Abstract
Background
Studies describing the clinical progression of animals with reverse patent ductus arteriosus (PDA) are lacking.
Objectives
To describe the signalment, presenting signs, echocardiographic features, and survival in a group of dogs and cats with bidirectional and continuous right‐to‐left PDA.
Animals
Forty‐six client‐owned animals included, comprising 43 dogs and 3 cats with bidirectional or continuous right‐to‐left PDA.
Methods
Retrospective multicenter study. Medical records and echocardiographic findings reviewed from animals diagnosed with bidirectional or continuous right‐to‐left PDA. Impact of ductal morphology, spectral Doppler flow profile, PCV, sildenafil treatment at presentation, sildenafil dose, severity of pulmonary hypertension, general anesthesia with or without surgery and the presence of right‐sided congestive heart failure (R‐CHF) on crude mortality rate were evaluated via Mantel‐Cox log rank comparison of Kaplan‐Meier survival curves. Univariable and multivariable Cox proportional hazards analysis was performed, and hazard ratio (HR) (95% confidence intervals [CI]) was presented.
Results
Hindlimb collapse was the most common presenting sign in dogs (n = 16). Clinical signs in cats were variable. Median survival time was 626 days in dogs (range 1‐3628 days). Dogs with R‐CHF had a shorter median survival time (58 days vs 1839 days, P = .03). Dogs treated with sildenafil at initial presentation survived longer (1839 days vs 302 days, P = .03), which was the only independent predictor of survival (HR 0.35, CI 0.15‐0.86, P = 0.021).
Conclusions and Clinical Importance
Dogs and cats with reverse PDA have a variable clinical presentation and prognosis. Survival time was longer in animals prescribed sildenafil at diagnosis. Dogs with R‐CHF at presentation have a worse overall outcome.
Keywords: congenital heart disease, polycythemia, right‐sided congestive heart failure, sildenafil
Abbreviations
- ASD
atrial septal defect
- CHF
congestive heart failure
- DCRV
double‐chambered right ventricle
- GA
general anesthesia
- LA
left atrium
- MDD
minimal ductal diameter
- PDA
patent ductus arteriosus
- PH
pulmonary hypertension
- PR
pulmonary regurgitation
- TR
tricuspid regurgitation
- TS
total solids
1. INTRODUCTION
Patent ductus arteriosus (PDA) is 1 of the most common congenital cardiac diseases in dogs, accounting for 11% to 30% of congenital heart defects. 1 , 2 , 3 , 4 It is less frequent in cats (representing only 3% of congenital heart defects 5 , 6 , 7 ).
The ductus arteriosus is a vessel that develops from the embryonic left sixth aortic arch. Typically, ductal flow decreases dramatically within the first 12 hours of life in the neonatal dog, ceasing altogether within 7 days, but in some individuals the ductus can remain patent. 8 Direction of blood flow through the PDA is determined by the relative resistances of the pulmonary and systemic vascular beds. Flow therefore typically proceeds from left‐to‐right, that is, from the aorta to the pulmonary artery. 9 In animals with severe pulmonary hypertension (PH), pulmonary vascular resistance can exceed systemic vascular resistance, leading to right‐to‐left shunting across the PDA, and mixing of deoxygenated blood in the descending aorta. There is a spectrum of disease, however, such that blood flow through the PDA can be continuously right‐to‐left or bidirectional, influenced by the severity of PH and systemic vascular resistance. Pulmonary hypertension and “shunt reversal” is a relatively uncommon complication, occurring in approximately 1% to 6% of dogs with PDA. 8 , 10 , 11 Cats appear to be more vulnerable to the development of PH, 12 with reverse or bidirectional shunting occurring more frequently in this species (~15%‐17% of cats with PDAs 13 , 14 ).
The etiology and timing of shunt reversal in dogs and cats with PH and PDA are poorly characterized and unpredictable. Increased endothelial shear stress due to augmented pulmonary blood flow can result in reactive vasoconstriction, progressive medial hypertrophy, intimal proliferation of the pulmonary vasculature, and shunt reversal (Eisenmenger's physiology). 1 , 15 , 16 In 1 colony of dogs, 17 PH and reversal of shunting developed within the first few weeks of life. Shunt reversal occurs after left‐sided congestive heart failure (CHF) in dogs 18 (but has not, as yet, been reported in cats) while others have presented with no prior indication of their congenital heart disease.
Polycythemia is a well‐recognized consequence of hypoxemia in animals with cyanotic congenital heart disease and the ensuing hyperviscosity, as opposed to CHF, is the most commonly reported cause of disease and death in animals with right‐to‐left PDA. 19 The need for frequent interventions (such as phlebotomy) to target polycythemia can be poorly tolerated by some animals, 15 , 20 and can result in serious owner concerns about the animal's quality of life.
There is paucity of information regarding the natural history and optimal treatment strategies for dogs and cats with reverse PDA. Information in the literature is limited to individual case reports, 21 , 22 , 23 , 24 small case series 9 , 11 , 15 , 25 or larger retrospective studies of PDA that include a small number of cases with continuous right‐to‐left or bidirectional flow through the ductus. 3 , 13 , 14 , 26 The aim of this study is to describe the signalment, presenting complaints, echocardiographic features, treatment, and survival time in a referral population of dogs and cats with bidirectional and continuous right‐to‐left PDA.
2. MATERIALS AND METHODS
This is a retrospective, multicenter cohort study. Ethical approval was sought from the Social Sciences Research Ethical Review Board (URN SR2019‐0080) at the Royal Veterinary College (RVC). Medical records from Southern Counties Veterinary Specialists, the RVC Queen Mother Hospital for Animals, Davies Veterinary Specialists and the University of Liverpool Small Animal Teaching Hospital were searched for dogs and cats examined between November 2003 and September 2019, using the key terms “reverse patent ductus arteriosus,” “bidirectional patent ductus arteriosus” and “right‐to‐left patent ductus arteriosus.” Dogs and cats were included in the study if they had been diagnosed with a continuous right‐to‐left or bidirectional shunting PDA by a board‐certified cardiologist or resident under their supervision. If the PDA was not visualized at the time of echocardiographic examination, a positive result after contrast echocardiography, assessed via the injection of agitated saline for a bubble study, was required to verify the presence of continuous right‐to‐left or bidirectional shunting. The term “reverse” PDA is used to describe cases with either continuous right‐to‐left or bidirectional PDA.
Information was collected regarding signalment, presenting signs, radiographic and echocardiographic findings, treatment and survival status.
