Abstract
Introduction: Since the declaration of coronavirus disease 2019 (COVID-19) as a pandemic, patients with dementia, were specifically vulnerable to the negative impact of the outbreak. Objective: To examine the association between lockdown amid COVID-19 pandemic and the rate of cognitive decline among patients with dementia and mild cognitive impairment (MCI). Methods: We conducted a cross-sectional observational study on patients with dementia and MCI who attended the outpatient clinic at Ibn Sina Hospital, the main tertiary neurology center in Kuwait, during the month of September 2020. The rate of cognitive decline, estimated by MMSE scores, was compared between the period prior to, and during lockdown. Results: We evaluated 36 consecutive patients with cognitive impairment (23 females [63.9%], mean age 71 ± 10.8 years, mean disease duration 34.6 ± 29 months). Eleven patients (30.6%) progressed to a more severe stage during the study period; 1 MCI (2.8%) converted to mild dementia, 6 (16.6%) mild to moderate, and 4 (11.1%) moderate to severe dementia. Monthly decline of MMSE scores before lockdown was 0.2 ± 0.1 points, while it was 0.53 ± 0.3 points during lockdown, which was statistically significant (p = .001). The most affected cognitive domain was the memory with a mean decline of 1.5 ± 0.8 points. Conclusions: This study provides “real-world” data suggesting rapid cognitive decline in patients with dementia during the lockdown period. Healthcare systems should pay more attention to this vulnerable group, to help them maintain their mental, physical and social well-being during this crisis.
Keywords: COVID-19, dementia, memory, lockdown, pandemic, cognition
Introduction
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected in Wuhan, China in late 2019. On March 11, 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) to be a pandemic of “alarming levels of spread and severity” (Guan et al., 2020). It has been documented through historical records that elderly populations in particular suffer the most during pandemics. Patients with chronic diseases, particularly those with dementia, were specifically vulnerable to the negative impact of this unprecedented healthcare crisis. Countries were forced to implement nationwide social distancing and lockdown measures, in order to slow down the spread of infection. However, these same measures exerted unintended secondary effects that could worsen the cognitive functions in this group of patients (Numbers & Brodaty, 2021). During the first 6 months of the pandemic, elderly patients were kept apart from their families and from the rest of the community, which has led to marked disruption of the social relationships of both patients and caregivers. Furthermore, with the cancellation of in-person hospital visits, dementia patients were more prone to inadequate quality medical care (Wang et al., 2020).
Dementia is a progressive neurocognitive disorder that affects memory, language, behavior, and the ability to perform everyday activities. It is one of the major causes of disability that significantly impacts the quality of life, as well as the ability to live independently (Kivimäki & Singh-Manoux, 2018). Cases of dementia are increasing globally due to the longer life expectancy of the world population. This rise by itself is considered a pandemic, with more than 50 million people affected worldwide, and one new case diagnosed every 3 seconds (Batsch & Mittelman, 2015). The association between dementia and COVID-19 pandemic increases morbidity and mortality in this population, and subjects these patients to the risk of significant harm. Little research has been conducted to evaluate the negative impact of pandemics on cognition, and to the best of our knowledge, only one study (Palermo et al., 2020) has been performed on COVID-19 pandemic in particular.
In this observational study, the overall aim was to investigate the impact of social distancing and lockdown measures amid COVID-19 pandemic on the rate of cognitive decline in patients with dementia and mild cognitive impairment attending outpatient clinic in the largest neurology tertiary hospital in Kuwait.
Methods
Participants
This observational, cross-sectional study, included all patients with cognitive impairment who attended the outpatient clinic in Ibn Sina Hospital, the main tertiary neurology center in Kuwait, during the month of September, 2020.
