretory PFPs under specific regulation.
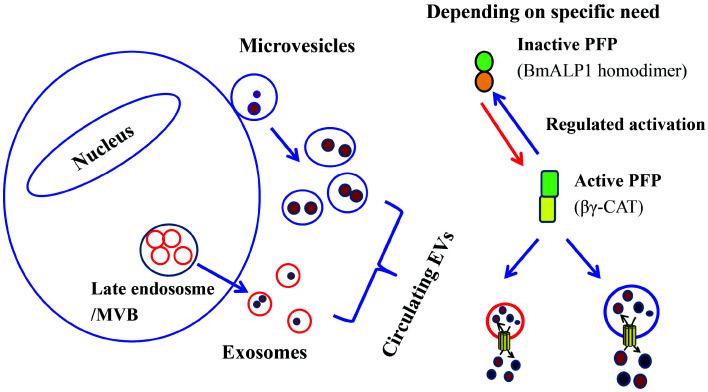
Accordingly, it would be interesting to study the direct action of the SELC protein βγ-CAT and its PFP homologues on EVs and their physiological relevance. Under proper conditions, the oligomerization and channel formation of PFPs in EVs may lead to the spatiotemporal release of EV cargo molecules to fit specific biological requirements in situ (Figure 3). Alternatively, the channels formed by PFPs may also serve to uptake specific material in cell surroundings, and the cell may acquire the materials taken up by fusion with the vesicles containing PFP channels, serving as an alternative way for cells to acquire extracellular materials (Figure 3). Thus, SELC proteins could extend their functions as secretory EV channels.
Figure 3.
Proposed direct action of SELC PFP on EVs
EVs comprised of exosomes and microvesicles circulate in biological fluids. They bear cargo molecules, like proteins and metabolites. Membrane active secretory PFPs, like SELC protein βγ-CAT, can be tightly regulated by specific regulators relying on distinct micro-environmental conditions (Figure 1). Subsequent oligomerization and channel formation of PFPs in EVs may lead to release of EV cargo molecules in a temporal and spatial manner to fit specific cellular and biological requirements in situ. Alternatively, channels formed by PFP can also be used to take up specific substances in the surrounding environment, and cells can obtain these substances by fusing with vesicles containing PFP channels, functioning as an alternative way for cells to acquire extracellular material.
Mechanism of SELC protein βγ-CAT endocytosis
The SELC protein βγ-CAT exerts cellular effects after endocytosis in the form of macropinocytosis. Macropinocytosis is believed to be a signal- and actin-dependent process that normally occurs in response to growth factor stimulation (Lim & Gleeson, 2011; Palm, 2019; Swanson & King, 2019). Interestingly, βγ-CAT targets AGSLs in lipid rafts to initiate stimulation and participation in macropinocytosis. The signals downstream of the lipid components and their relationship with cytoskeleton rearrangement are important open questions. Thus, the putative variation between classic macropinocytosis and that induced by βγ-CAT could be expected and should be emphasized.
Membrane pore/channel properties of SELC protein βγ-CAT
A question that needs to be addressed is the selectivity of channels formed by the SELC protein βγ-CAT for specific substance(s) in living cells. The pore size formed by an af-PFP is around 1.5 nm (Jia et al., 2016; Liu et al., 2008; Podobnik et al., 2016), similar to that formed by aerolysin. Aerolysin pores have been applied as nano-sensors in biomolecular detection and identification (Ouldali et al., 2020; Wang et al., 2018). Peptides, unfolded proteins, nucleic acids, and polysaccharide chains can be transported through aerolysin pores (Ouldali et al., 2020; Wang et al., 2018). This raises the possibility that these substances could potentially be transported through the pore formed by the endogenous SELC protein βγ-CAT.
