Abstract
Background
Identifying the cause of intracerebral hemorrhage (ICH) is relevant to optimize its management. We aimed to assess the applicability and utility of the Edinburgh CT criteria for cerebral amyloid angiopathy (CAA) in an unselected cohort of hospitalized patients.
Patients and Methods
We retrospectively applied the Edinburgh criteria to the first available brain CTs of patients hospitalized for a first-ever lobar ICH in the district of L’Aquila from 2011 to 2017. ICH characteristics and outcomes were compared according to the presence of the Edinburgh CT criteria, including associated subarachnoid hemorrhage (aSAH) and finger-like projections (FLPs). The outcome of ICH in-hospital mortality was assessed with multivariate logistic regression analysis. We adopted the Edinburgh criteria, age, NIHSS and Glasgow Coma Scale scores, systolic blood pressure, antiplatelet treatment, ICH volume, and intraventricular extension on admission as covariates.
Results
Of 178 patients with lobar ICH, 52 (29.2%) had aSAH+FLPs, 60 (33.7%) aSAH only, 1 (0.6%) FLPs, and 65 (36.5%) none. Patients with aSAH+FLPs were older (79.0 ± 9.2 years) than those with only one criterion or none (74.0 ± 15.3 and 72.2 ± 13.8 years, respectively; P = 0.020). Patients with aSAH+FLPs also had more severe ICH at onset, higher in-hospital case-fatality (log rank test P = 0.003) and higher mRS scores at discharge (P < 0.001) as compared to those fulfilling one or none of the Edinburgh criteria. Low Glasgow Coma Scale score was the only factor independently associated to in-hospital case-fatality (odds ratio per point increase 0.51; 95% confidence interval, 0.32–0.91; P = 0.021).
Discussion
Our data suggest the applicability of the Edinburgh CT criteria in a hospital setting. The presence of those criteria reflects ICH clinical severity.
Conclusions
Applying the Edinburgh CT criteria might help refining the diagnosis and improving the management of patients with lobar ICH.
Keywords: Intracerebral hemorrhage, amyloid angiopathy, imaging, computed tomography, stroke, prognosis
Introduction
Intracerebral hemorrhage (ICH) is the second most frequent and the most fatal type of stroke.1 ICH has a high short-term fatality2 that did not substantially decrease over the last decades.3,4 Identifying the cause of ICH is relevant to establish the prognosis and optimize the management of the disease. ICH is usually distinguished into lobar and non-lobar. According to the classical view, most cases of non-lobar ICH are attributed to hypertensive angiopathy, while most cases of lobar ICH are attributed to cerebral amyloid angiopathy (CAA).5 However, CAA causes relatively few lobar ICHs, and its diagnosis of certainty is still only pathologic (based on postmortem histopathological examination).6
The Edinburgh criteria for the diagnosis of probable CAA provide a combined computed tomography (CT)- and genetic-based tool to identify the patients with lobar ICH with the highest likelihood to have underlying CAA. A major limitation of those criteria is the unavailability of apolipoprotein E genotyping in many clinical settings. However, the use of CT-based criteria only can predict a probability of moderate or severe CAA up to 97% according to the original model, which compared the criteria against pathologic findings.7
Aims
We aimed to evaluate the applicability and the utility of Edinburgh CT criteria in an unselected cohort of patients hospitalized for lobar ICH.
Methods
Study design and population
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) to report the results of the present study (see Supplementary Material). The present hospital-based study is nested within a prospective population-based registry including patients with first-ever stroke and transient ischemic attack in the population of 298,343 inhabitants8 of the district of L’Aquila, central Italy. We included patients residing in the district of L’Aquila and hospitalized for a first-ever ICH from January 1, 2011 to December 31, 2017. The population is served by four public hospitals, all of which have 24/7 availability of brain CT. Two hospitals have neurology wards, one a neurosurgical ward, all have intensive care units and general medicine wards.
We initially recruited patients who were assigned the ICD-9-CM code 431, which indicates spontaneous hemorrhage within the brain parenchyma. We then validated ICH diagnoses according to the definition of neurological deficits documented by neuroimaging indicating the presence of an intraparenchymal hemorrhage.9,10 We only included patients with first-ever ICH; patients with hemorrhagic transformation of cerebral infarction or history of previous stroke were excluded.
As the study is observational, patients were treated according to routine clinical practice and following national and international guidelines.