Inclusion criteria were (i) patients with a diagnosis of mild cognitive impairment (MCI), Alzheimer dementia (AD), frontotemporal dementia (FTD), Lewy body dementia (DLB), Parkinson’s disease dementia (PDD), vascular dementia (VaD), or mixed dementia (MD), (ii) cognitive functions were assessed and recorded using MMSE at disease onset, and during the month prior to lockdown (February 2020). Patients were excluded if their records were incomplete, or their cognitive impairment was secondary to other diseases (e.g., normal pressure hydrocephalus, subdural hematoma, brain tumors).
Measures
Data of demographics (age, gender, marital status, educational level), and clinical characteristics (dementia type, duration, severity, medications, compliance to treatment, medical comorbidities, and comorbid depression) were included.
We evaluated cognitive decline using mini-mental state examination (MMSE) scores. The MMSE has a maximum score of 30, with five different domains of cognitive functions: (1) Orientation, with a maximum of 10 points, (2) Memory, with a maximum of 6 points, (3) Attention and calculation, with a maximum of 5 points, (4) Language, with a maximum of 8 points, and (5) Design copying, with a maximum of 1 point. MMSE score denotes severity of cognitive impairment as follows; mild: MMSE 21 to 24, moderate: MMSE 10 to 20, severe: MMSE less than 10. The MMSE was conducted and scored by one neurologist with experience in dementia for each patient (authors; I.I.I and W.A.K). Disease progression and the rate of cognitive decline (the estimated MMSE point decrease per month per period) were used as the main clinical outcomes in this study.
The rate of cognitive decline during lockdown period (from penultimate MMSE assessment in the month prior to lockdown, and last MMSE assessment after lockdown) was compared to the rate of decline from disease onset to the penultimate MMSE assessment.
The lockdown was implemented by the state of Kuwait at midnight of March 11, 2020, and a full nationwide lockdown was implemented on May 10, 2020.
We evaluated comorbid depression using the Patient Health Questionnaire-9 (PHQ-9) scores. It is a 9-item depression screening module, with a score ranging from (0 to 27); 0 to 4 is normal, 5 to 9 is mild, 10 to 14 is moderate, 15 to 19 is moderately severe, and 20 to 27 is severe depression. We explored the association of several sociodemographic and clinical factors, with the rate of cognitive decline in the cohort.
The study was approved by the Institutional Review Board Committee. All patients and/or caregivers provided written informed consent to participate in the study.
Statistical Analysis
Data were analyzed using IBM SPSS software package version 25.0 (Armonk, NY: IBM Corp). The Kolmogorov–Smirnov, Shapiro, and D’Agstino tests were used to verify the normality of distribution of variables. Wilcoxon signed ranks test was assessed for comparison between the two periods before and after lockdown for abnormally distributed quantitative variables. Mann–Whitney test was used to compare between the two groups for not normally distributed quantitative variables. Kruskal Wallis test was used to compare different groups for not normally distributed quantitative variables. Spearman coefficient was used to correlate between quantitative variables. Significance of the obtained results was set at p < .05 level.
Results
We evaluated 36 consecutive patients with cognitive impairment (23 females (63.9%), 20 married (55.6%), mean age 71 ± 10.8 years, mean disease duration 34.6 ± 29 months) during the month of September 2020. The demographic and clinical characteristics are summarized in Table 1. Of them, 17 patients (47.2%) had Alzheimer’s disease, 7 (19.4%) had mixed dementia, 7 (19.4%) had Parkinson’s disease dementia, 3 (8.3%) had mild cognitive impairment, and 2 (5.6%) had Lewy body dementia. No patient had FTD or pure VaD during the study period. Treatment compliance was reported by most patients (88.9%), with memantine (55.6%), and rivastigmine (50%) being the most frequently prescribed drugs.
Table 1.