After endocytosis with βγ-CAT, the solutes in extracellular fluids could be transported to the cytosol or intercellular spaces (Figure 2). The transport of antigen peptides to the cytosol via the βγ-CAT pore has been observed previously (Deng et al., 2020). Alternatively, substances in cytosol, like unfolded proteins, nucleic acids, or lipids, could be transported to βγ-CAT-vesicles for subsequent vesicular transport within or out of the cell (Figure 2).
Regulation of SELC protein βγ-CAT in various stages
The SELC protein βγ-CAT is likely to be tightly regulated, however, this requires further investigation. In the extracellular milieu, positive and negative regulatory elements exist for the assembly and disassociation of the βγ-CAT complex in toads; for example, its paralog BmALP3 (Wang et al., 2020). In addition, putative positive regulator(s) for the promotion of βγ-CAT assembly is the focus of ongoing studies.
In the process of βγ-CAT oligomerization, micro-environmental parameters, like membrane lipid compositions or pH, may influence PFP oligomerization and channel formation. Weak acidic environments (pH 5.5–6.5) facilitate βγ-CAT α-subunit (BmALP1) oligomerization (Ye, 2020), in accordance with a fact that βγ-CAT α-subunit (BmALP1) prefers to oligomerize and form channels on endolysosomes (Li et al., 2017; Xiang et al., 2014).
Growing evidence suggests that some unknown elements may regulate the opening or closing of channels formed by the SELC protein βγ-CAT. The βγ-CAT α-subunit (BmALP1) is an af-PFP with two βγ-crystallin domains fused at its N-terminal part, which can bind to nucleotides (He et al., 2008b). Adenosine triphosphate (ATP) and guanosine triphosphate (GTP) block fluorescent dye efflux out of the BmALP1 channels, implying that nucleotide binding may lead to channel closure (Ye, 2020).
The C-terminal cysteine residue in the βγ-CAT α-subunit (BmALP1), which is conserved in vertebrate af-PFPs, is the site involved in the regulation of βγ-CAT assembly in the extracellular milieu (Wang et al., 2020). However, after BmALP1 oligomerization and channel formation on endolysosomes, it is also possible that the cysteine residue acts as a key site in sensing intracellular conditions (like redox states) and in regulating the properties of the channels formed.
Potential application of SELC protein βγ-CAT
Molecular probe in cell biology: As the first example of a SELC protein, the SELC pathway of βγ-CAT suggests that the protein may be an extremely valuable molecular probe, especially in cell biology. Following the cellular path of βγ-CAT could result in exciting discoveries concerning endocytosis and exocytosis.
The βγ-CAT α-subunit (BmALP1) can exist in multiple forms, including monomers, homodimers, and BmTFF3 complexes (Liu et al., 2008; Wang et al., 2020). Consequently, how to detect active βγ-CAT with physiological functions is an important question. Quantitative hemolysis assay could help detect active βγ-CAT. Alternatively, monoclonal antibodies against βγ-CAT could be developed to detect the biologically active complex. In addition, as βγ-CAT works in the form of oligomers in the membrane, it would be necessary to determine the number of monomers that form oligomers, which is a subject of ongoing study.
Therapeutic agent: βγ-CAT is highly effective at stimulating immune responses to counteract pathogen infections (Deng et al., 2020; Li et al., 2017; Xiang et al., 2014) and to promote wound healing (Gao et al., 2019). βγ-CAT may reduce excessive epithelial hyperplasia and prevent scar formation by the activation of cell death signals (Gao et al., 2019). These properties make the protein an ideal candidate as a therapeutic agent in clinical settings. However, βγ-CAT drug development is currently hindered by the relatively low quantity obtained by purification from skin secretions.
Adjuvant in vaccine preparation: A big challenge for a vaccine adjuvant is its ability to induce cellular immunity against extracellular pathogens or malignant cells. DC cross-presentation plays a vital role in vaccine development (Coffman et al., 2010; Ho et al., 2018). The SELC protein βγ-CAT may strongly stimulate MHC-I-mediated cross presentation. Particularly, βγ-CAT stimulates robust EV release from DCs, which rapidly activates T lymphocytes (Deng et al., 2020). These properties render βγ-CAT a potent adjuvant candidate.