Data collection and follow-up
Clinical data were recorded on standardized forms and stored in a computerized database. Basic information included medical history, routine laboratory and instrumental tests. The National Institutes of Health Stroke Scale (NIHSS) score11 on admission and the modified Rankin Scale (mRS) score12 at discharge were also recorded.
While extended follow-up of the cohort is still ongoing to record long-term outcome events and mortality, in-hospital outcomes were analyzed in the present study.
Radiological assessment
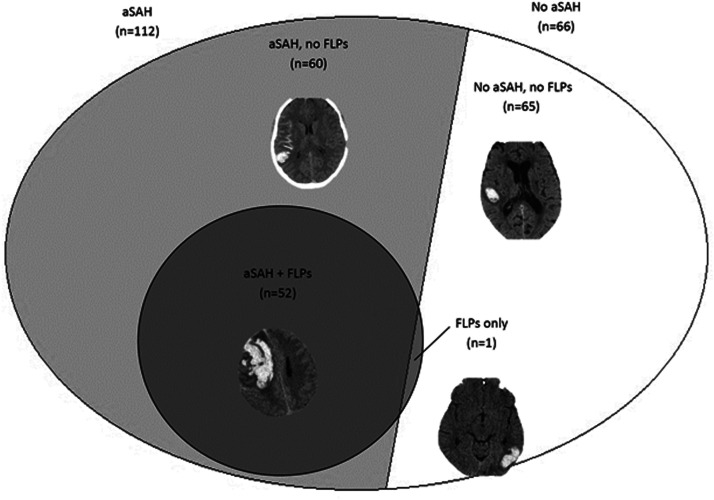
All the assessments were performed retrospectively on the first available brain CTs. Times from symptom onset to hospital arrival were used to estimate the times from symptom onset to brain CT. CT-confirmed cases of ICH were assessed independently by a neurologist and a radiologist trained in the assessment of the Edinburgh criteria by an online tool.13 CT assessments were performed on tridimensional images of the first brain CT performed by the patients, obtained with muptiplanar reformatting. Lobar, non-lobar, or uncertain location was adjudicated according to the Cerebral Haemorrhage Anatomical RaTing InStrument (CHARTS).14 For lobar ICH, the presence of associated subarachnoid hemorrhage (aSAH) and finger-like projections (FLPs) was assessed; aSAH was defined as an extra-axial collection of blood in the subarachnoid space, while FLPs were defined as elongated extensions arising from the hematoma, longer than they are wide, regardless of whether they extended to the cortex or not (Figure 1).7 Assessments were performed by two independent raters (EC and ET). Disagreements were resolved by consensus; primary analyses were performed on the assessments after consensus resolution. ICH volumes were estimated by a single operator (EC) according to the ABC/2 method.15
Figure 1.
Overlap between associated subarachnoid hemorrhage (aSAH) and finger-like projections (FLPs) in patients with lobar intracerebral hemorrhage (ICH).
Ethical aspects
The study was approved by the Internal Review Board of the University of L’Aquila. Every effort was made to obtain informed consent from the study participants.
Statistical analysis
Descriptive statistics are reported as absolute numbers with percentages, mean±SD or median with interquartile range (IQR). Groups were compared using the Pearson χ2 test or the Student’s t test for normally distributed variables, and with the Mann-Whitney U test for non-normally distributed variables. Interrater agreements beyond chance were calculated using Cohen’s kappa. Two-sided statistical significance was set at a P level <0.05. To assess normality of the distribution of variables, the Kolmogorov-Smirnov test was used. To assess the factors associated to in-hospital case-fatality, multivariate logistic analyses were performed with the Edinburgh criteria and the variables commonly included in ICH prognostic scores,16 including age, NIHSS and Glasgow Coma Scale scores, systolic blood pressure, antiplatelet treatment, ICH volume and intraventricular extension. All those variables were recorded on admission.
Statistical analyses were performed with SPSS software, version 20.
Results
During the study period, we identified 494 patients with ICH (288 males; 58.3%), with a mean age of 74.4 ± 13.2 years. All patients performed at least one brain CT. ICH location was lobar in 178 patients (36.0%), non-lobar in 258 (52.2%), and uncertain in the remaining 58 (11.8%).
Among the 178 patients with lobar ICH, 52 patients (29.2%) had aSAH+FLPs, 60 (33.7%) had aSAH only, 1 (0.6%) FLPs only, and 65 (36.5%) none (Figure 1). Interrater agreement was almost perfect for both aSAH (k = 0.860; P < 0.001) and FLPs (k = 0.905; P < 0.001).
