Abstract
Green tea (GT) treatment was evaluated for its effect on the immune and antineoplastic response of elderly acute myeloid leukemia patients with myelodysplasia-related changes (AML-MRC) who are ineligible for aggressive chemotherapy and bone marrow transplants. The eligible patients enrolled in the study (n = 10) received oral doses of GT extract (1000 mg/day) alone or combined with low-dose cytarabine chemotherapy for at least 6 months and/or until progression. Bone marrow (BM) and peripheral blood (PB) were evaluated monthly. Median survival was increased as compared to the control cohort, though not statistically different. Interestingly, improvements in the immunological profile of patients were found. After 30 days, an activated and cytotoxic phenotype was detected: GT increased total and naïve/effector CD8+ T cells, perforin+/granzyme B+ natural killer cells, monocytes, and classical monocytes with increased reactive oxygen species (ROS) production. A reduction in the immunosuppressive profile was also observed: GT reduced TGF-β and IL-4 expression, and decreased regulatory T cell and CXCR4+ regulatory T cell frequencies. ROS levels and CXCR4 expression were reduced in bone marrow CD34+ cells, as well as nuclear factor erythroid 2–related factor 2 (NRF2) and hypoxia-inducible factor 1α (HIF-1α) expression in biopsies. Immune modulation induced by GT appears to occur, regardless of tumor burden, as soon as 30 days after intake and is maintained for up to 180 days, even in the presence of low-dose chemotherapy. This pilot study highlights that GT extracts are safe and could improve the immune system of elderly AML-MRC patients.
Keywords: green tea, polyphenols, acute myeloid leukemia with myelodysplasia-related changes, immune response, reactive oxygen species
Introduction
Acute myeloid leukemia (AML) is one of the most common hematological malignancies, primarily affecting older adults, with a median age at diagnosis of 69 years. AML may occur as a progression of myelodysplastic syndromes (MDS), denominated AML with myelodysplasia-related changes (AML-MRC). AML-MRC has a worse outcome than de novo AML.1 Elderly AML-MRC patients are usually not candidates for bone marrow (BM) transplants nor eligible for intensive chemotherapy.2 Hypomethylating agents such as azacytidine are the current mainstay of therapy for advanced MDS and AML in the elderly; however the overall survival is not satisfactory and hematological responses occur in no more than 30% of patients.3 Treatment options for these patients are based on reduced intensity chemotherapy, such as low-dose cytarabine, best supportive care possible, and/or enrollment in clinical trials4 which encourages the search for new therapeutic approaches.
Polyphenols are natural compounds distributed in fruits, vegetables, cereals, oils, and especially in tea, the most consumed beverage in the world, second only to water for its human health-promoting characteristics. These compounds are known to have potential biological activity against cancers, diabetes, and cardiovascular disease.5 Among the common polyphenol-rich sources, green tea (GT) extract has been reported to modify proliferation, apoptosis, and other hallmarks of carcinogenesis.5,6 GT extracts contains approximately 50% of polyphenols (known as catechins) and epigallocatechin-3-gallate (EGCG) is generally considered to be the main active catechin in green tea.7 Oral preparations of GT catechins have been reported to present an acceptable safety profile in healthy individuals,8,9 which validates GT as an attractive candidate for AML-MRC.
Studies have reported that GT catechins have antitumor effects on hematologic malignancies such as chronic lymphocytic leukemia.10-13 Pre-clinical studies have reported anti-proliferative and pro-apoptotic effects of GT catechins in myeloid leukemia cell lines,14-17 AML xenograft mice14,15,18 and leukemia experimental models.19 However, clinical studies of the effects of GT catechins on AML are unavailable. Moreover, commercial GT products have been widely promoted and are consumed by a significant number of patients, rendering the determination of the effect of GT extract on AML-MRC important.
Herein, we investigated the potential role of GT extract on the immune and antineoplastic response of elderly AML-MRC patients, ineligible for aggressive chemotherapy and BM transplants.
Methods
Study Design and Participants
Elderly AML-MRC patients unfit for conventional chemotherapy and BM transplants using subcutaneous low-dose cytarabine were enrolled in this non-randomized, interventional, open-label, single arm, phase II pilot study. Patients diagnosed from October 2012 to December 2015 at the Hematology and Transfusion Medicine Center, University of Campinas Brazil were recruited and enrolled by the hematologists who assigned the participants to interventions. Eligibility criteria were patients over 60 years of age with AML-MRC according the 2016 World Health Organization classification; Eastern Cooperative Oncology Group performance status 0 to 3; informed consent, personally signed (or by the legal responsible person) and dated, to participate in the study; capability of complying with the study procedures and follow-up examinations; latest chemotherapy regimen at least 60 days before. No use of hydroxyurea was allowed. Exclusion criteria included patients with psychiatric disorders that could interfere with consent, study participation, or follow-up; chronically impaired renal function (creatinine clearance <30 ml/minute); both inadequate liver function (serum alanine aminotransferase and aspartate aminotransferase >2.5 × upper limit of normality) and total bilirubin >1.5 × upper limit of normality, if not caused by leukemic infiltration were excluded. Patients with known human immunodeficiency virus disease or active hepatitis C infection; diagnosed with another malignancy; history of organ allograft; patients with an indication for and capable of undergoing a non-myeloablative transplant procedure; cardiac disease resulting in heart failure (New York Heart Association class III or IV); unstable coronary artery disease (myocardial infarction more than 6 months prior to study entry was permitted); serious cardiac ventricular arrhythmias requiring anti-arrhythmic therapy (beta blockers or digoxin were permitted) were also excluded. All patients provided written informed consent for the study participation and data collection. Patient sample collections, patient consent and recruitment were in accordance with the protocols approved by the Institutional Ethics Committee of the University of Campinas (#14290713.8.0000.5404) and were registered in the Brazilian Registry of Clinical Trials (RBR-2tstxd). This study was conducted according to the Declaration of Helsinki.