All echocardiographic measurements were performed by a board‐certified cardiologist or supervised cardiology resident. Where available, echocardiographic data recorded included the presence or absence of subjective concentric right ventricular hypertrophy (right ventricular wall thickness subjectively equal to or exceeding left ventricular wall thickness), flattening of the interventricular septum and concurrent congenital cardiac disease. Left atrial (LA) size was quantified using the LA diameter to aortic root diameter ratio (LA : Ao), measured from the right parasternal short axis view at the level of the heart base, the first frame after aortic valve closure. 27
Ductal morphology was characterized according to the Miller classification system. 28 The inner edge‐to‐inner edge method was utilized to measure ductal dimensions: The minimal ductal diameter (MDD) was considered the width of the ductus at its most narrow diameter. 28 , 29 Results of ductal spectral Doppler interrogation were recorded. Where present, maximal tricuspid regurgitation (TR) and pulmonary regurgitation (PR) velocities (Vmax) were used to quantify pulmonary systolic and mean diastolic pressure using the modified Bernoulli equation (the instantaneous pressure gradient = 4v, 2 where v is the maximal velocity measured in m/s 30 , 31 ). Estimated right atrial pressure was not accounted for, so minimum values for systolic pulmonary artery pressure were recorded. Pulmonary hypertension was confirmed when peak velocities exceeded 3 m/s, for TR, or 2.2 m/s, for PR, in the absence of right ventricular outflow tract obstruction. 30 , 32 , 33
Contrast echocardiography, performed via injection of agitated saline into a peripheral vein, was used to visualize the passage of contrast through the abdominal aorta. 30 , 34 Visualization of contrast within the descending aorta was required for verification of reverse PDA in cases where spectral Doppler interrogation of the ductus was not available, in the absence of a concurrent right‐to‐left intracardiac shunt. Cases with concurrent intracardiac right‐to‐left shunting, determined by the presence of echodense contrast within the left side of the heart, were recorded. 34 The presence or absence of arrhythmias was determined from available paper ECG recordings, ECGs, or both recorded concurrently to echocardiographic examinations.
Packed cell volume and total solids (TS) were determined by centrifugation of fresh whole blood in heparinized microcapillary tubes and were recorded at the time of presentation. 9
Where additional diagnostic tests were performed to explore for possible causes of PH, details of the tests and results were recorded. Available thoracic radiographs were reviewed by a board‐certified radiologist or supervised resident at the time of presentation. Information on cardiac size and radiographic lung pattern were obtained from radiographic reports.
Details of medications prescribed at initial presentation were recorded. This included treatment with sildenafil (Viagra, Pfizer, U.S. Pharmaceuticals, New York, New York), myelosuppressive agents, and any other oral or parenteral medications. When phlebotomy was performed, preprocedural and postprocedural PCV were documented in addition to the total volume of blood removed. Information on any surgical or minimally invasive interventional procedures were documented.
Survival data were obtained from clinical records. Where the date or cause of death was not documented, referring practices were contacted between August and September 2019 to establish the outcome for each dog and cat.
2.1. Statistical analysis
Descriptive statistical analysis was performed on the data collected from the medical records and analyses were performed using commercially available software (BM SPSS Statistics 21.0 for Windows 7, IBM (UK) Ltd, Portsmouth, UK; GraphPad Prism 6, GraphPad Software Inc, San Diego, California). Continuous data were assessed for normality using the Shapiro‐Wilk test. Normally distributed data are presented as mean (±SD) and nonnormally distributed data are reported as median [range]. Median PCV values were compared between dogs grouped according to initial presenting signs using the Kruskal‐Wallis test, with the Dunn test used for post hoc pairwise comparisons.
Kaplan‐Meier survival curves were generated to assess the impact of ductal morphology, PCV, sildenafil administration at presentation, sildenafil dose, severity of PH, anesthesia (with or without surgery) and the presence of R‐CHF on median survival time. Data were censored if the animal was still alive or lost to follow‐up at the end of the study period and survival times were reported as medians (range). Differences between groups were analyzed using the Logrank (Mantel‐Cox) test.
For the Cox proportional hazards model, variables were also dichotomized using cut‐offs derived from the median values and were taken forward to the multivariable analysis as categorical data. Variables with a P value <.2 on univariable analysis were entered into a multivariable Cox regression analysis using a manual forward selection method to identify independent predictors of survival. The variable with the lowest P value was entered into the model at each iteration. The order in which variables were entered into the model was based on the strength of their association, with covariates with the lowest P values from the previous step entered first. Hazard ratios (HR) and 95% confidence intervals (CI) for the Cox regression were presented. A statistically significant result was defined as a P value <.05.
3. RESULTS
Forty‐six animals met the inclusion criteria, comprising 43 dogs and 3 cats. Signalment data are summarized in Table 1. Of the dogs, the breeds represented were Jack Russell Terrier (n = 9), Crossbreed (n = 5), Chihuahua (n = 3), Pembroke Welsh Corgi (n = 3), Border Terrier (n = 2), Shetland Sheepdog (n = 2), Patterdale Terrier (n = 2), and 1 each of the following: Border Collie, Greyhound, Havanese, Hungarian Vizsla, Italian Spinoni, Labarador Retriever, Lakeland Terrier, Lhasa Apso, Miniature Dachshund, Norwich Terrier, Old English Sheepdog, Pyrenean Shepherd, West Highland White Terrier, Yorkshire Terrier, Dachshund, Toy Poodle, and Shih Tzu. Two Siamese cats and 1 Domestic Shorthair were included in the study.
TABLE 1.
Demographic features and presenting signs for dogs and cats with reverse patent ductus arteriosus (PDA)
| Demographic and presenting signs | ||
|---|---|---|
| Variable | Dogs, n = 43 | Cats, n = 3 |
| Age (months) | 11.0 [44.0‐140.0] | 42.7 (± 22.7) |
| Females | 34 | 2 |
| Body weight (kg) | 6.6 [3.9‐9.4] | 3.4 |
| Presenting signs | ||
| Free from clinical signs | 4 | 1 |
| Hind limb collapse | 16 | — |
| Exercise intolerance | 9 | — |
| Abdominal distension | 3 | 1 |
| Signs of neurological disease | 3 | — |
| Syncope | 3 | — |
| Tachypnoea, dyspnoea, or both | 1 | 1 |
| Cough | 2 | — |
| Signs of gastrointestinal disease | 2 | — |
Note: Normally distributed data are presented as mean (±SD) and nonnormally distributed data are reported as median [range].
Receiving services at presentation included cardiology (n = 25), internal medicine (n = 11), neurology (n = 6), and emergency critical care (n = 4). Initial presenting signs were recorded for all 46 cases and are summarized in Table 1. The most common presenting signs in dogs were hindlimb collapse (n = 16) and exercise intolerance (n = 9). The 3 dogs with abdominal distension had ascites secondary to R‐CHF. The neurological signs reported in 3 dogs included forebrain signs (circling), acute onset vestibular signs and seizures. Presenting complaints in cats included tachypnoea (n = 1), abdominal distension (n = 1) and 1 cat was free of clinical signs.
