Abstract
Background
Dogs with protein‐losing enteropathy (PLE) are at risk of developing a hypercoagulable state, but the prevalence of hypercoagulability in dogs with chronic enteropathies (CE) and normal serum albumin concentration is unknown.
Hypothesis
Dogs with CE are predisposed to a hypercoagulable state as assessed by thromboelastography (TEG) independent of serum albumin concentration.
Methods
Dogs with chronic gastrointestinal signs from suspected inflammatory CE between 2017 and 2019 were included. Thirty‐eight were evaluated; every dog had a CBC, serum biochemistry panel, and abdominal imaging performed. The Canine Inflammatory Bowel Disease Activity Index (CIBDAI) was calculated. Thromboelastography was performed at presentation, and reaction time (R), kinetic time (K), α‐angle, maximal amplitude (MA), and global clot strength (G) were recorded. Dogs were considered hypercoagulable if the G value was ≥25% above the reference interval.
Results
Seventeen of 38 (44.7%; 95% confidence interval [CI], 28.6‐61.7%) dogs with CE were hypercoagulable. The G value did not differ between the 19 dogs with normal (≥28 g/L) serum albumin concentrations (9.05 kdyn/cm2; 95% CI, 7.26‐10.84; SD 3.71) and 19 dogs with hypoalbuminemia (11.3 kdyn/cm2; 95% CI, 9.04‐13.6, SD; 4.7; P = .11). The G value was negatively correlated with hematocrit, serum albumin concentration, and duration of signs and positively correlated with age.
Conclusions and Clinical Importance
Dogs with CE and normal serum albumin concentration can be hypercoagulable as measured by TEG.
Keywords: canine, gastrointestinal thromboembolism, inflammation, thromboelastography
Abbreviations
- AT
antithrombin
- CE
chronic enteropathies
- CIBDAI
Canine Inflammatory Bowel Disease Activity Index
- G value
global clot strength
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- K
kinetic time
- MA
maximal amplitude
- PLE
protein‐losing enteropathy
- PLN
protein‐losing nephropathy
- R
reaction time
- TEG
thromboelastography
1. INTRODUCTION
Chronic enteropathy (CE) is a common diagnosis in dogs presented for chronic diarrhea, with 1 study documenting a prevalence of inflammatory enteropathy in 70% of dogs with chronic diarrhea, and many dogs respond to dietary management (food‐responsive enteropathy). 1 Protein‐losing enteropathy (PLE) represents a more severe category of gastrointestinal (GI) disorders and may be associated with inflammation, GI lymphoma, and intestinal lymphangiectasia. Failure to resolve hypoalbuminemia in dogs with PLE has been negatively associated with prognosis. 2 , 3 A hypercoagulable state has been associated with PLE in dogs, resulting in thromboembolism. 4 , 5
Thromboelastography (TEG) is a validated method in veterinary medicine for overall assessment of coagulation by measuring clot formation, clot strength, and fibrinolysis. 6 Various diseases have been associated with a hypercoagulable state in dogs as assessed by TEG including chronic hepatopathies, spontaneous hyperadrenocorticism, immune‐mediated hemolytic anemia, protein‐losing nephropathy (PLN), and PLE. 5 , 7 , 8 , 9 , 10
Hypercoagulability in dogs with PLN and PLE may be attributed to the loss of antithrombin (AT) through the kidneys and GI tract, respectively. A previous study suggested that dogs with PLE are hypercoagulable as measured by TEG, but hypercoagulability did not appear to be associated with the severity of hypoalbuminemia or AT concentration, and dogs remained hypercoagulable after the initiation of treatment despite improvement in serum albumin concentration. 5 However, only 9 dogs had repeated TEG between 4 and 24 days after initiation of treatment, and these dogs were being treated with prednisolone, which itself may cause hypercoagulability. Similarly, no correlation was found between AT concentration and TEG parameters in dogs with PLN. 10 It is therefore possible that dogs are predisposed to a hypercoagulable state independent of AT concentration. The prevalence of hypercoagulability in dogs with CE but with normal serum albumin concentrations has not been investigated. Documentation of a hypercoagulable state in dogs with CE and normal serum albumin could highlight the role of the GI tract in systemic inflammation, and it may identify TEG as a potential tool in the diagnosis and monitoring of dogs with CE.