Study Protocol
AML-MRC enrolled patients (n = 10) received 2 capsules of GT extract totaling 1000 mg once daily for at least 6 months and/or until progression. Subcutaneous low-dose cytarabine (10 mg/m2 every 12 hour for 14 consecutive days) was introduced according to tolerance; frail patients received GT alone. Each flask of cytarabine contained sterile solution of 100 mg/ml cytarabine for intravenous and subcutaneous use (Accord Farmacêutica Ltda., Brazil). GT extract (from the leaves of Camellia sinensis (L.) Kuntze), standardized to 50% polyphenols, 30% catechins, 15% EGCG by HPLC (Galena, Brazil), was packed in capsules containing 500 mg. Microbiological and toxicological analyses were carried out to control the presence of eventual degradation products, heavy metals, or any other unacceptable contaminants. The application of the GT standardized extract dose of 1000 mg once daily was based on phase I/II clinical trials.12,13
BM and peripheral blood (PB) were evaluated monthly for safety and immune cell modifications. Responses were classified in accordance to the International Working Group for therapeutic trials in AML.20 Clinical evaluations and laboratory studies for potential toxicities, including serum liver enzymes, bilirubin, creatinine, uric acid, were performed weekly. Sample size estimation was performed according to the 2-stage Simon design with a futility threshold of 10 patients for the first part.
For survival comparison, we retrospectively analyzed clinical data from 12 AML-MRC patients (control cohort) diagnosed from December 2008 to January 2017 at the same Institution and treated by the same team with the same conventional care regimens including transfusion therapy and, when possible, low-dose cytarabine chemotherapy. Patients with early death (before 1 month of therapy) were excluded from the cohort.
Flow Cytometry Analysis
BM and PB samples were incubated for 30 minutes at room temperature with the following specific antibodies: CD3-Peridinin-chlorophyll-protein (PerCP); CD4-Fluorescein isothiocyanate (FITC) or tandem fluorochrome composed of R-phycoerythrin (PeCy5); CD8-FITC or R-phycoerythrin (PE); CD14-FITC; CD16-PE or Alexa®647; CD19-PeCy5; CD25-FITC or Allophycocyanin (APC); CD27-PE; CD34-FITC; CD38-PE; CD45-PE, PerCP or APC; CD45RA-APC; CD45RO-APC; CD56-FITC; CD184 (CXCR4)-APC. After incubation, red blood cells were lysed using FACS™ lysing solution (BD Biosciences). For intracellular staining, cells were fixed with 4% paraformaldehyde, and then suspended in permeabilization solution (BD Biosciences) for 30 minutes at room temperature before labelling with granzyme B-PE or APC and perforin-APC (e-Bioscience), or an isotype control antibody (BD Biosciences). For forkhead box P3 (FOXP3)-PE staining, cells were incubated with FOX Buffer solution (BD Biosciences) following the manufacturer’s instructions. Unless specified, all antibodies were from BD Biosciences. For ROS measurement, cells were incubated with 25 µmol/l of 2′,7′-dichlorofluorescein diacetate (DCFDA)-FITC at 37°C for 30 minutes. Fluorescence analysis was performed using a FACS Calibur and analyzed with BD FACS Diva or FlowJo software (Becton Dickinson).
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from PB mononuclear cells using the RNeasy Micro Kit (Qiagen). cDNA was synthesized using Revert Aid H Minus First Strand cDNA Synthesis Kit (Fermentas). All samples were assayed with cDNA, SYBR Green Master Mix PCR (Fermentas), and specific primers in the ABI 7500 Sequence Detection System (Applied-Biosystem). The values of the relative quantification of gene expression were calculated using the equation 2−ΔΔCT.21 A negative “no template” control was included for each primer pair. Amplification specificity was verified using a dissociation curve at the end of each run. Three replicas were run on the same plate for each sample. Sense and antisense primers were designed to be complementary to the sequences contained in different exons (sequences of genes at Supplemental Table S1).
Stromal Cell-derived Factor-1α (SDF-1α) Quantification
SDF-1α was measured in the serum of patients by RD Luminex Screening Human Magnetic Assay (LXSAHM-04 CXCL12/SDF-1 alpha (BR20) using Bio Plex 200 instrument equipped with Bio-Plex Manager software version 6.0.
Immunofluorescence Microscopy
Confocal imaging was carried out using primary antibodies against nuclear factor erythroid 2–related factor 2 (NRF2) Alexa fluor 555-conjugated anti-mouse secondary antibodies. Cells were immobilized on cover slips previously treated with poly-L-lysine (1 mg/ml), fixed with 4% paraformaldehyde for 15 minutes and permeabilized in PBS containing 0.5% Triton-X-100 for 15 minutes. The cells were then blocked with 3% BSA in PBS and incubated with the primary (overnight, 4°C) and secondary (2 hour, room temperature) antibodies. Slides were mounted using the Prolong Gold antifade reagent with DAPI (Molecular Probes) and examined at the National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABIC) at University of Campinas, using a Zeiss LSM 780-NLO confocal on an Axio Observer Z.1 microscope (Carl Zeiss AG). Images were collected using 1024 × 1024 image format and 63× optical zoom. In the absence of primary antibodies, staining of secondary antibodies (negative controls) failed to produce any significant staining.
Immunohistochemistry Staining
NRF2 and hypoxia-inducible factor 1α (HIF-1α) protein expression was demonstrated in paraffin-embedded sections using conventional immunohistochemical techniques. Briefly, 4 µm sections of BM biopsy specimens were deparaffinized, hydrated and endogenous peroxidase was blocked with H2O2. Antigen retrieval was performed by boiling the slides in 0.1 mM citrate buffer, pH 6.0, in a microwave oven for 12 minutes. After cooling, the sections were incubated with the monoclonal antibodies of interest, overnight, at 4°C. The reaction was detected with the streptavidin-biotin-peroxidase complex and stained with diaminobenzidine. Counterstaining was performed with Meyer’s hematoxylin. Images were collected using a 40× objective connected to a light microscope (Olympus CBA, Olympus America).