Only 1 dog had a continuous murmur prior to referral. Auscultation abnormalities at presentation included a split or loud second heart sound in 15 dogs, 4 of which had a concurrent heart murmur. Eighteen dogs had a heart murmur in the absence of S2 abnormalities. Three dogs had a diastolic component in addition to a systolic component and 1 of these dogs also had a loud S2. Murmur point of maximum intensity was variably reported. Ten dogs had no abnormalities reported after cardiac auscultation. Two cats had heart murmurs and 1 had a split second heart sound. Fifteen animals (14 dogs and 1 cat) had a murmur reported in their previous history. Differential cyanosis was assessed in 26 dogs and present in 11 out of 26 dogs.
Electrocardiography was performed in 20 dogs and right mean electrical axis deviation was the most frequently reported abnormality (n = 11). Additional electrocardiographic abnormalities included isolated ventricular premature complexes (n = 1), paroxysmal supraventricular tachycardia (n = 1) and sinus arrhythmia with second degree atrioventricular block in a dog that had been sedated with butorphanol prior to echocardiographic examination.
Packed cell volume and TS recorded on presentation were 60% (±13.13) and 63.35 g/L (±7.95), respectively. The median PCV was compared between dogs grouped according to 4 common presenting signs (hindlimb collapse, exercise intolerance, neurological signs, and dogs who were asymptomatic at presentation). Dogs who had neurological signs at presentation had a significantly higher PCV compared to those who were asymptomatic (P = .01, Figure 1).
FIGURE 1.
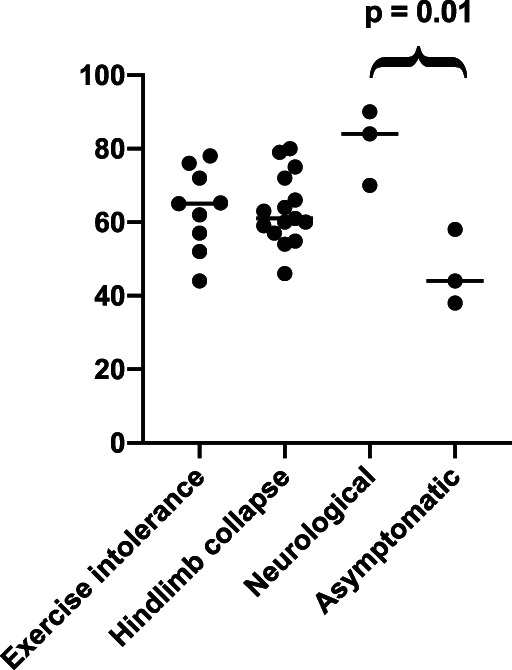
Median PCV values of dogs grouped according to initial presenting signs of hindlimb collapse, signs of neurological disease, exercise intolerance, and dogs who were asymptomatic
Thoracic radiographic reports were available for review in 32 animals (30 dogs and 2 cats). Abnormalities included right‐sided cardiac enlargement in 30 animals, with normal pulmonary opacity (17 dogs and 1 cat) and increased pulmonary opacity (11 dogs and 1 cat), respectively. Bilateral cardiac enlargement with a bronchointerstitial pattern was present in 1 dog and a diffuse interstitial lung pattern with no reported cardiac enlargement in another dog. Of the 12 animals with right‐sided cardiac enlargement and increased pulmonary opacity, the following lung patterns were described: interstitial (n = 1), bronchointerstitial (n = 5), patchy pulmonary infiltrate (n = 3, including 1 cat), alveolar pattern (n = 1), and a nonspecific increase in pulmonary opacity (n = 2). In 16 cases, dilation of the main pulmonary artery was reported.
Echocardiography was performed in all 46 animals. Direct visualization of the ductus was achieved and recorded in 39/46 cases. The distribution of ductal morphology types is presented in Table 2. Two cats had a type III and 1 cat had a type IIb ductus. The mean minimal ductal diameter of the entire study sample was 6.14 mm (±2.78). Spectral Doppler flow profiles were available for review in 35 animals (32 dogs and 3 cats) and showed continuous right‐to‐left shunting and bidirectional flow in 16 dogs each, respectively. All 3 cats had bidirectional flow across the ductus. Concentric right ventricular hypertrophy was present in 40 dogs and 3 cats. Septal flattening was assessed in 30 animals (29 dogs and 1 cat) and was present in all cases. Left atrial size according to LA : Ao for the entire group was 1.28 (±0.26) (see Table 3). Additional congenital cardiac abnormalities included tricuspid valve dysplasia in 4 dogs, patent foramen ovale (right‐to‐left shunting), persistent left cranial vena cava and mitral stenosis in 1 dog each, respectively. The severity of mitral stenosis was not described. A bubble study was performed to verify the presence of the persistent left cranial vena cava, through the injection of agitated saline via the left cephalic vein. One cat had a double‐chambered right ventricle (DCRV) and right‐to‐left shunting across an atrial septal defect (ASD).
TABLE 2.
Ductal morphology grouped according to the Miller classification system in dogs and cats
| Ductal morphology | Dogs | Cats |
|---|---|---|
| IIa | 2 | — |
| IIb | 9 | 1 |
| III | 15 | 2 |
| Unavailable a | 17 | — |
Unable to access echo to confirm patent ductus arteriosus (PDA) morphology (n = 8), PDA not visualized at the time of echo (n = 7), and poor‐quality image of the ductus (n = 2).
TABLE 3.
Specific echocardiographic measures of pulmonary hypertension, ductal diameter, and left atrial size in dogs and cats
| Echocardiographic findings | |
|---|---|
| Variable | Result |
| MDD type II | 5.30 mm (±2.45) |
| MDD type III | 6.85 mm [4.90‐8.87] |
| MDD overall | 6.14 mm (±2.78) |
| TR maximal velocity | 5.14 m/s (±0.72) |
| PR maximal velocity | 3.90 m/s (±0.59) |
| LA : Ao | 1.28 (±0.26) |
Note: Normally distributed data are presented as mean (±SD) and nonnormally distributed data are reported as median [range].
Abbreviations: LA : Ao, left atrial to aortic ratio; MDD, minimal ductal diameter; PR, pulmonic regurgitation; TR, tricuspid regurgitation..
Contrast echocardiography was performed in 36 dogs and 2 cats. In the remaining 7 dogs and 1 cat, spectral Doppler profiles across the ductus were available for review for verification of continuous right‐to‐left or bidirectional shunting. A positive result, determined by the presence of contrast in the abdominal aorta, was present in 33 dogs. In 2 dogs, contrast could not be visualized in the descending aorta, but bidirectional flow was confirmed across the ductus on spectral Doppler traces. In 1 dog and 2 cats, the aorta was not assessed during contrast echocardiography (only the heart for concurrent intracardiac shunting); however, spectral Doppler profiles were also available for these cases.