Patients with aSAH+FLPs were older than those with only one criterion or none (79.0 ± 9.2, 74.0 ± 15.3, and 72.2 ± 13.8 years, respectively; P = 0.020; Table 1). The relative proportion of patients with aSAH+FLPs was highest (39.4%) in patients aged 75–84 years and lowest (6.3%) in those aged <55 years, whereas the relative proportion of patients with none of the criteria was highest in patients aged <55 years (50.0%) and lowest in those aged ≥85 years (21.6%; P = 0.038; Supplemental Figure 1). Sex distribution was similar across groups as well as distribution of risk factors (Table 1). On hospital admission, patients with aSAH+FLPs had lower mean systolic blood pressure (151.4 ± 28.8 mmHg) as compared to patients with only one criterion (154.2 ± 31.9 mmHg) or none (166.7 ± 30.1 mmHg; P = 0.034; Table 1). Referring to treatment history, the proportion of patients on antiplatelets was higher in patients with aSAH+FLPs (48.1%) than in the other two groups (27.9% and 29.2%, respectively; P = 0.044; Table 1). Intraventricular extension of ICH was more prevalent in patients with aSAH+FLPs (34.6%) as compared with those with one (23.0%) or none of them (10.8%; P = 0.007; Table 1).
Table 1.
Characteristics of the included patients with lobar intracerebral hemorrhage in the present study.
| All lobar ICHs (n = 178) |
aSAH+FLPs (n = 52) |
aSAH or FLPs only (n = 61) |
No aSAH or FLPs (n = 65) |
P value | |
|---|---|---|---|---|---|
| Male, n (%) | 89 (50.0) | 30 (57.7) | 29 (47.5) | 30 (46.2) | 0.414 |
| Age, mean ± SD | 74.8 ± 13.4 | 79.0 ± 9.2 | 74.0 ± 15.3 | 72.2 ± 13.8 | 0.020 |
| Time from symptom onset to hospital arrival | 0.293 | ||||
| ≤4.5 hours | 66 (37.1) | 17 (32.7) | 24 (39.3) | 25 (38.5) | |
| >4.5 hours | 50 (28.1) | 10 (19.2) | 19 (31.1) | 21 (32.3) | |
| Not available | 62 (34.8) | 25 (48.1) | 18 (29.5) | 19 (29.2) | |
| Systolic blood pressure at onset, mean ± SD | 158.0 ± 30.9 | 151.4 ± 28.8 | 154.2 ± 28.8 | 166.7 ± 30.1 | 0.034 |
| Diastolic blood pressure at onset, mean ± SD | 86.5 ± 17.2 | 81.8 ± 15.4 | 86.6 ± 15.4 | 90.0 ± 19.4 | 0.074 |
| Intraventricular extension of ICH, n (%) | 39 (21.9) | 18 (34.6) | 14 (23.0) | 7 (10.8) | 0.007 |
| Risk factors, n (%) | |||||
| Arterial hypertension | 135 (75.8) | 36 (69.2) | 46 (75.4) | 53 (81.5) | 0.301 |
| Hypercholesterolemia | 34 (19.1) | 13 (25.0) | 8 (13.1) | 13 (20.0) | 0.270 |
| Diabetes mellitus | 35 (19.7) | 12 (23.1) | 8 (13.1) | 15 (23.1) | 0.284 |
| Atrial fibrillation | 30 (16.9) | 5 (9.6) | 9 (14.8) | 16 (24.6) | 0.146 |
| Coronary heart disease | 39 (21.9) | 15 (28.8) | 9 (14.8) | 15 (23.1) | 0.188 |
| Cigarette smoking | 15 (8.4) | 5 (9.6) | 6 (9.8) | 4 (6.2) | 0.709 |
| Alcohol abuse | 16 (9.0) | 1 (1.9) | 8 (13.1) | 7 (10.8) | 0.103 |
| Ongoing treatment at onset, n (%) | |||||
| Statins | 30 (16.9) | 12 (23.1) | 7 (11.5) | 11 (16.9) | 0.260 |
| Antihypertensives | 120 (67.4) | 30 (57.7) | 43 (70.5) | 47 (72.3) | 0.201 |
| Antiplatelets | 61 (34.3) | 25 (48.1) | 17 (27.9) | 19 (29.2) | 0.044 |
| Anticoagulants | 32 (18.0) | 7 (13.5) | 12 (19.7) | 13 (20.0) | 0.302 |
aSAH indicates associated subarachnoid hemorrhage; FLPs, finger-like projections; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation.