Plasma EGCG Levels
Plasma EGCG levels were measured at the end of the first month of treatment. Blood samples was collected into tubes containing sodium heparin and plasma was removed after centrifugation. Plasma was then aliquoted into cryotubes with a solution containing 20% acid ascorbic with 0.05% EDTA and stored at −80°C until analysis. Plasma levels of EGCG were then measured at the Institute of Chemistry-IQ/UNICAMP, using an established HPLC procedure.22
Statistical Analysis
Analysis was performed using GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA). Wilcoxon test was used for comparisons of the studied parameters before and after 30 days. Comparison of the studied parameters before and after 180 days was not performed given the small n of patients; values are demonstrated as mean [min-max]. Survival of patients was analyzed by Log-rank test (Kaplan–Meier method). A P value of <.05 was considered statistically significant.
Results
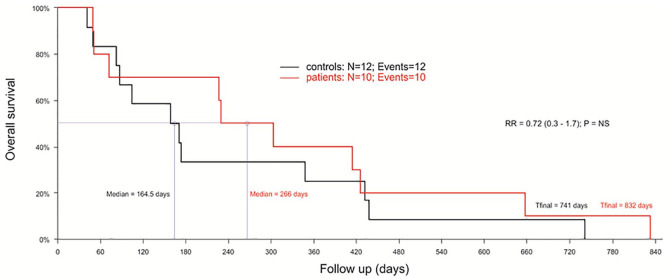
This study was carried out in 10 AML-MRC patients, median age 77 years (min-max: 64-87). The characteristics of patients are presented in Supplemental Table S2. Cytogenetic risk according R-IPSS23 revealed a 60% of cases with intermediate risk or worse. During the study, hospitalization rates were low, with a median of 12 (5-58) days whereas the control cohort (median age 72 years [min-max: 61-82]) presented a median of 19 days of hospitalization (data not shown). Median survival of GT-treated patients was 266 days, which was increased compared to the control cohort (164 days), though not statistically different (Figure 1).
Figure 1.
GT treatment effect on survival of elderly AML-MRC patients (n = 10) in red, compared with AML-MRC controls (n = 12), in black. The control cohort comprised patients diagnosed from December, 2008 to January, 2017 at the same Institution and attended by the same team. Log-rank test (Kaplan-Meier method).
Abbreviations: AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; GT, green tea; NS, non-significant.
According to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCTCAE 4.0) 4 patients presented grade 1 nausea during the first 2 weeks of GT treatment with complete resolution after the first month. One patient had grade 2 alkaline aminotransferase elevation 15 days after cytarabine initiation and was diagnosed with hepatic abscess; however, he was successfully treated with antibiotics and reinitiated GT treatment afterwards without further complications. In addition, plasma levels of EGCG after 30 days of treatment with GT averaged 2.03 µM (1.1 µg/ml).
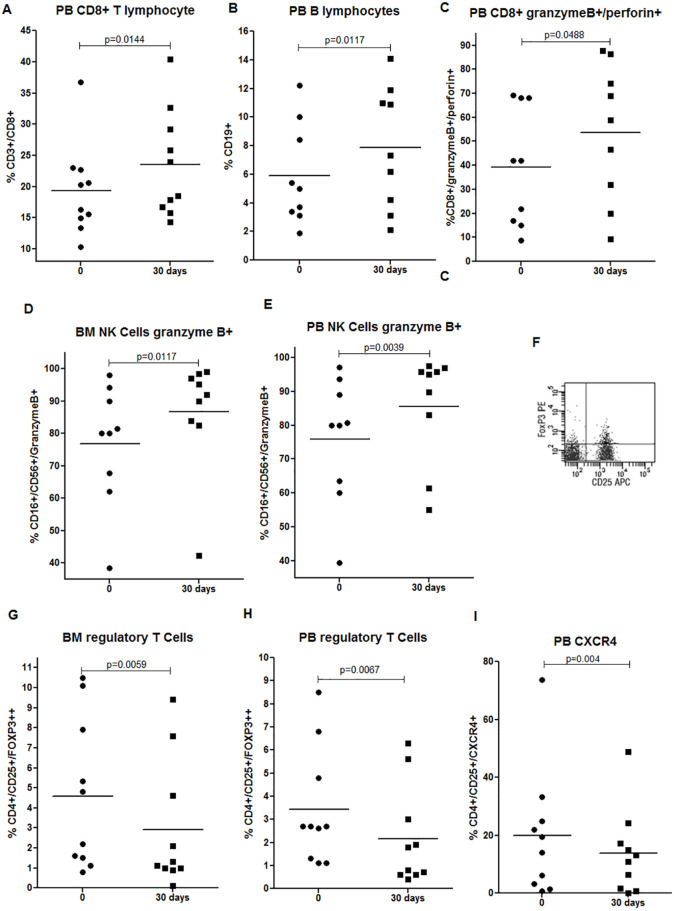
Important changes in the immunological profile of patients could be observed as soon as 30 days after treatment with GT. Improvement of the cytotoxic response was observed: a significant increase in the frequencies of PB CD8+ T and B cells in PB (Figure 2A-B), granzyme B/perforin+ CD8+ T cells in PB (Figure 2C), and increased frequencies of granzyme B+ NK cells in both PB and BM (Figure 2D-E). The population of CD4+CD25+FOXP3++ T cells or regulatory T (Treg) cells, as a percentage of total CD4+ T cells, was further evaluated by flow cytometry using the gating strategy as demonstrated in Figure 2F. Treg cells were reduced in both BM and PB after 30 days of treatment (Figure 2G-H). CXCR4 has been described as the specific trafficking receptor responsible for Treg homing to the BM niche, a reservoir for Treg cells that traffics through the SDF-1α/CXCR4 signals.24 We detected a reduction of CXCR4+ Treg PB cells after 30 days (Figure 2I), which can provide a less immunosuppressive BM microenvironment. In addition, we observed a significant reduction in the mRNA expression of immunosuppressive molecules such as TGF-β (mean 1.04 [1.0-1.1] vs 0.43 [0.054-1.0]; P = .03) and IL-4 (mean 1.22 [1.0-1.6] vs 0.35 [0.013-0.69]; P = .03) after 30 days, and no changes were detected in IL-1β and TNF-α (P > .05).
Figure 2.