Distribution of the Studied Cases According to Different Parameters (n = 36).
| No. (%) | Mean ± SD | Median (min.–max.) | |
|---|---|---|---|
| Gender | |||
| Male | 13 (36.1) | ||
| Female | 23 (63.9) | ||
| Age (years) | 71 ± 10.8 | 70 (40–91) | |
| Educational level | |||
| Primary school or less | 10 (27.8) | ||
| High school | 15 (41.6) | ||
| University | 11 (30.6) | ||
| Diagnosis | |||
| MCI | 3 (8.3) | ||
| PDD | 7 (19.4) | ||
| DLB | 2 (5.6) | ||
| Alzheimer’s disease | 17 (47.2) | ||
| Mixed dementia | 7 (19.4) | ||
| Disease duration (months) | 34.6 ± 29 | 24 (11–140) | |
| Marital status | |||
| Single | 3 (8.3) | ||
| Married | 20 (55.6) | ||
| Divorced | 3 (8.3) | ||
| Widow | 10 (27.8) | ||
| Treatment | |||
| No | 4 (11.1) | ||
| Yes | 32 (88.9) | ||
| Memantine | 20 (55.6) | ||
| Donepezil | 8 (22.2) | ||
| Rivastigmine | 18 (50) | ||
| Comorbidity | |||
| No | 10 (27.8) | ||
| Yes | 26 (72.2) | ||
| Cancer | 1 (2.8) | ||
| Diabetes mellitus | 15 (41.7) | ||
| Hypertension | 20 (55.6) | ||
| Stroke | 5 (13.9) | ||
| Thyroid disease | 1 (2.8) | ||
| Depression | 9 (25) | ||
| COVID 19 infection | 1 (2.8) | ||
| Cognitive domains decline | |||
| Orientation | 0.4 ± 0.5 | 0 (0–1) | |
| Memory | 1.5 ± 0.8 | 1.5 (0–3) | |
| Attention and calculation | 0.5 ± 0.6 | 0 (0–2) | |
| Language | 0.2 ± 0.4 | 0 (0–1) | |
| Design copying | 0.2 ± 0.4 | 0 (0–2) | |
| Compliance to treatment | 31 (86.1) | ||
| MMSE scores | |||
| Initial | 20.9 ± 3.9 | 22 (10–26) | |
| Before lockdown | 17.3 ± 4.9 | 18 (8–25) | |
| MCI | 3 (8.3) | ||
| Mild dementia | 13 (36.1) | ||
| Moderate dementia | 17 (47.2) | ||
| Severe dementia | 3 (8.3) | ||
| After lockdown | 13.9 ± 4.9 | 14 (5–24) | |
| MCI | 2 (5.6) | ||
| Mild dementia | 8 (22.2) | ||
| Moderate dementia | 19 (52.8) | ||
| Severe dementia | 7 (19.4) | ||
| PHQ-9 | 3.9 ± 3.5 | 3 (0–16) | |
Twenty-six patients (72.2%) had medical co-morbidities; 20 (55.6%) had hypertension, 15 (41.7%) had diabetes mellitus, 5 (13.9%) had history of stroke, 1 (2.8%) had thyroid disease, and one patient (2.8%) had breast cancer. Depression was found in nine patients (25%), and mean PHQ-9 was 3.9 ± 3.5 points. Only one patient had history of COVID-19 infection in our study, with an uncomplicated course.
Before lockdown, three patients (8.3%) had MCI, 13 (36.1%) had mild dementia, 17 (47.2%) had moderate dementia, and 3 (8.3%) had severe dementia. After lockdown, two patients (5.6%) had MCI, 8 (22.2%) had mild dementia, 19 (52.8%) had moderate dementia, and 7 (19.4%) had severe dementia. Disease severity and stage was changed in 11 patients (30.6%) during the study period; 1 MCI (2.8%) converted to mild dementia, 6 (16.6%) mild converted to moderate, and 4 (11.1%) moderate converted to severe dementia. Mean MMSE at disease onset was 20.9 ± 3.9, mean penultimate MMSE prior to lockdown (disease duration; 28.4 ± 21 months) was 17.3 ± 4.9, and mean MMSE after lockdown was 13.9 ± 4.9.