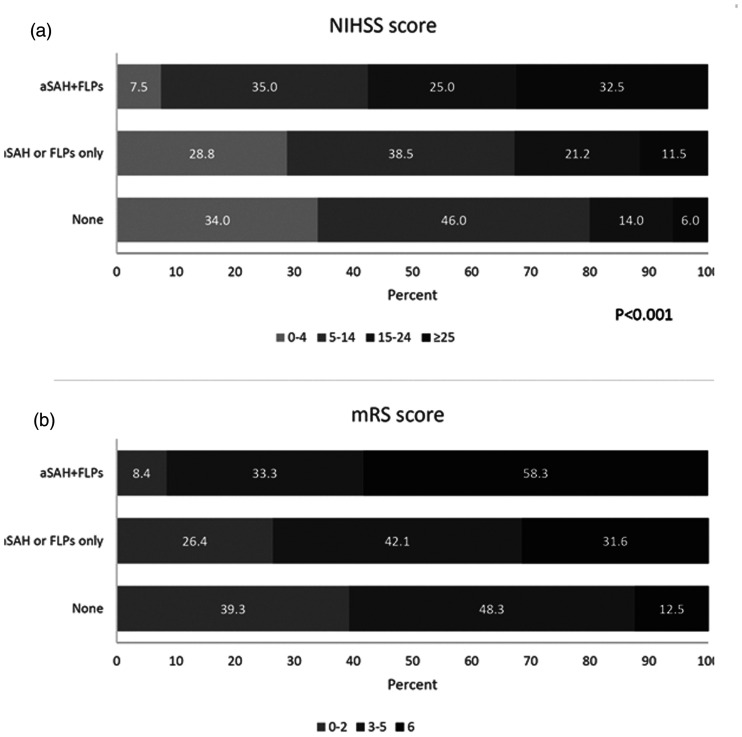
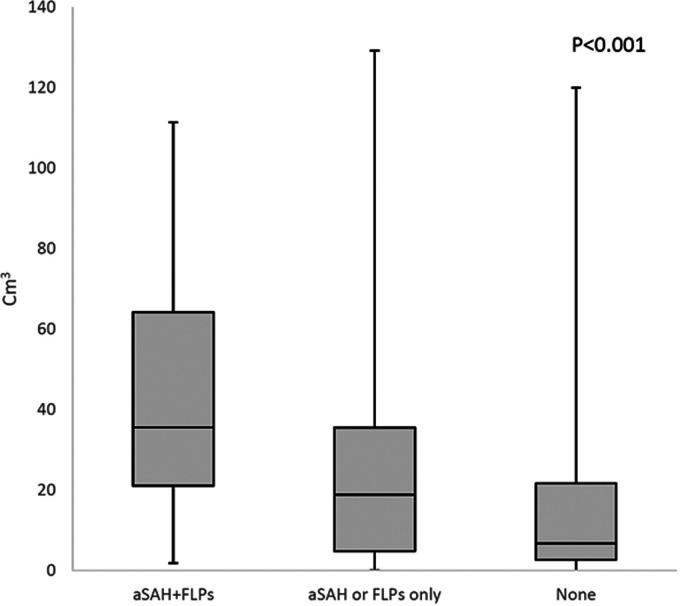
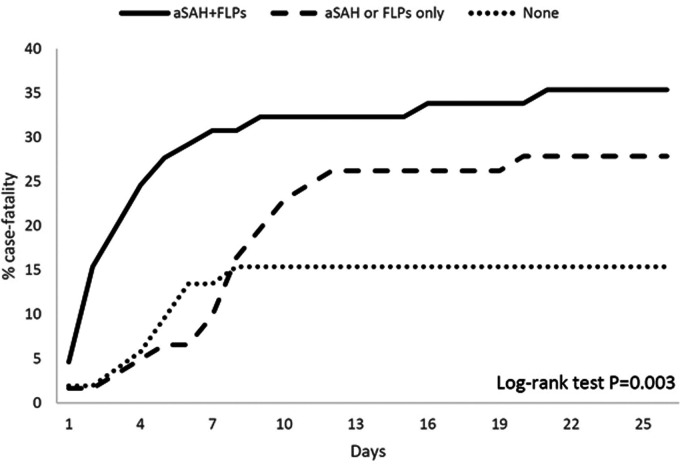
ICH was more severe at onset in patients with aSAH+FLPs compared with those fulfilling one or none of the Edinburgh criteria, as shown by a higher NIHSS score (P < 0.001; Figure 2(a)) and by higher median ICH volumes (P < 0.001; Figure 3). Those same patients also had worse in-hospital outcomes as shown by higher in-hospital case-fatality (log rank test P = 0.003; Figure 4) and higher mRS scores at discharge (P < 0.001; Figure 2(b)).