CONCLUDING REMARKS
Secretory PFPs have been identified in organisms from all kingdoms of life. Most studies on PFPs have focused on their role in cell death, including virulence factors of pathogens or effectors of host immune systems (Dal Peraro & Van Der Goot, 2016; Delbridge et al., 2016; Kovacs & Miao, 2017; Liu & Lieberman, 2020; Merle et al., 2015; Zhang, 2015). To benefit from amphibian research models, sufficient amounts of af-PFP and TFFs can be identified and purified from the skin secretions of B. maxima, especially in their natural form. Studies have illustrated an unexpected interaction network among extracellular af-PFPs (paralog regulation of BmALP1 by BmALP3) and TFFs (formation of SELC protein complex βγ-CAT) depending on environmental conditions.
Importantly, one of the formation modes of regulated SELC protein βγ-CAT is oxygen tension dependent. The PFP complex acts in the endocytic pathway by channel formation on endolysosomes, including stimulating cell macropinocytosis, which can facilitate substance uptake and exchange as well as vesicular transport. On the other hand, cell exocytosis would be induced and/or modulated in the presence of SELC proteins depending on cell contexts and surroundings. These cellular effects are in accordance with the biological requirements of the toad in material acquisition and exchange, vesicular transport, water-oxygen balance, and metabolic adjustment, whilst maintaining mucosal barrier homeostasis and fulfilling immune defense.
Our research identified an unexpected SELC pathway conducted by a secretory PFP in cell endocytic and exocytic systems with relevant physiological effects. With this newly obtained evidence, future studies should illustrate the possible conservation and variation of these novel cellular strategies and SELC pathways in interactions with and adaptations to the environment in other living organisms (especially vertebrates) and clarify their physiological relevance. Such studies will eventually lead to novel strategies and methods to combat human diseases, like cancer, atherosclerosis, neurodegeneration, and immune abnormalities.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.Z., Q.Q.W., Z.Z., and C.J.D. conceived and conceptualized the study; Y.Z. wrote the manuscript. Q.Q.W., Z.Z., and C.J.D. critically discussed and revised the manuscript for important intellectual content. Q.Q.W. and C.J.D. edited the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31572268, U1602225, 31872226) and Yunling Scholar Program to Y.Z.
References
- 1.Antonescu CN, McGraw TE, Klip A Reciprocal regulation of endocytosis and metabolism. Cold Spring Harbor Perspectives in Biology. 2014;6(7):a016964. doi: 10.1101/cshperspect.a016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, Zeitz M, et al Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells . The Journal of Infectious Diseases. 2011;204(8):1283–1292. doi: 10.1093/infdis/jir504. [DOI] [PubMed] [Google Scholar]
- 3.Banjara S, Suraweera CD, Hinds MG, Kvansakul M The Bcl-2 family: ancient origins, conserved structures, and divergent mechanisms. Biomolecules. 2020;10(1):128. doi: 10.3390/biom10010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbeau TR, Lillywhite HB Body wiping behaviors associated with cutaneous lipids in hylid tree frogs of Florida. The Journal of Experimental Biology. 2005;208(Pt 11):2147–2156. doi: 10.1242/jeb.01623. [DOI] [PubMed] [Google Scholar]