GT treatment on lymphocytes and NK cells of elderly AML-MRC patients. (A-B) Frequencies of PB CD8+ T and B cells. (C) Frequencies of PB granzyme B+/perforin+ CD8+ T cells. (D-E) Frequencies of BM and PB granzyme B+ NK cells. (F) Representative gating strategy of Treg cells (double positive CD25+/FOXP3++ population selected as the percentage of CD4+ cells) by flow cytometry. (G-H) Frequencies of BM and PB Treg cells. (I) Frequencies of PB CXCR4+ Treg cells. BM and PB samples of 10 AML-MRC patients were analyzed before and after 30 days by flow cytometry. Wilcoxon test.
Abbreviations: AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; BM, bone marrow; GT, green tea; NK, natural killer; PB, peripheral blood; Treg, regulatory T cells.
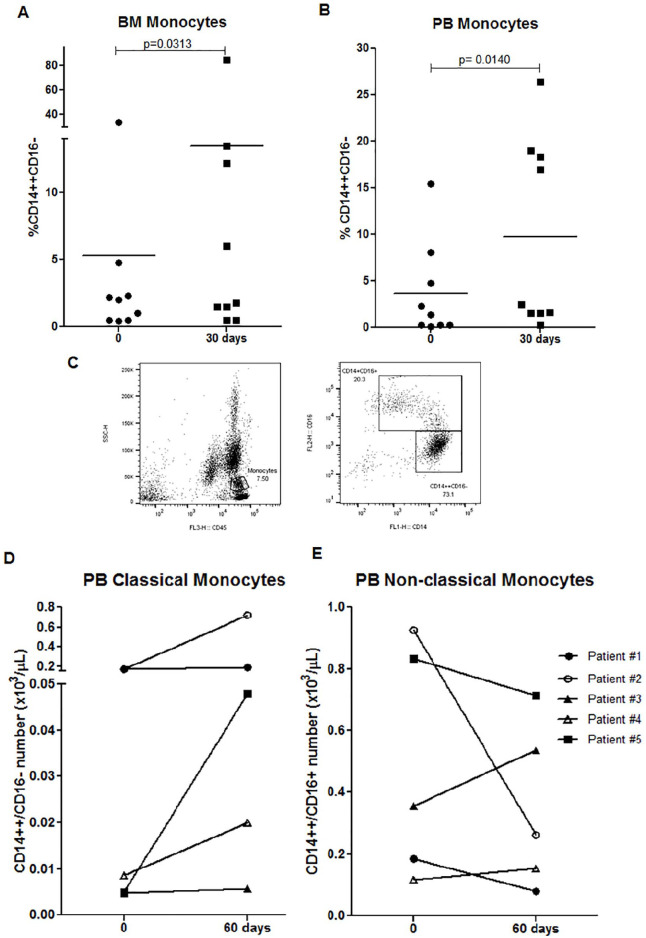
Regarding the monocyte population, our results showed a significant increase of BM and PB monocytes after 30 days (Figure 3A-B). By presenting antigens to T cells, monocytes assume an important role in both innate and adaptive immune response. Using flow cytometry with antibodies against CD14 and CD16, human blood monocytes were classified into classical (CD14++CD16−) and intermediate/non-classical (CD14+CD16+) monocytes, as shown in Figure 3C. Our results show an increase of PB classical monocytes in 3 patients after 60 days of treatment (Figure 3D) with no changes after 30 days (data not shown). As shown in Figure 3D-E, 2 patients who presented a greater increase of classical monocytes also showed a reduction of intermediate/non-classical monocytes. Moreover, a significant increase of intracellular ROS levels was detected in PB (mean 479 [318-640] vs 1939 [784-4025]; P = .031) and BM classical monocytes (mean 557 [340-775] vs 2354 [1158-5734]; P = .03) after 30 days.
Figure 3.
GT treatment on monocytes of elderly AML-MRC patients. (A-B) Frequencies of BM and PB monocytes of 10 AML-MRC patients were analyzed before and after 30 days by flow cytometry. (C) Representative gating strategy of classical (CD14++CD16−) and intermediate/non-classical (CD14+CD16+) monocytes by flow cytometry. (D-E) Number of PB classical and non-classical monocytes from 5 AML-MRC patients analyzed before and 60 days after treatment by flow cytometry. Wilcoxon test.
Abbreviations: AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; BM, bone marrow; GT, green tea; PB, peripheral blood.
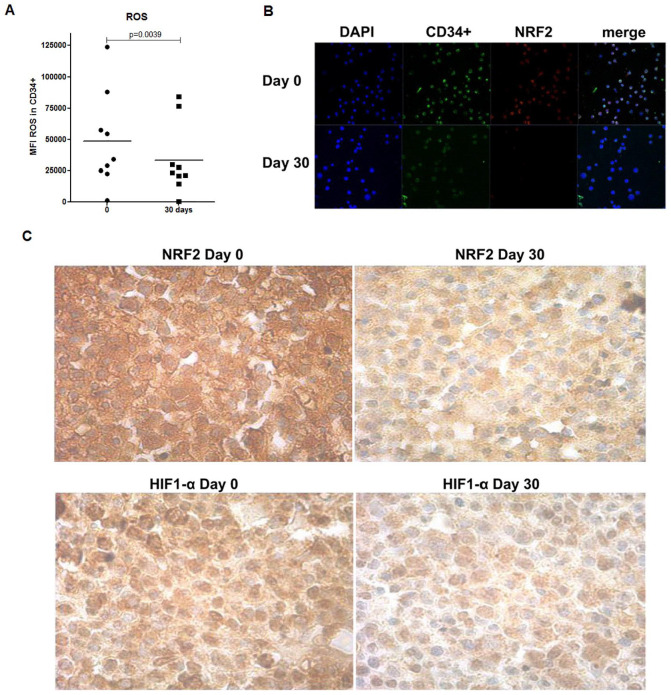
In order to evaluate GT antioxidant capacity, we measured ROS levels in BM CD34+ leukemia cells, using the DCFH-DA-based assay. As shown in Figure 4A, 30 days treatment resulted in a significant decrease of ROS in BM CD34+ cells, corroborating the assumption that GT can protect cells and tissues from oxidative damage by scavenging oxygen-free radicals. The ROS-rich environment has also been described to have inappropriate constitutive NRF2 in addition to HIF-1α activation, leading to increase of leukemic cell survival and chemotherapy resistance.25 As shown in Figure 4, 30 days GT treatment induced a drastic decrease of NRF2 expression in CD34+ cells (Figure 4B) as well as in the biopsies obtained from the BM of patients (Figure 4C). We also detected a reduction in the expression of HIF-1α in the BM biopsies (Figure 4C).