Our aim was to evaluate the prevalence of hypercoagulability in dogs with chronic CE with normal serum albumin concentration in comparison to dogs with hypoalbuminemia. We hypothesized that dogs with CE and normal serum albumin concentration would also be hypercoagulable as measured by TEG. Secondary aims were to assess the association of hypercoagulability with other clinicopathological variables in this population.
2. MATERIALS AND METHODS
2.1. Study population
Client‐owned dogs presented for investigation of chronic GI signs were enrolled at the Small Animal Referral Hospital, Langford Vets, prospectively between July 2018 and September 2019. Ethical approval for the study was obtained (VIN/18/041). Data of dogs that met the inclusion criteria between June 2017 and July 2018 were included retrospectively. All adult dogs (≥12 months) with chronic (≥2 weeks) GI signs (diarrhea with or without vomiting or decreased appetite) were eligible for inclusion into the study. Dogs that had received corticosteroids or nonsteroidal anti‐inflammatory drugs within 2 weeks were excluded. Primary CE was diagnosed based on (a) exclusion of infectious enteropathies (negative fecal culture and parasitology or fenbendazole 50 mg/kg PO q24h for 5 days) and (b) exclusion of extra‐GI diseases (serum biochemical variables [Langford Diagnostic Services], and abdominal ultrasound or computed tomography [CT]). All dogs had a CBC available for review. A bile acid stimulation assay was performed if biochemical abnormalities consistent with hepatic dysfunction were observed (decreased urea, cholesterol, or glucose concentration) or decreased liver size was suspected on abdominal imaging. A serum basal cortisol concentration was measured if findings on CBC were suggestive of hypoadrenocorticism (eg, lymphocytosis, eosinophilia) and subsequent ACTH stimulation test if basal serum cortisol concentration was <55 nmol. 11 Exclusion criteria included a bile acid stimulation assay consistent with hepatic dysfunction (postprandial serum bile acid concentration increased compared with laboratory reference ranges) or ACTH stimulation test results compatible with hypoadrenocorticism. Dogs were excluded if neoplasia was identified on histopathology or if there was a high degree of suspicion on abdominal imaging (eg, focal mass lesion, focal or multifocal loss of intestinal wall layering). Forty‐two dogs were enrolled: 4 dogs were excluded because neoplasia was confirmed as the final diagnosis (tubulo‐villus adenoma, n = 1; large cell lymphoma, n = 1) and corticosteroids administered within 2 weeks of TEG (n = 2) with 38 dogs meeting the inclusion criteria (21 retrospectively, 17 prospectively).
Hypoalbuminemia was defined as serum albumin concentration < 28 g/L and normoalbuminemia as ≥28 g/L. In cases wherein hypoalbuminemia was documented, urinalysis with urine : protein creatinine ratio was performed to exclude concurrent PLN. Evidence of inflammation on histopathology of endoscopic and surgical biopsy specimens and type of inflammation was collected when available. Other data recorded were hematocrit (HCT) and platelet counts because they could affect the TEG results. Hematocrit and platelet counts were considered normal if the values were within the laboratory reference intervals (HCT, 0.37‐0.57 L/L; and platelet count, 143‐400 × 109/L). All dogs with low platelet count on the analyzer had a manual blood film examined to evaluate for platelet clumps. Blood for serum cobalamin concentration measurement was collected and patients were grouped as hypocobalaminemic if the values were below the lower reference interval of the laboratory or normal if the values were within or above the reference interval (204‐290 pmol/L). The Canine Inflammatory Bowel Disease Activity Index (CIBDAI) was calculated and categorized as normal (0‐3), mild (4, 5), moderate (6–8), or severe (≥9). 12
2.2. Hemostatic analysis
Blood samples were obtained by jugular venipuncture using a 21G needle into a 5‐mL syringe and then 2.5 mL of the sample was decanted into a 2.5 mL citrate tube. The sample was allowed to stand for 30 to 90 minutes before analysis at room temperature. Analysis was performed by trained operators who were veterinary residents in internal medicine or emergency and critical care or by intensive care veterinary technicians. The samples were kaolin‐activated and analyzed following the manufacturer's instructions and were in compliance with previously established guidelines 13 using a TEG 5000 Thromboelastograph Hemostasis Analyzer system (Haemonetics) to obtain the reaction time (R), kinetic time (K), α‐angle, maximal amplitude (MA), and global clot strength (G).