The monthly decline of MMSE scores before lockdown (from disease onset to penultimate visit) was 0.2 ± 0.1 points, while the monthly MMSE decline during lockdown period was 0.53 ± 0.3 points, which was statistically significant (p = .001). Findings are illustrated in Table 2 and Figure 1. We calculated a mean of decline of MMSE scores of (1.2 points/6 months) before lockdown, and (3.1 points/6 months) during the lockdown period from March to August 2020. The most affected cognitive domain during lockdown was the memory with a mean decline of (1.5 ± 0.8) points, followed by attention and calculation (0.5 ± 0.6) points, orientation (0.4 ± 0.5) points, design copying (0.2 ± 0.4) points, and language (0.2 ± 0.4) points.
Table 2.
Comparison between the Two Studied Periods According to Monthly Decline of MMSE Scores (n = 36).
| MMSE | Monthly decline from initial assessment to before lockdown | Monthly decline from before to after lockdown | Z | p Value |
|---|---|---|---|---|
| Mean ± SD | 0.2 ± 0.1 | 0.53 ± 0.3 | 4.997* | <.001* |
| Median (min.–max.) | 0.2 (0–0.6) | 0.5 (0–1.3) |
Z = Wilcoxon signed ranks test.
p-Value for comparing between monthly decline of MMSE from initial to before lockdown, and from before to after lockdown.
Statistically significant at p ≤ .05.
Figure 1.
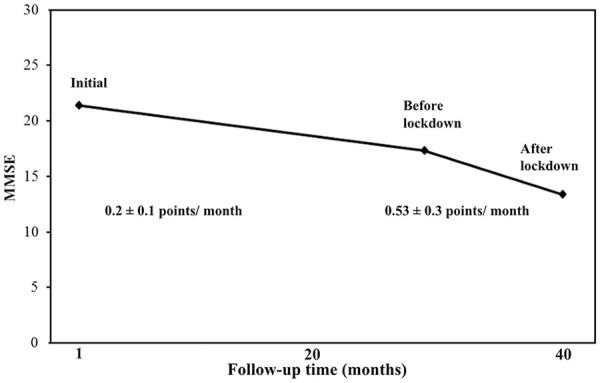
Monthly decline of MMSE scores before and during lockdown during the pandemic.
However, we found no statistically significant correlation between the rate of decline of cognitive functions during the lockdown period and age (rs = 0.242, p = .156), gender (U = 0.023, p = .880), marital status (H = 0.465, p = .927), educational level (H = 0.542, p = .815), dementia type (H = 5.870, p = .209), dementia severity (H = 0.135, p = .098), disease duration (rs = 0.186, p = .278), medical comorbidity (U = 122.0, p = .794), comorbid depression (U = 115.0, p = .830), and treatment compliance (U = 47.0, p = .175).
Discussion
In this study, the cognitive functions in patients with dementia and MCI declined more rapidly during lockdown, in comparison to the pre-pandemic period. Around third of patients with dementia converted to a more severe stage, and one MCI patient converted to dementia.
The definition of “rapid” or “aggressive” decline in cognitive functions is still debatable in the literature. The operational threshold of decline of MMSE scores ranged from 3 points/year (Carcaillon et al., 2007), to 3 points/6 months (Soto et al., 2008). However, in clinical practice, any drop in MMSE scores that exceeds the average points drop of (2.5–3/year) in AD and (1/year) in MCI could be considered as “rapid” (Schmidt et al., 2011; Tosto et al., 2014). In our study, a mean drop of 3.1 points in 6 months would be considered “rapid” by most definitions, and it was statistically significant. It showed that an accelerated rate of cognitive decline occurred during lockdown period amid the outbreak. However, this accelerated decline was not correlated with any of the assessed clinical variables in our cohort, probably because of the small number of patients enrolled. To the best of our knowledge, only one study evaluated the real-world impact of COVID-19 pandemic on cognitive functions. However, it evaluated only patients with Parkinson’s disease (Palermo et al., 2020). Similar to our findings, the mean MMSE scores declined from (January–February 2020) to (June–July 2020) from 25.5 ± 1.4 to 24.9 ± 1.3 in MCI, and from 21.1 ± 1.9 to 20.8 ± 2.0 in PDD patients, with one MCI subject converted to PDD.