Figure 4.
GT treatment on BM cells and biopsies of elderly AML-MRC patients. (A) ROS levels in BM CD34+ cells expressed as MFI were analyzed by flow cytometry. (B) Representative image of NRF2 expression in BM CD34+ cells analyzed by immunofluorescence microscopy. Images were collected using 63× optical zoom. (C) Representative image of NRF2 and HIF-1α protein expression in BM biopsy specimens analyzed before by immunohistochemistry using a 40× objective. BM samples of 10 AML-MRC patients were analyzed before and after 30 days of treatment.
Abbreviations: AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; BM, bone marrow; GT, green tea; MFI, mean fluorescence intensity; ROS, reactive oxygen species.
The follow-up of patients who received GT alone or in combination with cytarabine are presented in Table 1. After 180 days, the improvement of cytotoxic response can still be observed: GT alone or in combination with cytarabine increased the frequencies of total and naïve/effector CD8+ T cell, and granzyme B/perforin+ CD8+ T cells in both PB and BM. A reduction of Treg cells in the PB and BM was also observed after 180 days, in the presence or absence of cytarabine, parallel to a decrease of CXCR4+ PB Treg cells. In the BM, CXCR4+ CD34+ leukemia cells were reduced in the presence or absence of cytarabine. As expected, further increases in ROS in the BM CD34+ cells were induced by cytarabine; importantly GT treatment was able to reduce ROS in these cells after 180 days, in the presence or absence of cytarabine. The only difference detected was in the serum levels of SDF-1α; GT alone was able to reduce SDF-1α levels though not in the presence of cytarabine (Table 1).
Table 1.
GT Treatment in Combination or Without Low-Dose Cytarabine in AML-MRC Patients After 180 days.
| Parameters | GT (n = 2)† | GT in combination with cytarabine (n = 3)§ | ||
|---|---|---|---|---|
| 0 | 180 d | 0 | 180 d | |
| Peripheral blood | ||||
| CD8 T lymphocyte | ||||
| % CD8 T cell | 15.6 [14.9-16.3] | 24.5 [20-29] | 19.5 [15.6-22.7] | 25.5 [22.8-29.6] |
| % CD8 T naïve | 20.6 [18.6-22.6] | 35.5 [22-49] | 18.5 [16-22.8] | 30.4 [29.9-31.4] |
| % CD8 T effector | 13.9 [12.1-15.7] | 19.4 [19.1-19.7] | 23 [21.3-24.3] | 34.6 [23.2-42.4] |
| % CD8+ granzyme B+/perforin+ | 12.4 [8.8-16] | 42 [35-48.9] | 44.3 [21.7-69.3] | 65.1 [53.3-71] |
| Regulatory T cell | ||||
| % Treg | 1.9 [1.1-2.7] | 0.2 [0.2-0.2] | 3.6 [1.3-6.8] | 1.0 [0.5-1.8] |
| % CXCR4+ Treg | 46.7 [19.5-73.8] | 10.1 [5.2-15] | 10.4 [3.1-21.9] | 2.4 [1-4.5] |
| Natural killer cell | ||||
| % NK granzyme B+ cells | 70 [60-80] | 87 [81-93] | 77.8 [63.6-89] | 89.8 [83.6-97.2] |
| % NK perforin+ cells | 75.5 [75-76] | 87.9 [84-91.7] | 71.2 [68-76.3] | 83 [74-92.2] |
| SDF-1α (µg/ml) | 528.3 [282-775] | 308.1 [169.7-446.4] | 645.8 [439.4-797] | 628.1 [514.6-716.4] |
| Bone marrow | ||||
| CD8 T lymphocyte | ||||
| % CD8 T cell | 18.5 [17-20] | 25.0 [25-25] | 16.5 [14.4-20.1] | 26.1 [23.1-30] |
| % CD8 T naïve | 24.7 [12.9-36.4] | 52.6 [34.3-70.9] | 27.0 [23-34.1] | 38.3 [34-44.8] |
| % CD8 T effector | 25.2 [24.2-26.1] | 41.5 [38-45] | 24.5 [9.8-35.8] | 32.2 [18.4-45] |
| % CD8+ granzyme B+/perforin+ | 17.5 [9.0-26] | 36.0 [31-41] | 40.4 [33-53.6] | 61.4 [48.9-75] |
| Regulatory T cell | ||||
| % Treg | 1.4 [1.1-1.6] | 0.2 [0.2-0.2] | 6.9 [4.8-10.5] | 1.1 [0.7-1.8] |
| Natural killer cell | ||||
| % NK granzyme B+ cells | 80.0 [80-80] | 84.9 [82.7-87] | 79.7 [67.7-90] | 87.7 [82.2-90.8] |
| % NK perforin+ cells | 69.0 [65-73] | 82.7 [73-92.3] | 64.3 [60.5-67] | 83.2 [75-95.4] |
| CD34 cell | ||||
| % CXCR4+ CD34+ cell | 24.0 [22-26] | 5.0 [5-5] | 26.2 [13.3-37.3] | 13.8 [5.9-20.2] |
| MFI ROS in CD34+ cell | 31 402 [28 886-33 919] | 346.5 [227-466] | 89 870 [57 702-123 975] | 71 221 [30 037-142 000] |
Data are given as mean [min-max]. Bone marrow and peripheral blood samples of AML-MRC patients receiving green tea in combination (n = 3) or without (n = 2) low-dose cytarabine were analyzed before and after 180 days of treatment by flow cytometry. Serum levels of SDF-1α were detected using Bio Plex instrument.