Pulmonary hypertension was confirmed in 34 cases (32 dogs and 2 cats) via spectral Doppler interrogation of tricuspid and PR velocities. Mean TR velocity and PR velocity were 5.14 m/s (±0.72) and 3.90 m/s (±0.59), respectively. Investigations to establish the etiology of PH were performed in 14 dogs and included Baermann fecal flotation (n = 6), IDEXX Angio Detect (n = 1), Baermann and IDEXX Angio Detect (n = 5), Baermann and SNAP 4Dx serology screen for Dirofilaria immitis (n = 1), d‐dimers and urine‐protein‐creatinine ratio (n = 1). Results were unremarkable apart from in 1 dog with unexplained proteinuria, in which urine sediment evaluation and culture were both negative. In this dog, hypercoagulability or potential thromboembolic disease could not be confirmed or rejected as a possible cause of PH.
Thirty‐two dogs and 1 cat received sildenafil on presentation. The median total dose of sildenafil was 3.0 mg/kg/d [1.8‐5.1]. Twenty‐three animals received additional medications including pimobendan (n = 8), bosentan (n = 1), furosemide either alone (n = 1), or in combination with benazepril, clopidogrel, aspirin, pimobendan and spironolactone (n = 4), fenbendazole alone (n = 1), or in combination with potentiated amoxicillin or trimethroprim sulphonamide (n = 2), hydroxyurea alone (n = 2), or with terbutaline (n = 1) and 1 dog was treated with clopidogrel alone (n = 1). One dog presenting with seizures was treated with phenobarbital.
Therapeutic phlebotomy was performed in 13 dogs, the PCVs of which ranged from 65% to 90% with a mean of 73.9%. The total volume of blood collected was only available for 8/13 dogs, with a median value of 10.0 mL/kg [8.9‐19.0]. Medical leech phlebotomy was performed in 1 dog (a Pembroke Welsh Corgi) after traditional phlebotomy had been performed on 3 occasions, reducing her PCV from 67% to 56%. Records of total PCV reduction after phlebotomy were only available for 4 dogs: 2 Jack Russell Terriers, from 78% to 67% and 72% to 60%, respectively, and 1 Norwich Terrier, from 70% to 58%. A Patterdale Terrier underwent phlebotomy, which reduced his PCV 90% to 67%, but the volume removed was not recorded.
Survival information was available for 38 animals. The remaining 8 were lost to follow‐up. At the time of data collection, 12 animals were alive and 26 had died. The median survival time for dogs was 626 days (range 1‐3628, Figure 2). The median survival time was not calculated in cats due to the low numbers included. Their individual survival times were 1, 8, and 2473 days.
FIGURE 2.
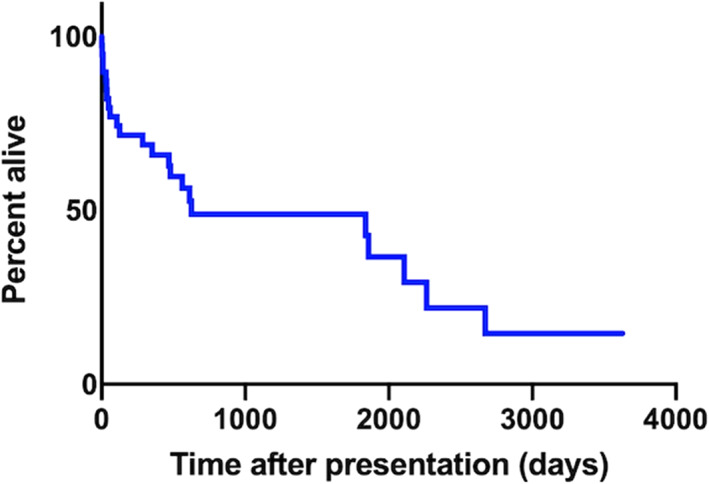
Kaplan‐Meier curve to show overall survival of dogs with bidirectional or right‐to‐left shunting patent ductus arteriosus (PDA)
Sixteen dogs were euthanised. The reasons for euthanasia included progressive dyspnoea (n = 3), unspecified progressive clinical signs (n = 7), haemorrhagic gastroenteritis and hypovolaemic shock (n = 1), owner decision not to treat at time of diagnosis (n = 1), renal disease (n = 1), seizures (n = 1), but was not recorded in 2 dogs. Five dogs died without euthanasia. The cited cause of death included a road traffic accident (n = 1), unexpected death after collapse at home (n = 1) and death due to unknown cause in 3 dogs. Five animals died during or shortly after general anesthesia (GA). In 3 of these cases, closure of the ductus had been attempted. A West Highland White Terrier developed progressive dyspnoea after surgical ligation of the ductus and cardiopulmonary arrest occurred 7 days after surgery. Minimally invasive closure of the ductus was attempted using the Amplatz canine duct occluder in a greyhound, but the procedure was aborted due to instability under GA. Surgical closure of the ductus was attempted in the same dog 4 weeks later, but it developed ventricular fibrillation at the time of ductal ligation and died. One cat experienced major hemorrhage at the time of ductal ligation and died in theater.
Two animals died during GA that was unrelated to any attempt to close the ductus: One dog (a Shih Tzu) died after intubation for surgical management of a compressive spinal cord lesion between L4 and L7 due to suspected hemorrhage. Frank blood was visualized in the endotracheal tube and the surgery was not attempted. The dog died shortly afterwards, and signs were attributed to a coagulopathy. The cat with concurrent right‐to‐left shunting ASD and DCRV was anesthetized for cardiac catheterisation and transoesophageal echocardiography. She developed third‐degree atrioventricular block and profound hypoxemia under anesthesia and died after failed cardiopulmonary resuscitation. Five of the 6 animals in which shunt closure was attempted received sildenafil preoperatively; the cat who died after hemorrhage during duct ligation did not receive sildenafil. The sildenafil dose range was 2.5 to 15 mg/kg/d. Successful ductal ligation via surgical ligation was achieved in 3 cases: a Siamese cat and Chihuahua, who were both alive at the time of data collection (follow‐up 2473 and 966 days, respectively), and a Jack Russell Terrier who was lost to follow‐up, but was last known to be alive at 74 days postsurgery.
Neither ductal flow (whether bidirectional or continuous right‐to‐left) or ductal morphology (type II vs type III) influenced outcome (P = .78 and P = .25, respectively). Likewise, TR velocity (>5.14 m/s) and PCV at presentation (>60%) had no impact on outcome (P = .36 and P = .62, respectively). Dogs that were treated with sildenafil at presentation lived longer than those without (1839 days vs 302 days, P = .03, Figure 3); however, the dose of sildenafil did not impact survival (562 days vs 1839 days, P = .91, Figure 4). Dogs with R‐CHF at presentation had a worse survival (58 days vs 1839 days, P = .03, Figure 5). When comparing dogs that underwent GA with or without surgical closure of the ductus to those who did not, there was no significant difference in outcome (P = .11).