The lockdown measures are believed to worsen cognitive functions in patients with dementia by several means. Social deprivation and the lack of interaction with families and friends during home confinement can be considered a major cause. Evidence from longitudinal studies suggest that participation in social activity is a potential modifiable risk factor for cognitive decline. The more socially active older adults, the less cognitive decline they experience, and vice versa (James et al., 2011). Some studies further suggest that multi-sensory cognitive stimulation, termed “environmental enrichment,” can induce hippocampal neurogenesis and synaptic plasticity through epigenetic changes, in addition to increase brain resilience to stress and cognitive reserve (Hampel and Vergallo, 2020).
Research have also shown that perceived social isolation (i.e., loneliness) is a risk factor for poor overall cognitive performance, faster cognitive decline, and increased depression (Tilvis et al., 2004, Wilson et al., 2007). A recent Dutch study (Van Tilburg et al., 2020) on 1,679 community-dwelling participants aged 65 to 102 years showed significantly higher levels of loneliness during lockdown, compared to pre-pandemic period. Moreover, the pandemic can be perceived at a societal level as a source of major stress. Ongoing stress creates a vicious circle that is known to accelerate dementia progression (Peavy et al., 2012). Similar to other stressors, fear of COVID-19 contagion can causes cognitive impairments in different domains namely memory and learning (Schwabe et al., 2012), attention (Dutra et al., 2018), and executive domains (Zarrabian & Hassani-Abharian, 2020). In our study, memory impairment was the most affected cognitive domain. This was similar to a recent study (Stewart et al., 2020) that assessed the impact of loneliness on cognitive functions, where working memory in particular was found to be the most significantly affected. This can put dementia patients at higher risk of COVID-19 infection, through difficulties in remembering safety measures, such as wearing masks, understanding the public health messages, or by restlessness and wandering.
Several studies have documented deterioration in the mental health of normal population, as well as patients with chronic illnesses during lockdown (Al-Hashel & Ismail, 2020; Galea et al., 2020). A recent study (El Haj et al., 2020) on 58 patients with AD showed significantly higher levels of depression and anxiety during lockdown in comparison to pre-pandemic period. Patients with MCI or mild dementia can develop anxiety, apathy or depression, while those with severe dementia can develop behavioral symptoms, such as agitation, or wandering (Brown et al., 2020). Moreover, a review article published in October 2020 (Devita et al., 2020) have shown that, in older adults with dementia, psychiatric symptoms caused by social isolation amid COVID-19 lockdown are linked to more severe neuropsychiatric and behavioral disturbances.
Another additional factor is the enforced lack of physical activity, due to movement restrictions. In a meta-analysis of 15 prospective studies, Sofi et al. (2011) documented an inverse relationship between physical activity and cognitive function. Furthermore, cancellation of in-person hospital visits, the interruption of cognitive training interventions and physiotherapy programs, and limited knowledge of telecommunication, can increase the complex impact of both COVID-19 pandemic and dementia.
Strengths and Limitations
To the best of our knowledge, this is the first study to provide “real-world” data regarding the impact of COVID-19 pandemic on patients with different types of dementia. We evaluated the overall rate of cognitive decline in those patients, in addition to the preferential effect on different cognitive domains. However, we are aware that this study had some limitations. First, the number of patients is small, and the sample came from a single-center, which might limit the generalizability of our results. This initial cross-sectional observational study requires replication in a larger cohort before it can be substantively interpreted.