CD8 T cell (CD3+CD8+), CD8 T naïve (CD8+CD45RA++CD27+), CD8 T effector (CD8+CD45RA++CD27−), Treg (CD4+CD25+FOXP3++), CXCR4+ Treg (CD4+CD25+CXCR4+), NK granzyme B+ cells (CD16+CD56+granzyme B+), NK perforin+ cells (CD16+CD56+perforin+).
Abbreviations: AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; MFI, mean fluorescence intensity.
Two patients did not receive low-dose cytarabine during this study and were treated with green tea alone.
Low-dose cytarabine was introduced in 5 out of 10 studied patients, of which, 3 started cytarabine at day 60 and 1 started at day 150; 1 patient passed away before completing treatment and 1 patient lacked in measurements.
Discussion
In this study, GT was administered in association with low-dose chemotherapy using cytarabine or in monotherapy for 10 elderly AML-MRC patients, ineligible for aggressive chemotherapy and BM transplants, during at least 6 months and/or until progression. Tea polyphenols at high doses may trigger pro-oxidant activity and exhibit toxic effects on hepatocytes.9,26 However, in our study, no related toxicity was detected. Moreover, plasma EGCG levels detected in patients after 30 days of GT treatment were in accordance with other studies.9,12,13 In a phase 1 and phase 2 trial of daily oral polyphenon E capsules at a dose of 2000 mg containing approximately 200 mg of EGCG, in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia, the median trough total plasma EGCG level after 1 month of therapy was 40.4 ng/mL (ranged from 2.9 to 3974 ng/mL) and 188.6 ng/mL (ranged from 0.001 to 9.56 μM), respectively.12,13 In another study, the AUC of free EGCG was 158.4 ± 89.8 minutes μg/ml for the once daily 800 mg Polyphenon E treatment.9 In addition, median survival of GT-treated patients presents an increase (266 days), though not statistically when compared to the control cohort (164 days). This is not surprising given that the size our sample might not have been sufficient to enable the detection of differences as not only does the enrollment of these patients present a challenge, but a number of patients pass away before the end of the study.
Important modifications in the immune profile of AML-MRC patients receiving GT were observed as soon as 30 days after treatment, and were maintained over time. Significant increases of naïve and effector CD8+ T cells, and NK cells with a high cytotoxic phenotype (as measured by the expression of granzyme B and perforin) were observed. The activation of T and NK cells by tea polyphenols has been reported; GT has been described to affect the immune response in a murine leukemia mouse model by increasing levels of T cell and macrophage cell surface markers.27 Increased activity of CD4+ T cells and enhanced cytotoxicity of NK cells have also been reported in vivo.28 Moreover, EGCG enhanced CD8+ T cell-mediated antitumor immunity induced by DNA vaccination.29 These results lead us to presume that GT induced an activated and cytotoxic phenotype which could facilitate the response against leukemia cells. Treg cells are known to maintain immunological tolerance by actively suppressing immune response, and may impair antitumor responses. Higher levels of these cells have been reported in AML and in high-risk MDS,24,30 and are correlated with reduced remission rates,31 which corroborates our hypothesis that Treg reduction induced by GT is a desirable event for leukemia cell burden control.
We further identified an increased number of classical monocytes with no changes in the intermediate/non-classical subpopulations. Classical monocytes are linked to the phagocytosis process and to a high capacity of presenting antigen to T cells. Pronounced expansion of intermediate/non-classical subpopulations is related to the progression of acute leukemia and could indicate the severity of disease.32 Our results also showed increased intracellular levels of ROS in classical monocytes from the PB and BM. ROS are recognized as important intracellular signaling molecules involved in redox regulation within the cells of the immune system. Phagocytic cells are known to be activated under oxidative conditions. This activation is mediated by the NADPH oxidase system resulting in a marked increase in oxygen consumption and consequent superoxide anion production.33 Therefore GT treatment seems to have induced monocyte activation.
Notably, AML-MRC patients displayed reduced frequencies of CXCR4+ Treg PB cells after 30 days, which can provide a less immunosuppressive BM microenvironment. SDF-1α, also named CXCL12, attracts Treg homing to BM through CXCR4/SDF-1α signals.24 We further observed decreased frequencies of BM CD34+CXCR4+ cells which could benefit patients, since the disruption of SDF-1α/CXCR4 axis in AML cells may inhibit multiple pro-survival signals, thereby producing an anti-leukemia effect.34 The relationship between SDF-1α/CXCR4 axis and ROS has been demonstrated repeatedly.34-36 In steady-state, SDF-1α/CXCR4 interactions maintain quiescence of stem cells and may limit ROS levels36; in contrast, ROS increases the expression of CXCR4 in cancer cells through HIF-1α activation.35,37 HIF-1α is a transcription factor for CXCR4, regulated by hypoxia and ROS.35 CXCR4 is additionally considered a target for NRF2, another transcription factor regulated by ROS.38 NRF2 is constitutively activated in AML and its abnormal activation has a cytoprotective role enabling cell survival by upregulating anti-apoptotic genes.39 The ROS-rich environment has further been described to have inappropriate constitutive NRF2 in addition to HIF-1α activation, leading to increased cell survival and chemotherapy resistance.25 Interestingly, our results showed that GT treatment decreased intracellular levels of ROS in BM CD34+ cells, as well as reduced NRF2 expression in CD34+ cells. Notably, reduced expression of NRF2 and HIF-1α were visualized in the BM biopsies after 30 days. One possible explanation would be that GT impaired ROS production in CD34+ cells and, consequently, inhibited HIF-1α and NRF2 ultimately causing a reduction in CXCR4 expression. These results are corroborated by studies in which GT causes inhibition of HIF-1α activation in cancer cells.19,40,41
The precise mechanism underlying ROS overproduction in leukemic cell remains unknown. Constitutive activation of NADPH oxidase system, reduced antioxidant defense,42 elevated leukocyte mitochondrial DNA content43 are possible mechanisms involved in ROS overproduction. ROS is an important inducer of a key cytokine accounting for the differentiation, proliferation and functions of Treg and NK cells.43 Abundant ROS production leads to an increase of peripheral Treg cell, an important suppressor of antitumor immunity, possibly through induction of TGF-β1.43-45 Our findings demonstrate a significant reduction in the mRNA expression of immunosuppressive molecules such as TGF-β and IL-4 with no effects on IL-1β and TNF-α, in parallel to decreased Treg cells observed during GT treatment which corroborates this hypothesis.