Figure 2.
Distribution of (a) National Institutes of Health Stroke Scale score categories on hospital admission and (b) modified Rankin Scale scores at hospital discharge according to the presence of Edinburgh criteria for probable cerebral amyloid angiopathy.
Figure 3.
Box plot of intracerebral hemorrhage volume according to the presence of Edinburgh criteria for probable cerebral amyloid angiopathy in patients with lobar intracerebral hemorrhage. Boxes indicate interquartile ranges.
Figure 4.
Kaplan-Meier curves of cumulative case-fatality according to the presence of Edinburgh criteria for probable cerebral amyloid angiopathy in patients with lobar intracerebral hemorrhage.
The multivariate logistic analysis performed on age, NIHSS score on admission, systolic blood pressure on admission, Edinburgh CT criteria, Glasgow Coma Scale score on admission, and ICH volume showed that low Glasgow Coma Scale score (OR per point increase, 0.54; 95% CI, 0.32–0.91; P = 0.021) was the only factor independently associated to in-hospital case-fatality (Table 2).
Table 2.
Multivariate logistic regression analysis of predictors of in-hospital case-fatality.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age, per 10 years | 2.76 | 0.95-7.99 | 0.061 |
| NIHSS score, per point | 1.11 | 0.93-1.32 | 0.233 |
| Systolic BP on admission, per mmHg | 0.97 | 0.93-1.00 | 0.066 |
| Antiplatelets on admission | 0.23 | 0.03-1.78 | 0.158 |
| Intraventricular extension | 1.97 | 0.30-12.95 | 0.482 |
| Edinburgh criteria | |||
| aSAH+FLPs | 3.20 | 0.14-74.95 | 0.470 |
| aSAH only | 3.00 | 0.21-43.68 | 0.421 |
| None | 1.00 | Ref. | |
| ICH volume, per cm3 | 1.02 | 0.98-1.06 | 0.381 |
| GCS score on admission, per point increase | 0.54 | 0.32-0.91 | 0.021 |
aSAH indicates associated subarachnoid hemorrhage; FLPs, finger-like projections; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; NIHSS, National Institutes of Health Stroke Scale.
Discussion
Our study showed that the CT-based radiological Edinburgh criteria are fully applicable in the routine clinical setting in patients who have lobar ICH even in the absence of specifically designed neuroimaging studies. The application of those criteria allows identifying a subgroup of patients with a poor short-term outcome. The higher short-term fatality of lobar ICH in the presence of the Edinburgh criteria is interesting as it might provide an easy tool for prognostication.
We found a substantial overlap between the presence of FLPs and aSAH (Figure 1); in detail, all but one patient with FLPs also had aSAH, while one third of patients with lobar ICH had aSAH only. The presence of aSAH+FLPs was associated with higher age, lower systolic blood pressure, and a higher prevalence of antiplatelet treatment, in line with CAA-related lobar ICH, which is usually age-dependent, non-hypertensive in nature, and associated with antithrombotic medication.17 By consequence, our findings indirectly support the reliability of the Edinburgh CT criteria. Compared with the presence of aSAH+FLPs and none of the criteria, the presence of aSAH only was associated with intermediate age and blood pressure at onset, clinical severity, median volume, and case-fatality. Those clinical and radiological characteristics are consistent with the medium probability of moderate or severe CAA associated with aSAH only as compared with the high probability associated with aSAH+FLPs.7
At hospital admission, patients with aSAH+FLPs had the highest stroke severity, while patients with one criterion had intermediate severity and those with none of the criteria had the lowest severity. As expected, the distribution of ICH volume was consistent with clinical severity. The higher severity and volume of lobar ICH in patients with aSAH+FLPs compared with those without are in line with previous findings.18,19 In-hospital mortality was more than doubled in patients with aSAH+FLPs and almost doubled in patients with one criterion as compared with those with none of them (Figure 4). This is in line with other evidence showing that indirect signs of CAA are markers of poor prognosis.19 Notably, aSAH and FLPs might be more easily detectable in larger than in smaller lobar ICHs, which provides a further link between the presence of those criteria and ICH prognosis.