FIGURE 3.
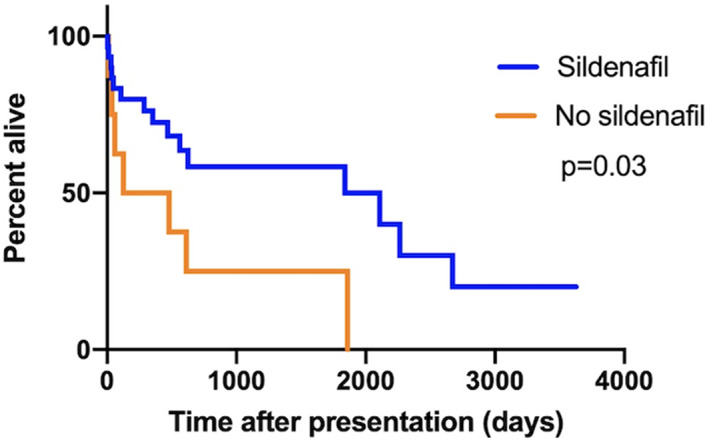
Kaplan‐Meier curves to explore the difference in median survival time between dogs treated with sildenafil and those not treated with sildenafil in dogs with bidirectional or right‐to‐left shunting patent ductus arteriosus (PDA)
FIGURE 4.
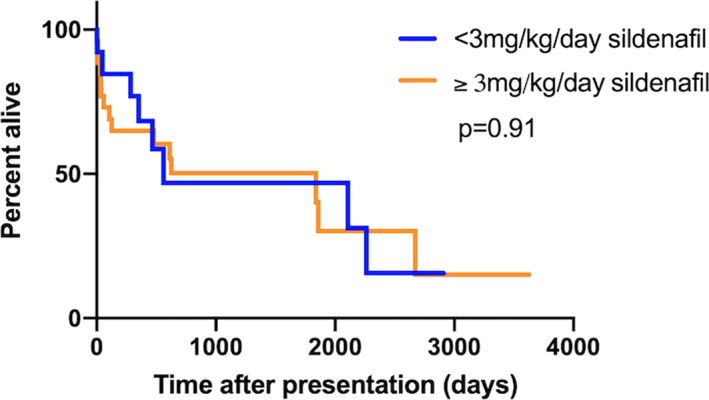
Kaplan‐Meier curves to explore the difference in median survival time between dogs receiving sildenafil dose <3 mg/kg/d and those receiving sildenafil dose ≥3 mg/kg/d in dogs with bidirectional or right‐to‐left shunting patent ductus arteriosus (PDA)
FIGURE 5.
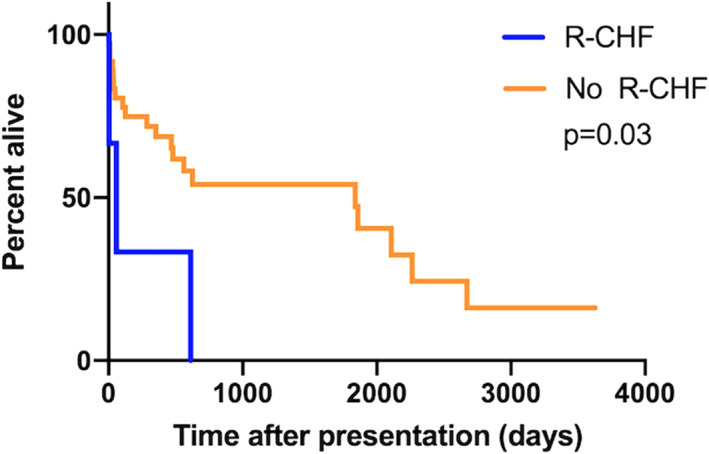
Kaplan‐Meier curves to explore the difference in median survival time between dogs in right‐sided congestive heart failure (R‐CHF) compared to those without R‐CHF in dogs with bidirectional or right‐to‐left shunting patent ductus arteriosus (PDA)
Presence of R‐CHF (P = .04), treatment with sildenafil (P = .021), age ( = 0.06), and minimum ductal diameter (P = 0.20) were evaluated in multivariable Cox regression. Treatment with sildenafil was the only independent predictor of crude mortality rate (HR 0.35, CI 0.15‐0.86, P = .02).
4. DISCUSSION
This multicenter study describes the natural history and outcome of dogs and cats with reverse PDA. In agreement with previous reports, presenting signs were variable. 9 , 15 It is therefore unsurprising that animals were referred to a variety of disciplines, including neurology, internal medicine, and emergency critical care. This wide range of clinical presentations adds to the diagnostic challenge in evaluating dogs and cats with reverse PDA.
Survival times for dogs with R‐CHF were short, although this was a complicating factor in only a small number of cases. Right heart function is challenging to determine with echocardiography due to the complex geometry of the right ventricle 35 , 36 ; however, the presence of R‐CHF is suggestive of secondary right‐sided myocardial dysfunction in these animals (although the impact of concurrent right‐sided congenital disease, such as TVD, cannot be excluded). The development of R‐CHF is also a poor prognostic indicator in dogs with pulmonic stenosis (rather than PH), 37 , 38 , 39 and worse outcomes are similarly reported in people with R‐CHF due to Eisenmenger's physiology. 40 Interestingly, in this study, median survival time in dogs with bidirectional and right‐to‐left PDA without R‐CHF at the time of presentation was greater than 5 years, suggesting that dogs without R‐CHF can have a good prognosis despite having reverse PDA.