- 5.Broz P, Pelegrín P, Shao F The gasdermins, a protein family executing cell death and inflammation. Nature Reviews Immunology. 2020;20(3):143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 6.Burggren WW, Warburton S Amphibians as animal models for laboratory research in physiology. ILAR Journal. 2007;48(3):260–269. doi: 10.1093/ilar.48.3.260. [DOI] [PubMed] [Google Scholar]
- 7.Centeno FC, Antoniazzi MM, Andrade DV, Kodama RT, Sciani JM, Pimenta DC, et al Anuran skin and basking behavior: the case of the treefrog Bokermannohyla alvarengai (Bokermann, 1956) . Journal of Morphology. 2015;276(10):1172–1182. doi: 10.1002/jmor.20407. [DOI] [PubMed] [Google Scholar]
- 8.Chang CY, Thompson H, Rodman N, Bylander J, Thomas J Pathogenic analysis of Aeromonas hydrophila septicemia . Annals of Clinical and Laboratory Science. 1997;27(4):254–259. [PubMed] [Google Scholar]
- 9.Cirauqui N, Abriata LA, Van Der Goot FG, Dal Peraro M Structural, physicochemical and dynamic features conserved within the aerolysin pore-forming toxin family. Scientific Reports. 2017;7(1):13932. doi: 10.1038/s41598-017-13714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffman RL, Sher A, Seder RA Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conner SD, Schmid SL Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 12.Cossart P, Helenius A Endocytosis of viruses and bacteria. Cold Spring Harbor Perspectives in Biology. 2014;6(8):a016972. doi: 10.1101/cshperspect.a016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen PJ, Steinberg F To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nature Reviews Molecular Cell Biology. 2018;19(11):679–696. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- 14.Dal Peraro M, Van Der Goot FG Pore-forming toxins: ancient, but never really out of fashion. Nature Reviews Microbiology. 2016;14(2):77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 15.Dang LY, Rougé P, Van Damme EJM Amaranthin-like proteins with aerolysin domains in plants. Frontiers in Plant Science. 2017;8:1368. doi: 10.3389/fpls.2017.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Colibus L, Sonnen AFP, Morris KJ, Siebert CA, Abrusci P, Plitzko J, et al Structures of lysenin reveal a shared evolutionary origin for pore-forming proteins and its mode of sphingomyelin recognition. Structure. 2012;20(9):1498–1507. doi: 10.1016/j.str.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delbridge ARD, Grabow S, Strasser A, Vaux DL Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nature Reviews Cancer. 2016;16(2):99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 18.Deng CJ, Liu L, Liu LZ, Wang QQ, Guo XL, Lee WH, et al A secreted pore-forming protein modulates cellular endolysosomes to augment antigen presentation. FASEB Journal. 2020;34(10):13609–13625. doi: 10.1096/fj.202001176R. [DOI] [PubMed] [Google Scholar]
- 19.Doherty GJ, McMahon HT Mechanisms of endocytosis. Annual Review of Biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson JG, Porat-Shliom N, Cohen LA Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cellular Signalling. 2009;21(1):1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Carmeliet P Hypoxia and inflammation. The New England Journal of Medicine. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fivaz M, Abrami L, Tsitrin Y, Van Der Goot FG. 2001. Aerolysin from Aeromonas hydrophila and related toxins. In: van der Goot FG. Pore-Forming Toxins. Berlin, Heidelberg: Springer, 35−52.