The presence of aSAH+FLPs was not independently associated to lobar ICH case-fatality. However, as ICH volume is a continuous variable it is not easily manageable in routine clinical practice, while the application of yes/no criteria such as the Edimburgh CT criteria may easily allow to predict prognosis even in the emergency room. Currently available prognostic scores for ICH mostly include age, ICH volume, and Glasgow Coma Scale score at onset.16 aSAH+FLPs, which are easily identified on brain CT, are present in a subgroup of patients with old age and high ICH volume and might therefore be considered as indirect markers of poor prognosis, even if only in patients with lobar ICH. The radiological Edinburgh criteria might be more predictive of ICH recurrence19,20 than short-term outcomes, or may predict occurrence of further bleedings in patients who start antithrombotics.
Our study may give some insight on the yield of implementation of ApoE testing. The predicted probability of moderate or severe CAA according to CT findings, without ApoE genotyping,7 was 97% according to the original study (Supplemental Table 1) and would change little with positive or negative ApoE testing. Positive ApoE testing would have significantly increased the probability of moderate or severe CAA in the 33.7% of patients fulfilling only one of the Edinburgh radiological criteria, while negative testing would have ruled out CAA in the 34.3% of patients not fulfilling the radiological criteria (Supplemental Table 1). In summary, our results suggest that ApoE testing would be useful in two thirds of patients with lobar ICH.
We tested the potential role of the Edinburgh criteria in a hospital-based setting. The main strength of our study is the inclusion of a large series of patients with first-ever ICH confirmed by neuroimaging in a multicenter, real-life setting, allowing generalization to common clinical practice. However, our study suffers from several limitations due to its naturalistic design. The lack of necropsy examinations did not allow assessing whether the Edinburgh CT criteria really reflect CAA pathology in our patients. Besides, we did not perform ApoE testing, impairing the confirmation of our assumptions about the diagnostic yield of genetic testing. Moreover, we based our assessments on the first available brain CT for each patient; the assessment of follow-up imaging would have likely improved the prognostic evaluation of ICH21 and might have disclosed further CAA-related characteristics in some patients. Lastly, we limited our observations to the in-hospital outcomes, not including long-term follow-up for case-fatality and ICH recurrence.
Our study did not give insights on the potential added value of brain MRI over brain CT in the etiologic investigation of lobar ICH. In fact, it is desirable that patients with ICH are investigated with brain MRI in order to better characterize the underlying mechanisms of the hemorrhage.22,23 The use of brain MRI would have allowed the assessment of CAA markers such as cortical superficial siderosis and cerebral microbleeds,24 as well as other signs of microangiopathy25,26 and potential secondary causes of ICH. However, further studies are warranted to test the validity of the Edinburgh criteria in different settings.
In conclusion, our study suggests that the Edinburgh CT criteria for the diagnosis of probable CAA-related ICH are easily applicable in routine clinical practice. The application of those criteria may yield added value to management of patients with lobar ICH by refining their diagnosis and guiding the choice of treatments, such as restarting antiplatelets or anticoagulants after the event. Furthermore, the Edinburgh CT criteria reflect the clinical severity of ICH and are markers of in-hospital unfavorable outcomes. Implementation at the population-level of ApoE testing might be useful to better characterize the uncertain diagnosis of patients with lobar ICH who do not have a combination of aSAH and FLPs.
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_2396987320975736 for Clinical usefulness of Edinburgh CT criteria in patients with lobar intracerebral hemorrhage by Raffaele Ornello, Enrico Colangeli, Emanuele Tommasino, Cindy Tiseo, Giulia Perrotta, Ciro Scarpato, Martina Gentile, Leondino Mammarella, Carmine Marini, Francesca Pistoia, Alessandra Splendiani and Simona Sacco in European Stroke Journal
Acknowledgements
None.
Footnotes
Declaration of conflicting interests: RThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: O reports non-financial relationships with Novartis, Allergan, and Teva outside the submitted work; SS reports Dr Sacco reports personal fees and non-financial support from Allergan, personal fees and nonfinancial support from Abbott, personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from TEVA, personal fees from Medscape, other from Bayer, other from Pfizer, other from Medtronic, other from Starmed, other from Bristol-Myers Squibb, and other from Daiichi-Sankyo outside the submitted work; all the other Authors declare no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The study was approved by the Internal Review Board of the University of L’Aquila with the registration number 57/2019.
