Abstract
Data from recent dose-response toxicological studies suggest that the no-observed-adverse-effect-level (NOAEL) may depend upon whether hormesis is present. A further examination of these data supports this hypothesis by showing that the NOAEL was greater for living units (organisms or cells) showing hormesis than for living units showing no hormesis. For example, some cancer tissue cells may exhibit hormetic responses to an anticancer drug while some other cancer tissue cells may not. These findings suggest that living units showing hormesis may also be less susceptible than living units not showing hormesis. However, these findings are preliminary and cannot be generalized or assumed to be a norm yet. New studies are needed to evaluate how NOAEL shifts depending on the occurrence of hormesis.
Keywords: biphasic response, non-clinical risk assessment, organism tolerance limits, point of departure (POD), susceptibility screening, toxicological threshold
Main Text
The no-observed-adverse-effect-level (NOAEL) of the hormetic dose (Dose refers to both dose and exposure hereafter unless otherwise specified)-response relationship is an important point of departure (POD) in toxicology, ecotoxicology, environmental toxicology, and risk assessment as it can be used to set thresholds up to which doses are presumably safe, identifying how hazardous chemicals are and what their (un)acceptable doses are.1 NOAEL is also important in ecology, where it facilitates the quantitative estimation of biological plasticity and ranking of susceptibility/tolerance among groups of organisms at different levels of biological organization. NOAEL has therefore wide implications in various scientific (sub)disciplines.
A common definition of NOAEL is “the highest experimental point that is without adverse effect,” while a more accurate definition proposed is “the highest dose that does not cause toxicologically relevant increases in the frequency or severity of effects between exposed and control groups based on careful biological and statistical analysis.”2 While there is no standardized definition,2 it is clear that the NOAEL could be set at doses that have a negligible/minor negative effect. In the hormesis literature, the NOAEL is commonly considered to be the highest dose at which a dose-response curve intersects the control-response line, and is alternatively called zero equivalent point (ZEP), i.e. a point that is equivalent to the control3 (see also early works of Edward J. Calabrese1). As such, the NOAEL separates a hormetic dose-response curve into 2 parts: the zone with stimulatory effects (left of NOAEL) and the zone with inhibitory effects (right of NOAEL).
An important question remaining obscure is whether the occurrence of hormesis affects the NOAEL relative to when hormesis is absent (described by the threshold model). In a recent issue of Dose-Response, Aoishi and her colleagues evaluated quantitative characteristics of hormesis in breast cancer using a histoculture drug response assay.4 A unique feature of this research is the exposure of surgically-resected fresh tumor specimens from 22 patients (17 patients with invasive ductal carcinoma, 3 patients with mucinous carcinoma, and 2 patients with other “special-type” cancers) to 9 concentrations of paclitaxel (0 and 2-256 μg/mL) for 7 days. Hormesis appeared in 9 out of the 22 dose-response relationships (hereafter cases) (ν = 69.2%), and the maximum stimulation was in agreement with the broad hormesis literature, i.e. commonly up to 160% of control response (8/9 of hormesis cases) and all below 180%. While Aoishi et al. examined how ED50 varied between cases showing hormesis and cases not showing hormesis (typically following the threshold model), their study provides important evidence that the NOAEL also differs between the 2 groups.
We used the dose-responses relationships of Aoishi et al4 to estimate the NOAEL either at the typical ZEP (NOAEL-ZEP; stricter) or at a 5% inhibition (NOAEL-5%; less strict) using Adobe Photoshop CS4 Extended v.11 (Adobe Systems Incorporated, CA, USA). We compared NOAEL between groups using Mann-Whitney U test at a level of significance α = 0.05 (STATISTICA v.10; StatSoft Inc.). The median NOAEL-ZEP (U = 7.0, P < 0.001) and NOAEL-5% (U = 8.0, P < 0.001) were 5.61 and 2.72 times larger for the cases showing hormesis (n = 9) than for the cases showing no hormesis (n = 13). Therefore, it can be concluded that the NOAEL was, in general, larger for the cases showing hormesis than for the cases showing no hormesis (Figure 1), independently of the calculation.
Figure 1.