- 23.Galinier R, Portela J, Moné Y, Allienne JF, Henri H, Delbecq S, et al Biomphalysin, a new β pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni . PLoS Pathogens. 2013;9(3):e1003216. doi: 10.1371/journal.ppat.1003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galvin BD, Kim S, Horvitz HR Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33 . Genetics. 2008;179(1):403–417. doi: 10.1534/genetics.108.087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Q, Xiang Y, Chen ZM, Zeng L, Ma XT, Zhang Y βγ-CAT, a non-lens betagamma-crystallin and trefoil factor complex, induces calcium-dependent platelet apoptosis. Thromb Haemost. 2011a;105(5):846–854. doi: 10.1160/TH10-10-0690. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Xiang Y, Zeng L, Ma XT, Lee WH, Zhang Y Characterization of the βγ-crystallin domains of βγ-CAT, a non-lens βγ-crystallin and trefoil factor complex, from the skin of the toad Bombina maxima . Biochimie. 2011b;93(10):1865–1872. doi: 10.1016/j.biochi.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Gao ZH, Deng CJ, Xie YY, Guo XL, Wang QQ, Liu LZ, et al Pore-forming toxin-like protein complex expressed by frog promotes tissue repair. FASEB Journal. 2019;33(1):782–795. doi: 10.1096/fj.201800087R. [DOI] [PubMed] [Google Scholar]
- 28.Gu ZZ, Noss EH, Hsu VW, Brenner MB Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. Journal of Cell Biology. 2011;193(1):61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo XL, Liu LZ, Wang QQ, Liang JY, Lee WH, Xiang Y, et al Endogenous pore-forming protein complex targets acidic glycosphingolipids in lipid rafts to initiate endolysosome regulation. Communications Biology. 2019;2(1):59. doi: 10.1038/s42003-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurcel L, Abrami L, Girardin S, Tschopp J, Van Der Goot FG Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126(6):1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Haslam IS, Roubos EW, Mangoni ML, Yoshizato K, Vaudry H, Kloepper JE, et al From frog integument to human skin: dermatological perspectives from frog skin biology. Biological Reviews. 2014;89(3):618–655. doi: 10.1111/brv.12072. [DOI] [PubMed] [Google Scholar]
- 32.He YY, Liu SB, Lee WH, Zhang Y Melanoma cell growth inhibition by βγ-CAT, which is a novel non-lens betagamma-crystallin and trefoil factor complex from frog Bombina maxima skin . Toxicon. 2008a;52(2):341–347. doi: 10.1016/j.toxicon.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 33.He YY, Liu SB, Qian JQ, Lee WH, Zhang Y Mechanism of βγ-CAT cell nuclear transportation and selectively killing of tumor cells. Zoological Research. 2008b;29(4):386–398. doi: 10.3724/SP.J.1141.2008.00386. [DOI] [Google Scholar]
- 34.Ho NI, Huis in 'T Veld LGM, Raaijmakers TK, Adema GJ Adjuvants enhancing cross-presentation by dendritic cells: the key to more effective vaccines? Frontiers in Immunology. 2018;9:2874. doi: 10.3389/fimmu.2018.02874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia N, Liu N, Cheng W, Jiang YL, Sun H, Chen LL, et al Structural basis for receptor recognition and pore formation of a zebrafish aerolysin-like protein. EMBO Reports. 2016;17(2):235–248. doi: 10.15252/embr.201540851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jørgensen CB Amphibian respiration and olfaction and their relationships: from Robert Townson (1794) to the present. Biological Reviews of the Cambridge Philosophical Society. 2000;75(3):297–345. doi: 10.1017/S0006323100005491. [DOI] [PubMed] [Google Scholar]
- 37.Jouette J, Guichet A, Claret SB Dynein-mediated transport and membrane trafficking control PAR3 polarised distribution. eLife. 2019;8:e40212. doi: 10.7554/eLife.40212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klumperman J, Raposo G The complex ultrastructure of the endolysosomal system. Cold Spring Harbor Perspectives in Biology. 2014;6(10):a016857. doi: 10.1101/cshperspect.a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovacs SB, Miao EA Gasdermins: effectors of pyroptosis. Trends in Cell Biology. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Droguen PM, Claret S, Guichet A, Brodu V Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system . Development. 2015;142(2):363–374. doi: 10.1242/dev.113472. [DOI] [PubMed] [Google Scholar]