Informed consent: Every effort was made to obtain informed consent from the study participants.
Guarantor: SS.
Contributorship: RO and SS conceived the study. EC, ET, CT, GP, CS, MG, and LM were involved in patient recruitment and data analysis. FP and CM were involved in protocol development and gaining ethical approval. All authors reviewed and edited the manuscript and approved its final version.
ORCID iDs: Raffaele Ornello https://orcid.org/0000-0001-9501-4031
Emanuele Tommasino https://orcid.org/0000-0003-1523-8081
References
- 1.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8: 355–369. [DOI] [PubMed] [Google Scholar]
- 2.Sacco S, Marini C, Toni D, et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009; 40: 394–399. [DOI] [PubMed] [Google Scholar]
- 3.Sacco S, Ornello R, Degan D, et al. Declining incidence of intracerebral hemorrhage over two decades in a population-based study. Eur J Neurol 2016; 23: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 4.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and Meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke 1997; 28: 1418–1422. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues MA, Samarasekera N, Lerpiniere C, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol 2018; 17: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Istituto Nazionale di Statistica (ISTAT). Censimento della popolazione e delle abitazioni, https://dati.istat.it (2011, accessed 20 March 2020).
- 9.Mohr JP, Caplan LR, Melski JW, et al. The Harvard cooperative stroke registry: a prospective registry. Neurology 1978; 28: 754–762. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). Manual of the international statistical classification of diseases, injuries, and causes of death, 9th revision. Geneva (Switzerland): World Health Organization, 1977. [Google Scholar]
- 11.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 12.Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The University of Edinburgh. Edinburgh criteria for CAA-associated ICH training (ECCITING), www.ed.ac.uk/clinical-sciences/edinburgh-imaging/education-teaching/short-courses/training-tools/edinburgh-criteria-for-caa-associated-ich-training (accessed 20 March 2020).
- 14.Charidimou A, Schmitt A, Wilson D, et al. The cerebral haemorrhage anatomical RaTing inStrument (CHARTS): development and assessment of reliability. J Neurol Sci 2017; 372: 178–183. [DOI] [PubMed] [Google Scholar]
- 15.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996; 27: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 16.Zyck S, Du L, Gould G, et al. Scoping review and commentary on prognostication for patients with intracerebral hemorrhage with advances in surgical techniques. Neurocrit Care 2020; 33: 256–272. [DOI] [PubMed]
- 17.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012; 83: 124–137. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Arima H, Wu G, et al. Subarachnoid extension of intracerebral hemorrhage and 90-day outcomes in INTERACT2. Stroke 2014; 45: 258–260. [DOI] [PubMed] [Google Scholar]
- 19.Raposo N, Charidimou A, Roongpiboonsopit D, et al. Convexity subarachnoid hemorrhage in lobar intracerebral hemorrhage: a prognostic marker. Neurology 2020; 94: e968–e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roongpiboonsopit D, Charidimou A, William CM, et al. Cortical superficial siderosis predicts early recurrent lobar hemorrhage. Neurology 2016; 87: 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lun R, Yogendrakumar V, Demchuk AM, et al. Calculation of prognostic scores, using delayed imaging, outperforms baseline assessments in acute intracerebral hemorrhage. Stroke 2020; 51: 1107–1110. [DOI] [PubMed] [Google Scholar]
- 22.Cordonnier C, Demchuk A, Ziai W, et al. Intracerebral haemorrhage: current approaches to acute management. Lancet 2018; 392: 1257–1268. [DOI] [PubMed] [Google Scholar]
- 23.Schrag M, Kirshner H. Management of intracerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol 2020; 75: 1819–1831. [DOI] [PubMed] [Google Scholar]
- 24.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010; 74: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology 2018; 90: e119–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology 2017; 89: 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_2396987320975736 for Clinical usefulness of Edinburgh CT criteria in patients with lobar intracerebral hemorrhage by Raffaele Ornello, Enrico Colangeli, Emanuele Tommasino, Cindy Tiseo, Giulia Perrotta, Ciro Scarpato, Martina Gentile, Leondino Mammarella, Carmine Marini, Francesca Pistoia, Alessandra Splendiani and Simona Sacco in European Stroke Journal