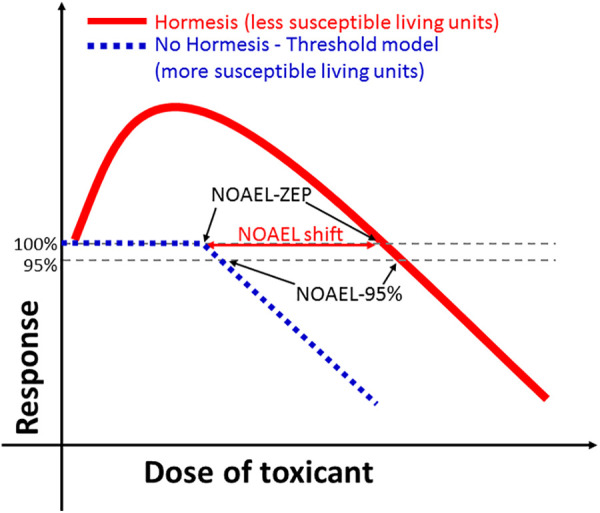
Hypothetical examples of dose-response relationships with either hormesis or no hormesis (e.g. threshold model). NOAEL-ZEP: no-observed-adverse-effect-level at the zero equivalent point (0% inhibition). NOAEL-5%: no-observed-adverse-effect-level at a 5% inhibition.
Studies evaluating whether hormesis can impact herbicide resistance evolution in weeds provide data supporting the findings discussed above based on the breast cancer study.4 For instance, different biotypes of Alopecurus myosuroides Huds. were exposed to 3 acetyl-coenzyme A carboxylase (ACCase) inhibitors, and dose-response relationships were studied among ACCase-sensitive and -resistance biotypes and different groups of individuals within biotype (subpopulation groups).5 A closer examination of the data reported in this paper5 reveals evidence suggesting the occurrence of a larger NOAEL for resistant groups showing hormesis compared with susceptible groups showing no hormesis. Such evidence can also be seen in further extensive studies assessing dose-response relationships in susceptible and resistant plant groups,6 while other studies using subpopulation groups with different degrees of susceptibility (percentiles) generally do not provide such evidence.7,8 It should be made clear that both susceptible and resistant organisms can exhibit hormesis.6
The preliminary evidence discussed herein makes the case that NOAEL may be smaller for organisms showing no hormesis than for organisms showing hormesis (Figure 1). The former may be potentially susceptible organisms while the latter may be relatively less susceptible or resistant organisms. This hypothesis cannot be generalized at this stage due to the striking lack of studies providing data for NOAEL shift in hormetic sub-populations in comparison to no-hormetic counterparts. However, this is a critical issue affecting dose-response evaluations, risk assessments, and organismal susceptibility/resistance screenings, indicating the need for new studies designed with the purpose to evaluate the NOAEL shift between organisms showing hormesis and organisms not showing hormesis.
Acknowledgments
Evgenios Agathokleous acknowledges multi-year support from The Startup Foundation for Introducing Talent of Nanjing University of Information Science & Technology (NUIST), Nanjing, China (No. 003080 to E.A.).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Evgenios Agathokleous  https://orcid.org/0000-0002-0058-4857
https://orcid.org/0000-0002-0058-4857
References
- 1. Calabrese EJ. Expanding the RfD concept to incorporate and optimize beneficial effects while preventing toxic responses from nonessential toxicants. Ecotoxicol Environ Saf. 1996;34(1):94–101. [DOI] [PubMed] [Google Scholar]
- 2. Dorato MA, Engelhardt JA. The no-observed-adverse-effect-level in drug safety evaluations: use, issues, and definition(s). Regul Toxicol Pharmacol. 2005;42:265–274. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose-Response. 2009;7(1):1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoishi Y, Yoshimasu T, Oura S, et al. Quantitative evaluation of hormesis in breast cancer using histoculture drug response assay. Dose-Response. 2019;17:1559325819896183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belz RG, Farooq MB, Wagner J. Does selective hormesis impact herbicide resistance evolution in weeds? ACCase-resistant populations of Alopecurus myosuroides Huds. As a case study. Pest Manag Sci. 2018;74(8):1880–1891. [DOI] [PubMed] [Google Scholar]
- 6. Belz RG. Herbicide hormesis can act as a driver of resistance evolution in weeds—PSII-target site resistance in Chenopodium album L. As a case study. Pest Manag Sci. 2018;74:2874–2883. [DOI] [PubMed] [Google Scholar]
- 7. Belz RG, Patama M, Sinkkonen A. Low doses of six toxicants change plant size distribution in dense populations of Lactuca sativa. Sci Total Environ. 2018;631-632:510–523. [DOI] [PubMed] [Google Scholar]
- 8. Belz RG, Sinkkonen A. Low toxin doses change plant size distribution in dense populations—Glyphosate exposed Hordeum vulgare as a greenhouse case study. Environ Int. 2019;132:105072. [DOI] [PubMed] [Google Scholar]