- 41.Li SA, Liu L, Guo XL, Zhang YY, Xiang Y, Wang QQ, et al Host pore-forming protein complex neutralizes the acidification of endocytic organelles to counteract intracellular pathogens. The Journal of Infectious Diseases. 2017;215(11):1753–1763. doi: 10.1093/infdis/jix183. [DOI] [PubMed] [Google Scholar]
- 42.Lim JP, Gleeson PA Macropinocytosis: an endocytic pathway for internalising large gulps. Immunology & Cell Biology. 2011;89(8):836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 43.Lindenbergh MFS, Stoorvogel W Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annual Review of Immunology. 2018;36:435–459. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 44.Liu LS, Zhao LY, Wang SH, Jiang JP Research proceedings on amphibian model organisms. Zoological Research. 2016;37(4):237–245. doi: 10.13918/j.issn.2095-8137.2016.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu SB, He YY, Zhang Y, Lee WH, Qian JQ, Lai R, et al A novel non-lens βγ-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS One. 2008;3(3):e1770. doi: 10.1371/journal.pone.0001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Lieberman J Knocking 'em dead: pore-forming proteins in immune defense. Annual Review of Immunology. 2020;38:455–485. doi: 10.1146/annurev-immunol-111319-023800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llanses Martinez M, Rainero E Membrane dynamics in cell migration. Essays in Biochemistry. 2019;63(5):469–482. doi: 10.1042/EBC20190014. [DOI] [PubMed] [Google Scholar]
- 48.Lukoyanova N, Hoogenboom BW, Saibil HR The membrane attack complex, perforin and cholesterol-dependent cytolysin superfamily of pore-forming proteins. Journal of Cell Science. 2016;129(11):2125–2133. doi: 10.1242/jcs.182741. [DOI] [PubMed] [Google Scholar]
- 49.Magalhães GS, Lopes-Ferreira M, Junqueira-De-Azevedo ILM, Spencer PJ, Araújo MS, Portaro FCV, et al Natterins, a new class of proteins with kininogenase activity characterized from Thalassophryne nattereri fish venom . Biochimie. 2005;87(8):687–699. doi: 10.1016/j.biochi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Manzano S, Megías Z, Martínez C, García A, Aguado E, Chileh T, et al Overexpression of a flower-specific aerolysin-like protein from the dioecious plant Rumex acetosa alters flower development and induces male sterility in transgenic tobacco . The Plant Journal. 2017;89(1):58–72. doi: 10.1111/tpj.13322. [DOI] [PubMed] [Google Scholar]
- 51.McGonigal R, Barrie JA, Yao DG, McLaughlin M, Cunningham ME, Rowan EG, et al Glial sulfatides and neuronal complex gangliosides are functionally interdependent in maintaining myelinating axon integrity. The Journal of Neuroscience. 2019;39(1):63–77. doi: 10.1523/JNEUROSCI.2095-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medzhitov R Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 53.Meldolesi J Exosomes and ectosomes in intercellular communication. Current Biology. 2018;28(8):R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 54.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT Complement system part I - molecular mechanisms of activation and regulation. Frontiers in Immunology. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno-Layseca P, Icha J, Hamidi H, Ivaska J Integrin trafficking in cells and tissues. Nature Cell Biology. 2019;21(2):122–132. doi: 10.1038/s41556-018-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, et al Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505(7481):103–107. doi: 10.1038/nature12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa M, Takabatake T, Takahashi TC, Takeshima K Metamorphic change in EP37 expression: members of the βγ-crystallin superfamily in newt. Development Genes and Evolution. 1997;206(7):417–424. doi: 10.1007/s004270050071. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa M, Takahashi TC, Takabatake T, Takeshima K Isolation and characterization of a gene expressed mainly in the gastric epithelium, a novel member of the ep37 family that belongs to the βγ-crystallin superfamily . Development, Growth & Differentiation. 1998;40(5):465–473. doi: 10.1046/j.1440-169x.1998.t01-2-00001.x. [DOI] [PubMed] [Google Scholar]