Secondly, cognitive functions were assessed using MMSE, which has some important shortcomings. It overlooks age, education, cultural and socioeconomic variables, in addition to decrease sensitivity in mild and severe dementia. It is also well-known for “ceiling effect” when used for poorly educated people and “floor effect” for those with higher education (Franco-Marina et al., 2010). Moreover, we applied the average MMSE decline to the whole sample, which might not account for individual variabilities. However, the advantages of the MMSE are that it is brief, widely accepted, easily administered in the clinic, and can be used in studies for comparison in a wide variety of settings and circumstances.
A third limitation, given our specific aims and methodology, is the lack of information concerning other additional clinical factors that could influence disease progression (e.g., brain imaging, laboratory investigations). Nevertheless, there was no change in neurological examination of those patients in comparison to their penultimate visits, and there was no history of acute deterioration or delirium.
Conclusion
Despite limitations, this study provides “real-world” data on the negative impact of COVID-19 pandemic and its related restrictive measures on patients with dementia and MCI. We have demonstrated a significant acceleration of cognitive decline in those patients, which needs to be confirmed in a larger cohort over a longer period of time. Patients with dementia faced drastic changes in their lives during lockdown, from social isolation to enforced physical inactivity, which could be responsbile for worsening of their psychological and cognitive status. The consequences of this pandemic must be closely monitored, and healthcare systems should pay more attention to this vulnerable group, to help them maintain their mental, physical and social well-being during this crisis. People with dementia should receive premeditated care and support from healthcare facilities, as well as caregivers, while they are kept safe at their homes. Keeping them connected (even if virtual), and using multi-sensory cognitive stimulation is more important now than ever.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ismail Ibrahim Ismail  https://orcid.org/0000-0002-9788-7044
https://orcid.org/0000-0002-9788-7044
References
- Al-Hashel J. Y., Ismail I. I. (2020). Impact of coronavirus disease 2019 (COVID-19) pandemic on patients with migraine: A web-based survey study. The Journal of Headache and Pain, 21(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsch N. L., Mittelman M. S. (2015). World Alzheimer report 2012. Overcoming the stigma of dementia. Alzheimer’s Disease International (ADI). [Google Scholar]
- Brown E. E., Kumar S., Rajji T. K., Pollock B. G., Mulsant B. H. (2020). Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. The American Journal of Geriatric Psychiatry, 28(7), 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcaillon L., Pérès K., Péré J. J., Helmer C., Orgogozo J. M., Dartigues J. F. (2007). Fast cognitive decline at the time of dementia diagnosis: A major prognostic factor for survival in the community. Dementia and Geriatric Cognitive Disorders, 23(6), 439–445. [DOI] [PubMed] [Google Scholar]
- Devita M., Bordignon A., Sergi G., Coin A. (2020). The psychological and cognitive impact of Covid-19 on individuals with neurocognitive impairments: Research topics and remote intervention proposals. Aging Clinical and Experimental Research, 33(3), 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra S. J., Marx B. P., McGlinchey R., DeGutis J., Esterman M. (2018). Reward ameliorates posttraumatic stress disorder-related impairment in sustained attention. Chronic Stress, 2, 2470547018812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M., Altintas E., Chapelet G., Kapogiannis D., Gallouj K. (2020). High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Research, 291, 113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Marina F., García-González J. J., Wagner-Echeagaray F., Gallo J., Ugalde O., Sánchez-García S., Espinel-Bermúdez C., Juárez-Cedillo T., Rodríguez M. A. V., García-Peña C. (2010). The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. International Psychogeriatrics, 22(1), 72. [DOI] [PubMed] [Google Scholar]