Estimated pulmonary arterial pressures were not predictors of death in this study sample, but are independently associated with survival in people with Eisenmenger syndrome, alongside World Health Organization functional class for PH and treatment with sildenafil. 41 Pulmonary hypertension can result from persistence of the foetal pulmonary circulation, development of acquired lesions or a combination of both mechanisms. 8 , 11 , 17 , 18 , 42 , 43 Several factors including the nitric oxide pathway can contribute to the development of acquired PH. 44 , 45 Sildenafil citrate facilitates nitric oxide‐cyclic guanosine monophosphate (cGMP)‐induced vasodilatation by inhibiting the degradation of cGMP. There is currently no licensed preparation available for use in dogs; however, a number of studies have described its use in dogs with PH. 25 , 32 , 46 , 47 Information regarding the use of sildenafil in a large group of dogs with reverse PDA was previously lacking, although in 1 small case series of 5 dogs with reverse PDA, 25 New York Heart Association functional class significantly improved after 1 and 3 months of sildenafil and PCV significantly decreased after 3 months of treatment. 25 In our study, treatment with sildenafil (but not dose) was the only independent prognostic predictor of survival in dogs. This is challenging to interpret given the study's retrospective design and the reasons for prescribing this medication to specific animals may be affected by unknown bias. These results are in agreement, however, with studies investigating treatment of people with Eisenmenger physiology, in whom the use of pulmonary vasodilators (bosentan and sildenafil) have been demonstrated to be independently associated with survival. 48 One study demonstrated that 1‐ and 3‐year survival rates are significantly higher in people treated with sildenafil vs those receiving conventional treatment (digoxin, diuretics, and anticoagulants). 41 In this study, sequential echocardiographic estimates of pulmonary arterial pressures after the introduction of sildenafil and any dose adjustments performed over time were inconsistently reported. Consequently, we are unable to assess the impact of these factors on survival, which is a limitation of this study.
In dogs and cats, there is a general consensus that ductal closure is contraindicated with right‐to‐left shunting PDAs. 9 Complete attenuation of the shunt precludes access of the right ventricular blood into the systemic circulation, markedly increasing right ventricular afterload, risking shock, and possible death. 8 Treatment has therefore focused on amelioration of clinical signs of hypoxemia and correction of polycythemia to reduce blood viscosity and improve tissue perfusion. 15
Previous studies have reported successful closure of PDA in animals with PH, but continuous left‐to‐right shunting was present in all cases. There are no reports of successful occlusion of right‐to‐left shunting PDAs in the literature. Six animals (4 dogs and 2 cats) in our study underwent attempted closure of the ductus. Five of the 6 animals in which shunt attenuation was attempted received loading treatment with sildenafil and all 6 had bidirectional shunting on spectral Doppler interrogation prior to closure. Three animals died during or in the immediate post‐operative period, but those that survived surgery with follow‐up (3) had good long‐term survival.
Determination of the reversibility of vascular lesions is key to the decision‐making process in people with congenital cardiac shunts with PH prior to closure. Lung biopsy, hemodynamic changes during test occlusion, and response to pulmonary vasodilators are used to evaluate the reversibility of pulmonary vascular disease. 49 , 50 The most severe vascular changes accompanying PH, such as fibrinoid necrosis or plexiform lesions, are considered irreversible. 8 , 17 , 51 People with severe histologic changes, poorly responsive pulmonary vascular beds, or worsened pulmonary arterial pressure with test occlusion are typically considered inoperable. 49 Test occlusion at the time of surgical ligation has been described in 1 cat with PH and continuous left‐to‐right shunting PDA. 12 Invasive right ventricular pressures were measured simultaneously and did not increase after test occlusion. Lung biopsy performed at the time of thoracotomy revealed histopathological arterial changes consistent with PH (smooth muscle hypertrophy of small pulmonary arteries) but no arteritis or plexiform lesions. 12 It could be postulated that reasons for excellent tolerance after PDA closure in this cat and in the animals who survived surgical ligation in our study, are due to reversible pulmonary vascular disease.
Treatment targeting secondary polycythemia has been considered the cornerstone of management of dogs with reverse PDA, aiming to maintain the PCV below a threshold of 65%. 8 Only 13 dogs received phlebotomy in our group and the clinical rationale (ie, whether based on PCV or clinical signs) was not always documented. The median presenting PCV in our study was relatively low (60%, 36%‐90%), which might explain why phlebotomy was infrequently recommended. In people with polycythemia caused by right‐to‐left cardiac shunts, phlebotomy is reserved for symptomatic patients, even when the patient PCV exceeds 70%. 52 As the effects of phlebotomy are transient, and sequelae such as iron deficiency can develop, phlebotomy is recommended for temporary relief of hypervisocity symptoms and not for hematocrit level per se. In summary, phlebotomy is not recommended routinely in asymptomatic/mildly symptomatic human patients, regardless of hematocrit. 53 , 54 , 55 Similarly, best practice for managing polycythemia in dogs remains unclear. Dogs with signs of neurological disease at presentation had a significantly higher PCV compared to those who were asymptomatic. This difference, however, should be interpreted with caution due to the small number of dogs included within both of these groups. All 3 dogs with signs of neurological disease underwent phlebotomy.
Three dogs were treated with hydroxyurea for the management of polycythemia, 2 of which underwent concurrent phlebotomy. Hydroxyurea is recommended for the treatment of polycythemia in dogs, cats, and humans. 15 , 56 Despite a number of potential advantages of hydroxyurea over phlebotomy in polycythemic animals (avoidance of weakness and reduced risk of iron deficiency), 57 adverse effects can include anorexia, vomiting, bone marrow hypoplasia, and alopecia. This, in combination with the study sample's relatively low PCV at presentation, might explain the infrequent use of myelosuppressive agents in this study group.
We know that studies utilizing multidimensional imaging to evaluate ductal geometry and dimensions have demonstrated that the Miller classification system (derived from angiographic studies), might be inappropriate in classifying ductal morphology due to the highly variable and unusual appearances of PDA in dogs. Unfortunately, transesophageal echo and angiography were performed infrequently, and therefore, more detailed evaluation of ductal geometry in these dogs and cats is lacking.
Reasons for attempting to describe morphology in this group, is that traditionally, dogs with a cylindrical, nontapering ductal morphology were considered to be at higher risk for developing right‐to‐left shunting compared to those with a tapering, funnel‐shaped ductus. 1 Thus, dogs with large, tubular PDA, and higher shunt fractions might have increased risk of developing Eisenmenger's physiology. It is interesting that in our study, however, a number of individuals were included with a type II or tapering ductal morphology (12 vs 17 with type III), albeit using a classification system that has inherent limitations. 28
There are several limitations of this study, many of which relate to its retrospective design. The images from a number of digitally stored echocardiographic examinations were corrupted and unavailable for review. In 7 cases, the PDA was not visualized at the time of echocardiographic assessment and diagnosis was based on compatible clinical signs, supportive echocardiographic findings and a positive result following bubble study, after exclusion of intracardiac right‐to‐left shunts. Consequently, relying on the latter for diagnosis could have led to the inclusion of other forms of pulmonary‐to‐systemic shunts or pulmonary arteriovenous malformations that result in microcavitations bypassing the pulmonary capillaries and entering the descending aorta. Ductal velocities generated from Doppler interrogation to estimate pressure gradients between the pulmonary artery and aorta were inconsistently recorded. Ampulla diameter data were variably recorded and therefore ratios such as MDD: ampulla diameter could not be calculated. Radiographic findings were taken directly from reports, which might result in underreporting of certain abnormalities. Introduction of medical treatment was uncontrolled and based on the preference of the clinician managing the case. Secondary causes of PH were infrequently investigated or reported. The clinical signs associated with reverse PDA can be extremely variable and differentiating between cardiac causes and noncardiac causes of death can be extremely challenging. Consequently, crude mortality rate rather than cardiac mortality was evaluated during our survival analysis. Longitudinal follow‐up varied according to clinician preference and owner compliance.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGEMENT
No funding was received for this study. Some of these results were presented at the 2020 Veterinary Cardiovascular Society (VCS) Annual meeting, April 1, 2020. The authors thank Dr Ruby Chang for her contribution to the statistical analyses.