- 59.Oggero S, Austin-Williams S, Norling LV The contrasting role of extracellular vesicles in vascular inflammation and tissue repair. Frontiers in Pharmacology. 2019;10:1479. doi: 10.3389/fphar.2019.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omersa N, Podobnik M, Anderluh G Inhibition of pore-forming proteins. Toxins. 2019;11(9):545. doi: 10.3390/toxins11090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouldali H, Sarthak K, Ensslen T, Piguet F, Manivet P, Pelta J, et al Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nature Biotechnology. 2020;38(2):176–181. doi: 10.1038/s41587-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palm W Metabolic functions of macropinocytosis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2019;374(1765):20180285. doi: 10.1098/rstb.2018.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palm W, Thompson CB Nutrient acquisition strategies of mammalian cells. Nature. 2017;546(7657):234–242. doi: 10.1038/nature22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang Y, Li CH, Wang SY, Ba W, Yu T, Pei GY, et al A novel protein derived from lamprey supraneural body tissue with efficient cytocidal actions against tumor cells. Cell Communication and Signaling. 2017;15(1):42. doi: 10.1186/s12964-017-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Podobnik M, Savory P, Rojko N, Kisovec M, Wood N, Hambley R, et al Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly. Nature Communications. 2016;7(1):11598. doi: 10.1038/ncomms11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian JQ, Liu SB, He YY, Lee WH, Zhang Y Acute toxicity of βγ-CAT, a naturally existing non-lens βγ-crystallin and trefoil factor complex from frog Bombina maxima skin secretions . Toxicon. 2008a;52(1):22–31. doi: 10.1016/j.toxicon.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Qian JQ, Liu SB, He YY, Lee WH, Zhang Y βγ-CAT, a non-lens βγ-crystallin and trefoil factor complex from amphibian skin secretions, caused endothelium-dependent myocardial depression in isolated rabbit hearts. Toxicon. 2008b;52(2):285–292. doi: 10.1016/j.toxicon.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 68.Rawat N, Pumphrey MO, Liu SX, Zhang XF, Tiwari VK, Ando K, et al Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight . Nature Genetics. 2016;48(12):1576–1580. doi: 10.1038/ng.3706. [DOI] [PubMed] [Google Scholar]
- 69.Record M, Silvente-Poirot S, Poirot M, Wakelam MJO Extracellular vesicles: lipids as key components of their biogenesis and functions. Journal of Lipid Research. 2018;59(8):1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero M, Keyel M, Shi GL, Bhattacharjee P, Roth R, Heuser JE, et al Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death & Differentiation. 2017;24(5):798–808. doi: 10.1038/cdd.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell AE, Sneider A, Witwer KW, Bergese P, Bhattacharyya SN, Cocks A, et al Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. Journal of Extracellular Vesicles. 2019;8(1):1684862. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadowski-Fugitt LM, Tracy CR, Christian KA, Williams JB Cocoon and epidermis of Australian Cyclorana frogs differ in composition of lipid classes that affect water loss . Physiological and Biochemical Zoology. 2012;85(1):40–50. doi: 10.1086/663695. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt F, Levin J, Kamp F, Kretzschmar H, Giese A, Bötzel K Single-channel electrophysiology reveals a distinct and uniform pore complex formed by α-synuclein oligomers in lipid membranes. PLoS One. 2012;7(8):e42545. doi: 10.1371/journal.pone.0042545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scita G, Di Fiore PP The endocytic matrix. Nature. 2010;463(7280):464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 75.Sekizawa Y, Kubo T, Kobayashi H, Nakajima T, Natori S Molecular cloning of cDNA for lysenin, a novel protein in the earthworm Eisenia foetida that causes contraction of rat vascular smooth muscle . Gene. 1997;191(1):97–102. doi: 10.1016/S0378-1119(97)00047-4. [DOI] [PubMed] [Google Scholar]
- 76.Shibata Y, Sano T, Tsuchiya N, Okada R, Mochida H, Tanaka S, et al Gene expression and localization of two types of AQP5 in Xenopus tropicalis under hydration and dehydration . American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2014;307(1):R44–R56. doi: 10.1152/ajpregu.00186.2013. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, Shibata Y, Ogushi Y, Okada R Molecular machinery for vasotocin-dependent transepithelial water movement in amphibians: aquaporins and evolution. The Biological Bulletin. 2015;229(1):109–119. doi: 10.1086/BBLv229n1p109. [DOI] [PubMed] [Google Scholar]