The fate of cells depends on the levels of ROS in the redox microenvironment. Endogenous ROS levels are elevated in tumor cells46 and there is increasing evidence that oxidative stress is crucial for leukemia development.47 Indeed, a change in the intracellular redox status, caused by ROS, may promote proliferation, genetic and epigenetic instability, immune evasion and survival of leukemic cells.42 ROS are also essential for the innate and adaptive immune response. ROS are a double-edged swords in the treatment of tumors since high levels of ROS may induce tumor cell death but can also induce tumor growing signals. Moreover, AML-MRC is a complex and diverse disease with distinct levels of ROS. The results presented herein showed decreased ROS levels in CD34+ cells confirming the antioxidant capacity of GT, which may be related to the positive modulation of the immune system found in studied patients.
Importantly, GT immune effects were maintained over time despite the presence of cytarabine. The only detected difference was the serum levels of SDF-1α; GT alone was able to reduce SDF-1α levels but not in the presence of cytarabine. Although GT did not affect SDF-1α levels in the presence of cytarabine, it appears to induce a decrease in Treg immunosuppressive cells, in addition to reducing CXCR4 expression in Treg cells, which could reverse the suppressor profile of BM microenvironment.
Conclusions
Taken together, these results lead us to presume that GT could induce an activated and cytotoxic phenotype of the immune system which could facilitate the response against leukemia cells. This hypothesis is based on the knowledge that the collaboration of innate and adaptive immune responses is very important in tumor immunology and immunosuppressive pathways cooperate to cause a state of immune tolerance. Therefore, the rapid dissemination of AML negatively impacts anti-leukemia immunity. There is evidence that tea polyphenols stimulate immune cells with increased T cell functions including activation, proliferation and production of cytokines.28,29,48 This information is scarce in AML patients. A study conducted in chronic lymphocytic leukemia patients revealed that 8 out 10 patients studied (80%) showed a reduction of lymphocytosis and absolute number of circulating Treg cells, and IL-10 and TGF-β serum levels declined throughout the GT intake period27; our results for cytokines evaluation are in agreement with these findings. Thus, we presume that the immunomodulatory effect of GT herein described could be valid for other haematological malignancies. In summary, this pilot study highlights that GT is safe and could improve the immune system of elderly AML-MRC patients.
Supplemental Material
Supplemental material, sj-pdf-1-ict-10.1177_15347354211002647 for Immunomodulatory Effect of Green Tea Treatment in Combination with Low-dose Chemotherapy in Elderly Acute Myeloid Leukemia Patients with Myelodysplasia-related Changes by Andrana K. Calgarotto, Ana L. Longhini, Fernando V. Pericole de Souza, Adriana S. Santos Duarte, Karla P. Ferro, Irene Santos, Victor Maso, Sara T. Olalla Saad and Cristiane Okuda Torello in Integrative Cancer Therapies
Acknowledgments
We thank Raquel S. Foglio for English review, Roberto Zulli for statistical analysis and Tereza Salles for her valuable technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/MCT) [grant number PQ 301676/2013-5]; the Fundação de Amparo à Pesquisa do Estado de São Paulo [grant numbers 11/51959-0, 12/06675-7]; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
ORCID iD: Cristiane Okuda Torello  https://orcid.org/0000-0002-1611-020X
https://orcid.org/0000-0002-1611-020X
Supplemental Material: Supplemental material for this article is available online.
Availability of Data and Materials: Data are available upon request.
References
- 1. Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908-1915. doi: 10.1200/JCO.2006.10.2731 [DOI] [PubMed] [Google Scholar]
- 2. Ikegawa S, Doki N, Kurosawa S, et al. Allogeneic hematopoietic stem cell transplant overcomes poor prognosis of acute myeloid leukemia with myelodysplasia-related changes. Leuk Lymphoma. 2016;57:76-80. doi: 10.3109/10428194.2015.1063148 [DOI] [PubMed] [Google Scholar]
- 3. Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131:515-524. [DOI] [PubMed] [Google Scholar]
- 4. Zeidan AM, Stahl M, Sekeres MA, Steensma DP, Komrokji RS, Gore SD. A call for action: increasing enrollment of untreated patients with higher-risk myelodysplastic syndromes in first-line clinical trials. Cancer. 2017;123:3662-3672. doi: 10.1002/cncr.30903 [DOI] [PubMed] [Google Scholar]
- 5. Mao X, Gu C, Chen D, Yu B, He J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget. 2017;8:81649-81661. doi: 10.18632/oncotarget.20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788-794. doi: 10.1182/blood-2003-08-2763 [DOI] [PubMed] [Google Scholar]
- 7. Stingl JC, Ettrich T, Muche R, et al. Protocol for MInimizing the Risk of Metachronous Adenomas of the CoLorectum with Green Tea Extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer. 2011;11:360. doi: 10.1186/1471-2407-11-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chow HH, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53-58. [PubMed] [Google Scholar]
- 9. Chow H-HS, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312-3319. [PubMed] [Google Scholar]
- 10. Nakazato T, Ito K, Ikeda Y, Kizaki M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res. 2005;11:6040-6049. doi: 10.1158/1078-0432.CCR-04-2273 [DOI] [PubMed] [Google Scholar]
- 11. Shanafelt TD, Lee YK, Call TG, et al. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30:707-712. doi: 10.1016/j.leukres.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 12. Shanafelt TD, Call TG, Zent CS, et al. Phase I trial of daily oral polyphenon E in patients with asymptomatic rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;27:3808-3814. doi: 10.1200/JCO.2008.21.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shanafelt TD, Call TG, Zent CS, et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119:363-370. doi: 10.1002/cncr.27719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Chen QS, Xu PP, et al. Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARα oncoprotein degradation. J Hematol Oncol. 2014;7:1-9. doi: 10.1186/s13045-014-0075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakazato T, Ito K, Miyakawa Y, et al. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica. 2005;90:317-325. [PubMed] [Google Scholar]