Greet V, Bode EF, Dukes‐McEwan J, Oliveira P, Connolly DJ, Sargent J. Clinical features and outcome of dogs and cats with bidirectional and continuous right‐to‐left shunting patent ductus arteriosus. J Vet Intern Med. 2021;35:780–788. 10.1111/jvim.16072
David J. Connolly and Julia Sargent are senior authors.
REFERENCES
- 1. Buchanan JW. Patent Ductus Arteriousus morphology, pathogenesis, types and treatment. J Vet Cardiol. 2001;3(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 2. Oliveira P, Domenech O, Silva J, Vannini S, Bussadori R, Bussadori C. Retrospective review of congenital heart disease in 976 dogs. J Vet Intern Med. 2011;25(3):477‐483. [DOI] [PubMed] [Google Scholar]
- 3. Tidholm A. Retrospective study of congenital heart defects in 151 dogs. J Small Anim Pract. 1997;38:94‐98. [DOI] [PubMed] [Google Scholar]
- 4. Schrope DP. Prevalence of congenital heart disease in 76,301 mixed‐breed dogs and 57,025 mixed‐breed cats. J Vet Cardiol. 2014;17(3):192‐202. [DOI] [PubMed] [Google Scholar]
- 5. Tidholm A, Ljungvall I, Michal J, Häggström J, Höglund K. Congenital heart defects in cats: a retrospective study of 162 cats (1996‐2013). J Vet Cardiol. 2015;17:S215‐S219. [DOI] [PubMed] [Google Scholar]
- 6. Liu SK. Pathology of feline heart diseases. Vet Clin North Am. 1977;7(2):323‐339. [DOI] [PubMed] [Google Scholar]
- 7. Hutton JE, Steffey MA, Runge JJ, McClaran JK, Silverman SJ, Kass PH. Surgical and nonsurgical management of patent ductus arteriosus in cats: 28 cases (1991–2012). J Am Vet Med Assoc. 2015;247(3):278‐285. [DOI] [PubMed] [Google Scholar]
- 8. Bonagura J, Lehmkuhl L. Chapter 24 congenital heart disease. In: Fox PR, Sisson D, Moïse NS, eds. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. Philadelphia: Saunders; 1999. [Google Scholar]
- 9. Côté E, Ettinger SJ. Long‐term clinical management of right‐to‐left (“reversed”) patent ductus arteriosus in 3 dogs. J Vet Intern Med. 2001;15(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 10. Kittleson MD. Chapter 12 patent ductus arteriosus. In: Kittleson MD, Kienle RD, eds. Small Animal Cardiovascular Medicine. St Louis, Missouri: Elsevier; 1998:603. [Google Scholar]
- 11. Oswald GP, Orton EC. Patent ductus arteriosus and pulmonary hypertension in related Pembroke Welsh corgis. J Am Vet Med Assoc. 1993;202(5):761‐764. [PubMed] [Google Scholar]
- 12. Novo‐Matos J, Hurter K, Bektas R, Grest P, Glaus T. Patent ductus arteriosus in an adult cat with pulmonary hypertension and right‐sided congestive heart failure: hemodynamic evaluation and clinical outcome following ductal closure. J Vet Cardiol. 2014;16(3):197‐203. [DOI] [PubMed] [Google Scholar]
- 13. Bascuñán A, Thieman Mankin KM, Saunders AB, et al. Patent ductus arteriosus in cats (Felis catus): 50 cases (2000‐2015). J Vet Cardiol. 2017;19(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 14. Wustefeld‐Janssens BG, Burrow R, Mõtsküla P, Martin M, Dukes‐McEwan J. Clinical findings and treatment outcomes for cats diagnosed with patent ductus arteriosus in the UK: a retrospective study of 19 cases (2004‐2012). Vet Rec. 2016;179(1):17. [DOI] [PubMed] [Google Scholar]
- 15. Moore KW, Stepien RL. Hydroxyurea for treatment of polycythemia secondary to right‐to‐left shunting patent ductus arteriosus in 4 dogs. J Vet Intern Med. 2001;15:418‐421. [PubMed] [Google Scholar]
- 16. Duffels MGJ, Engelfriet PM, Berger RMF, et al. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol. 2007;120(2):198‐204. [DOI] [PubMed] [Google Scholar]
- 17. Patterson DF, Pyle RL, Buchanan JW, Trautvetter E, Abt DA. Hereditary patent ductus arteriosus and its sequelae in the dog. Circ Res. 1971;29(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 18. Pyle RL, Park RD, Alexander AF, Hill BL. Patent ductus arteriosus with pulmonary hypertension in the dog. J Am Vet Med Assoc. 1981;178(6):565‐571. [PubMed] [Google Scholar]
- 19. Beijerink NJ, Oyama MA, Bonagura JB. Chapter 250 congenital heart disease. In: Ettinger SJ, Feldman EC EC, eds. Textbook of Veterinary Internal Medicine. St Louis, Missouri: Elsevier; 2017. [Google Scholar]
- 20. Buchanan JW, Patterson DF. Etiology of patent ductus arteriosus in dogs. J Vet Intern Med. 2003;17(2):167‐171. [DOI] [PubMed] [Google Scholar]
- 21. Ferasin L, Rizzo F, Darke PGG. Original investigation of right‐to‐left shunting patent ductus arteriosus in an Irish setter puppy. Vet J. 2007;173(2):443‐448. [DOI] [PubMed] [Google Scholar]
- 22. Kolm US, Amberger CN, Boujon CE, Lombard CW. Plexogenic pulmonary arteriopathy in a Pembroke welsh corgi. J Small Anim Pract. 2004;45(9):461‐466. [DOI] [PubMed] [Google Scholar]
- 23. Russell NJ, Irwin PJ, Hopper BJ, Olivry T, Nicholls PK. Acute necrotising pulmonary vasculitis and pulmonary hypertension in a juvenile dog. J Small Anim Pract. 2008;49(7):349‐355. [DOI] [PubMed] [Google Scholar]
- 24. Connolly DJ, Lamb CR, Boswood A. Right‐to‐left shunting patent ductus arteriosus with pulmonary hypertension in a cat. J Small Anim Pract. 2003;44(4):184‐188. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura K, Yamasaki M, Ohta H, et al. Effects of sildenafil citrate on five dogs with Eisenmenger's syndrome. J Small Anim Pract. 2011;52(11):595‐598. [DOI] [PubMed] [Google Scholar]
- 26. Saunders AB, Gordon SG, Boggess MM, Miller MW. Long‐term outcome in dogs with patent ductus arteriosus: 520 cases (1994‐2009). J Vet Intern Med. 2014;28(2):401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med. 2000;14:429‐435. [DOI] [PubMed] [Google Scholar]