- Galea S., Merchant R. M., Lurie N. (2020). The mental health consequences of COVID-19 and physical distancing: The need for prevention and early intervention. JAMA Internal Medicine, 180(6), 817–818. [DOI] [PubMed] [Google Scholar]
- Guan W. J., Ni Z. Y., Hu Y., Liang W. H., Ou C. Q., He J. X., Liu L., Shan H., Lei C.-L., Hui D. S. C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-c., Tang C.-l., Wang T., Chen P.-y., Xiang J., . . . Zhong N. S. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18),1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Vergallo A. (2020). The Sars-Cov-2 pandemic and the brave new digital World of Environmental Enrichment to prevent brain aging and cognitive decline. The Journal of Prevention of Alzheimers Disease 7(4), 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B. D., Wilson R. S., Barnes L. L., Bennett D. A. (2011). Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society, 17(6), 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäki M., Singh-Manoux A. (2018). Prevention of dementia by targeting risk factors. The Lancet, 391(10130), 1574–1575. [DOI] [PubMed] [Google Scholar]
- Numbers K., Brodaty H. (2021). The effects of the COVID-19 pandemic on people with dementia. Nature Reviews Neurology, 17(2), 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo G., Tommasini L., Baldacci F., Del Prete E., Siciliano G., Ceravolo R. (2020). Impact of coronavirus disease 2019 pandemic on cognition in Parkinson’s disease. Movement Disorders, 35(10), 1717–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy G. M., Jacobson M. W., Salmon D. P., Gamst A. C., Patterson T. L., Goldman S., Mills P. J., Khandrika S., Galasko D. (2012). The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Disease and Associated Disorders, 26(3), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Joëls M., Roozendaal B., Wolf O. T., Oitzl M. S. (2012). Stress effects on memory: an update and integration. Neuroscience & Biobehavioral Reviews, 36(7), 1740–1749. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Wolff M., Weitz M., Bartlau T., Korth C., Zerr I. (2011). Rapidly progressive Alzheimer disease. Archives of Neurology, 68(9), 1124–1130. [DOI] [PubMed] [Google Scholar]
- Sofi F., Valecchi D., Bacci D., Abbate R., Gensini G. F., Casini A., Macchi C. (2011). Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine, 269(1), 107–117. [DOI] [PubMed] [Google Scholar]
- Soto M. E., Andrieu S., Cantet C., Reynish E., Ousset P. J., Arbus C., Gillette-Guyonnet S., Nourhashémi F., Real FR Group., Vellas B. (2008). Predictive value of rapid decline in mini mental state examination in clinical practice for prognosis in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 26(2), 109–116. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Yu L., Glover C. M., Mottola G., Bennett D. A., Wilson R. S., Boyle P. A. (2020). Loneliness interacts with cognition in relation to healthcare and financial decision making among community-dwelling older adults. The Gerontologist, 60(8), 1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilvis R. S., Kähönen-Väre M. H., Jolkkonen J., Valvanne J., Pitkala K. H., Strandberg T. E. (2004). Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59(3),M268–M274. [DOI] [PubMed] [Google Scholar]
- Tosto G., Zimmerman M. E., Carmichael O. T., Brickman A. M. (2014). Predicting aggressive decline in mild cognitive impairment: The importance of white matter hyperintensities. JAMA Neurology, 71(7), 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tilburg T. G., Steinmetz S., Stolte E., van der Roest H., de Vries D. H. (2020). Loneliness and mental health during the COVID-19 pandemic: A study among Dutch older adults. The Journals of Gerontology: Series B. Advance online publication. 10.1093/geronb/gbaa111 [DOI] [PMC free article] [PubMed]
- Wang H., Li T., Barbarino P., Gauthier S., Brodaty H., Molinuevo J. L., Xie H., Sun Y., Rang Y., Yu E., Weidner W., Yu X. (2020). Dementia care during COVID-19. The Lancet, 395(10231), 1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Krueger K. R., Arnold S. E., Schneider J. A., Kelly J. F., Barnes L. L., Tang Y., Bennett D. A. (2007). Loneliness and risk of Alzheimer disease. Archives of General Psychiatry, 64(2), 234–240. [DOI] [PubMed] [Google Scholar]
- Zarrabian S., Hassani-Abharian P. (2020). COVID-19 pandemic and the importance of cognitive rehabilitation. Basic and Clinical Neuroscience, 11(2), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]