- 16. Ly BTK, Chi HT, Yamagishi M, et al. Inhibition of FLT3 expression by green tea catechins in FLT3 mutated-AML cells. PLoS One. 2013;8:e66378. doi: 10.1371/journal.pone.0066378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Britschgi A, Simon HU, Tobler A, Fey MF, Tschan MP. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-trans retinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br J Haematol. 2010;149:55-64. doi: 10.1111/j.1365-2141.2009.08040.x [DOI] [PubMed] [Google Scholar]
- 18. Calgarotto AK, Maso V, Junior GCF, et al. Antitumor activities of quercetin and green tea in xenografts of human leukemia HL60 cells. Sci Rep. 2018;8:3459. doi: 10.1038/s41598-018-21516-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torello CO, Shiraishi RN, Della Via FI, et al. Reactive oxygen species production triggers green tea-induced anti-leukaemic effects on acute promyelocytic leukaemia model. Cancer Lett. 2018;414:116-126. doi: 10.1016/j.canlet.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 20. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642-4649. doi: 10.1200/JCO.2003.04.036 [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402-408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 22. Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279:164-169. doi: 10.1006/abio.2000.4487 [DOI] [PubMed] [Google Scholar]
- 23. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454-2465. doi: 10.1182/blood-2012-03-420489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451-8455. doi: 10.1158/0008-5472.CAN-04-1987 [DOI] [PubMed] [Google Scholar]
- 25. Sison EAR, McIntyre E, Magoon D, Brown P. Dynamic chemotherapy-induced upregulation of CXCR4 expression: a mechanism of therapeutic resistance in pediatric AML. Mol Cancer Res. 2013;11:1004-1016. doi: 10.1158/1541-7786.MCR-13-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzanti G, Menniti-Ippolito F, Moro PA, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331-341. doi: 10.1007/s00228-008-0610-7 [DOI] [PubMed] [Google Scholar]
- 27. D’arena G, Simeon V, De Martino L, et al. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. Int J Immunopathol Pharmacol. 2013;26:117-125. doi: 10.1177/039463201302600111 [DOI] [PubMed] [Google Scholar]
- 28. Kim YH, Won Y-S, Yang X, et al. Green tea catechin metabolites exert immunoregulatory effects on CD4 + T cell and natural killer cell activities. J Agric Food Chem. 2016;64:3591-3597. doi: 10.1021/acs.jafc.6b01115 [DOI] [PubMed] [Google Scholar]
- 29. Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802-811. doi: 10.1158/0008-5472.CAN-06-2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Zheng J, Liu J, et al. Increased population of CD4+CD25high regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75:468-476. doi: 10.1111/j.1600-0609.2005.00537.x [DOI] [PubMed] [Google Scholar]
- 31. Szczepanski MJ, Szajnik M, Czystowska M, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325-3332. doi: 10.1158/1078-0432.CCR-08-3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang X-Q, Zhang L, Liu H-A, et al. Expansion of CD14(+)CD16(+) monocytes is related to acute leukemia. Int J Clin Exp Med. 2015;8:12297-12306. [PMC free article] [PubMed] [Google Scholar]
- 33. Segal AW. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol. 2008;40:604-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burger JA. CXCR4 in acute myelogenous leukemia (AML): when too much attraction is bad for you. Leuk Res. 2009;33:747-748. doi: 10.1016/j.leukres.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 35. Chetram MA, Hinton CV. ROS-mediated regulation of CXCR4 in cancer. Front Biol (Beijing). 2013;8:273-278. doi: 10.1007/s11515-012-1204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ludin A, Gur-Cohen S, Golan K, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21:1605-1619. doi: 10.1089/ars.2014.5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu Q, He Y, Ji J, et al. Hypoxia-inducible factor 1α (HIF-1α) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget. 2015;6:10893-10907. doi: 10.18632/oncotarget.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai JJ, Dudakov JA, Takahashi K, et al. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol. 2013;15:309-316. doi: 10.1038/ncb2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rushworth SA, Zaitseva L, Murray MY, Shah NM, Bowles KM, MacEwan DJ. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188-5198. doi: 10.1182/blood-2012-04-422121 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5:1227-1238. doi: 10.1158/1535-7163.MCT-05-0490 [DOI] [PubMed] [Google Scholar]
- 41. Gu J-W, Makey KL, Tucker KB, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell. 2013;5:9. doi: 10.1186/2045-824X-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hole PS, Zabkiewicz J, Munje C, et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood. 2013;122:3322-3330. doi: 10.1182/blood-2013-04-491944 [DOI] [PubMed] [Google Scholar]
- 43. He X, Qu F, Zhou F, et al. High leukocyte mtDNA content contributes to poor prognosis through ROS-mediated immunosuppression in hepatocellular carcinoma patients. Oncotarget. 2016;7:22834-22845. doi: 10.18632/oncotarget.8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jain M, Rivera S, Monclus EA, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288:770-777. doi: 10.1074/jbc.M112.431973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kraaij MD, Savage NDL, van der Kooij SW, et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci U S A. 2010;107:17686-17691. doi: 10.1073/pnas.1012016107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117:5816-5826. doi: 10.1182/blood-2011-01-326025 [DOI] [PubMed] [Google Scholar]
- 47. Sallmyr A, Fan J, Datta K, et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111:3173-3182. doi: 10.1182/blood-2007-05-092510 [DOI] [PubMed] [Google Scholar]
- 48. Huang A-C, Cheng H-Y, Lin T-S, et al. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo. 2013;27:627-634. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ict-10.1177_15347354211002647 for Immunomodulatory Effect of Green Tea Treatment in Combination with Low-dose Chemotherapy in Elderly Acute Myeloid Leukemia Patients with Myelodysplasia-related Changes by Andrana K. Calgarotto, Ana L. Longhini, Fernando V. Pericole de Souza, Adriana S. Santos Duarte, Karla P. Ferro, Irene Santos, Victor Maso, Sara T. Olalla Saad and Cristiane Okuda Torello in Integrative Cancer Therapies