- 78.Swanson JA, King JS The breadth of macropinocytosis research. Philosophical Transactions of the Royal Society B: Biological Sciences. 2019;374(1765):20180146. doi: 10.1098/rstb.2018.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szczesny P, Iacovache I, Muszewska A, Ginalski K, Van Der Goot FG, Grynberg M Extending the aerolysin family: from bacteria to vertebrates. PLoS One. 2011;6(6):e20349. doi: 10.1371/journal.pone.0020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tandon P, Conlon F, Furlow JD, Horb ME Expanding the genetic toolkit in Xenopus: approaches and opportunities for human disease modeling . Developmental Biology. 2017;426(2):325–335. doi: 10.1016/j.ydbio.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tuma PL, Hubbard AL Transcytosis: crossing cellular barriers. Physiological Reviews. 2003;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 82.Van Der Goot FG, Hardie KR, Parker MW, Buckley JT The C-terminal peptide produced upon proteolytic activation of the cytolytic toxin aerolysin is not involved in channel formation. Journal of Biological Chemistry. 1994;269(48):30496–30501. doi: 10.1016/S0021-9258(18)43841-0. [DOI] [PubMed] [Google Scholar]
- 83.Van Niel G, D'Angelo G, Raposo G Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 84.Varga JFA, Bui-Marinos MP, Katzenback BA Frog skin innate immune defences: sensing and surviving pathogens. Frontiers in Immunology. 2018;9:3128. doi: 10.3389/fimmu.2018.03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang QQ, Bian XL, Zeng L, Pan F, Liu LZ, Liang JY, et al A cellular endolysosome-modulating pore-forming protein from a toad is negatively regulated by its paralog under oxidizing conditions. The Journal of Biological Chemistry. 2020;295(30):10293–10306. doi: 10.1074/jbc.RA120.013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Gu LQ, Tian K The aerolysin nanopore: from peptidomic to genomic applications. Nanoscale. 2018;10(29):13857–13866. doi: 10.1039/C8NR04255A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu FF, Feng B, Ren Y, Wu D, Chen Y, Huang SF, et al A pore-forming protein implements VLR-activated complement cytotoxicity in lamprey. Cell Discovery. 2017;3(1):17033. doi: 10.1038/celldisc.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu LG, Hamid E, Shin W, Chiang HC Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annual Review of Physiology. 2014;76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiang Y, Yan C, Guo XL, Zhou KF, Li SA, Gao Q, et al Host-derived, pore-forming toxin-like protein and trefoil factor complex protects the host against microbial infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(18):6702–6707. doi: 10.1073/pnas.1321317111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye CJ. 2020. Identification and Study of Ion Channel Functions of Natural Bioactive Substances. Ph.D. dissertation, The University of Chinese Academy of Sciences, China.
- 92.Yokoyama H, Kudo N, Todate M, Shimada Y, Suzuki M, Tamura K Skin regeneration of amphibians: a novel model for skin regeneration as adults. Development, Growth & Differentiation. 2018;60(6):316–325. doi: 10.1111/dgd.12544. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y Why do we study animal toxins? Zoological Research. 2015;36(4):183–222. doi: 10.13918/j.issn.2095-8137.2015.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Yu GY, Wang YJ, Xiang Y, Gao Q, Jiang P, et al Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cellular and Molecular Life Sciences. 2011;68(22):3771–3780. doi: 10.1007/s00018-011-0678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang YX, Lai R, Lee WH, Zhang Y Frog albumin is expressed in skin and characterized as a novel potent trypsin inhibitor. Protein Science. 2005;14(9):2469–2477. doi: 10.1110/ps.051551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao F, Yan C, Wang X, Yang Y, Wang GY, Lee WH, et al Comprehensive transcriptome profiling and functional analysis of the frog (Bombina maxima) immune system . DNA Research. 2014;21(1):1–13. doi: 10.1093/dnares/dst035. [DOI] [PMC free article] [PubMed] [Google Scholar]