- 28. Miller MW, Gordon SG, Saunders AB, et al. Angiographic classification of patent ductus arteriosus morphology in the dog. J Vet Cardiol. 2006;8(2):109‐114. [DOI] [PubMed] [Google Scholar]
- 29. Claretti M, Lopez BS, Boz E, Martelli F, Pradelli D, Bussadori CM. Complications during catheter‐mediated patent ductus arteriosus closure and pulmonary balloon valvuloplasty. J Small Anim Pract. 2019;60(10):607‐615. [DOI] [PubMed] [Google Scholar]
- 30. Bonagura J, Luis Fuentes V. Chapter 8 echocardiography. In: Mattoon J, Nyland T, eds. Small Animal Diagnostic Ultrasound. St Louis, Missouri: Elsevier; 2014:217‐331. [Google Scholar]
- 31. Reinero C, Visser LC, Kellihan HB, et al. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J Vet Intern Med. 2020;34:549‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kellihan HB, Stepien RL. Pulmonary hypertension in dogs: diagnosis and therapy. Vet Clin North Am Small Anim Pract. 2010;40:623‐641. [DOI] [PubMed] [Google Scholar]
- 33. Schober KE, Baade H. Doppler echocardiographic prediction of pulmonary hypertension in West Highland white terriers with chronic pulmonary disease. J Vet Intern Med. 2006;20:912‐920. [DOI] [PubMed] [Google Scholar]
- 34. Arndt JW, Oyama MA. Agitated saline contrast echocardiography to diagnose a congenital heart defect in a dog. J Vet Cardiol. 2008;10:129‐132. [DOI] [PubMed] [Google Scholar]
- 35. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117(11):1436‐1448. [DOI] [PubMed] [Google Scholar]
- 36. Visser LC, Scansen BA, Schober KE, Bonagura JD. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: repeatability and reference intervals. J Vet Cardiol. 2015;17(2):83‐96. [DOI] [PubMed] [Google Scholar]
- 37. Locatelli C, Spalla I, Domenech O, Sala E, Brambilla PG, Bussadori C. Pulmonic stenosis in dogs: survival and risk factors in a retrospective cohort of patients. J Small Anim Pract. 2013;54(9):445‐452. [DOI] [PubMed] [Google Scholar]
- 38. Stafford Johnson M, Martin M. Results of balloon valvuloplasty in 40 dogs with pulmonic stenosis. J Small Anim Pract. 2004;45(3):148‐153. [DOI] [PubMed] [Google Scholar]
- 39. Bristow P, Sargent J, Luis Fuentes V, Brockman D. Surgical treatment of pulmonic stenosis in dogs under cardiopulmonary bypass: outcome in nine dogs. J Small Anim Pract. 2018;59:38‐44. [DOI] [PubMed] [Google Scholar]
- 40. Saha A, Balakrishnan KG, Jaiswal PK, et al. Prognosis for patients with Eisenmenger syndrome of various aetiology. Int J Cardiol. 1994;45:199‐207. [DOI] [PubMed] [Google Scholar]
- 41. Sun YJ, Yang T, Zeng WJ, et al. Impact of sildenafil on survival of patients with eisenmenger syndrome. J Clin Pharmacol. 2013;53(6):611‐618. [DOI] [PubMed] [Google Scholar]
- 42. Turk JR, Miller LM, Miller JB, Sande RD. Necrotizing pulmonary arteritis in a dog with patent ductus arteriosus. J Small Anim Pract. 1981;22(9):603‐608. [DOI] [PubMed] [Google Scholar]
- 43. Gavaghan BJ, Lapointe JM, Thomas WP. Acute onset of pulmonary necrotising arteritis in a dog with a left‐to‐right patent ductus arteriosus. Aust Vet J. 1998;76(12):786‐791. [DOI] [PubMed] [Google Scholar]
- 44. Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S20‐S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 46. Kellum HB, Stepien RL. Sildenafil citrate therapy in 22 dogs with pulmonary hypertension. J Vet Intern Med. 2007;21(6):1258‐1264. 10.1892/07-006.1. [DOI] [PubMed] [Google Scholar]
- 47. Bach JF, Rozanski EA, MacGregor J, Betkowski JM, Rush JE. Retrospective evaluation of sildenafil citrate as a therapy for pulmonary hypertension in dogs. J Vet Intern Med. 2006;20(5):1132‐1135. [DOI] [PubMed] [Google Scholar]
- 48. Diller GP, Körten MA, Bauer UMM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J. 2016;37(18):1449‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamaki S, Mohri H, Haneda K, Endo M, Akimoto H. Indications for surgery based on lung biopsy in cases of ventricular septal defect and/or patent ductus arteriosus with severe pulmonary hypertension. Chest. 1989;96(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 50. Yan C, Zhao S, Jiang S, et al. Transcatheter closure of patent ductus arteriosus with severe pulmonary arterial hypertension in adults. Heart. 2007;93(4):514‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagenvoort CA. Plexogenic arteriopathy. Thorax. 1994;49(suppl 1):S39‐S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perloff JK, Rosove MH, Child JS, Wright GB. Adults with cyanotic congenital heart disease: hematological management. Ann Intern Med. 1988;109(5):406‐413. [DOI] [PubMed] [Google Scholar]
- 53. Bridges ND, Perloff JK. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1994;89(2):911. [PubMed] [Google Scholar]
- 54. Galie N, Manes A, Palazzini M, et al. Management of pulmonary arterial hypertension associated with congenital systemic‐to‐pulmonary shunts and Eisenmenger's syndrome. Drugs. 2008;68(8):1049‐1066. [DOI] [PubMed] [Google Scholar]
- 55. Mebus S, Schulze‐Neick I, Oechslin E, et al. The adult patient with Eisenmenger syndrome: a medical update after Dana point part II: medical treatment—study results. Curr Cardiol Rev. 2010;6(4):356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peterson ME, Randolph JF. Diagnosis of canine primary polycythemia and management with hydroxyurea. J Am Vet Med Assoc. 1982;180(4):415‐418. [PubMed] [Google Scholar]
- 57. Hocking WG, Golde DW. Polycythemia: evaluation and management. Blood Rev. 1989;3(1):59‐65. [DOI] [PubMed] [Google Scholar]