Hypercoagulability was assessed as G value ≥25% above the upper reference interval (≥8.0 kdyn/cm2) of the analyzer. 14 As such, G value of ≥10.0 kdyn/cm2 was considered hypercoagulable.
2.3. Statistical analysis
For comparisons, groups were defined as hypoalbuminemic (<28 g/L) or normoalbuminemic (≥28 g/L). Variables compared between the 2 groups included TEG parameters (R, K, α‐angle, MA, G), HCT, serum cobalamin concentration, platelet count, age, duration of clinical signs, and CIBDAI. Distribution of clinical and clinicopathologic data was assessed using the Shapiro‐Wilk test. Normally distributed (parametric) variables were compared between the 2 groups using a Student's t‐test. Nonparametric variables were compared using a Mann‐Whitney U test. Results are reported as median ± range for nonparametric data and mean ± SD for normally distributed data.
Groups were also defined as hypercoagulable (G value ≥10.0 kdyn/cm2) or normocoagulable (G value <10.0 kdyn/cm2), and the following variables were compared between the 2 groups: HCT, serum albumin concentration, serum cobalamin concentration, platelet count, age, duration of clinical signs, and CIBDAI.
Pearson's (r) and Spearman's rank correlation coefficient (r s) were used to assess whether significant correlations were present between G value and patient and clinicopathologic variables for parametric data and nonparametric data, respectively. Patient and clinicopathological variables assessed for correlation with G value included HCT, serum albumin concentration, serum cobalamin concentration, platelet count, age, and CIBDAI score. Statistical analysis was performed using the IBM SPSS statistical software (version 26). Statistical significance was set at P < .05.
A post hoc binary sample size calculation was performed using the prevalence of hypercoagulability (G ≥ 10.0 kdyn/cm2) of normoalbuminemic and hypoalbuminemic dogs with an α level set at 0.05 and power at 80%.
3. RESULTS
3.1. Group characteristics
Nineteen dogs were hypoalbuminemic (median, 24.0 g/L; range, 12.9‐27.8 g/L) and 19 dogs had normal serum albumin concentration (median, 32.2 g/L; range, 28.2‐33.8 g/L). Breeds in the hypoalbuminemic group included 7 crossbreed dogs and 1 each of the following: bichon frise, Border terrier, Cairn terrier, cocker spaniel, greyhound, husky, Labrador retriever, poodle, Rhodesian ridgeback, Siberian husky, Staffordshire bull terrier, and Weimaraner. Breeds in the normoalbuminemic group included 8 crossbreeds, 3 German shepherd dogs, and 1 each of the following: bichon frise, boxer, cocker spaniel, Dalmatian, golden retriever, Newfoundland, springer spaniel, and Staffordshire bull terrier. There were 10 male dogs (neutered, n = 9; intact, n = 1) and 9 female dogs (neutered, n = 6; intact, n = 3) in the hypoalbuminemic group, and 10 male dogs (neutered, n = 7; intact, n = 3) and 9 female dogs (neutered, n = 8; intact, n = 1) in the normoalbuminemic group. Three dogs in each of the hypoalbuminemic and normoalbuminemic groups did not have histopathology of GI biopsy specimens. The remaining 32 dogs with histopathology available all had histopathology consistent with inflammatory enteropathy; 2 dogs (1 in each of the hypoalbuminemia and normoalbuminemia groups) had lacteal dilatation suspected to be secondary to inflammation, but idiopathic lymphangiectasia could not be ruled out. Four dogs in the normoalbuminemic group had received cobalamin supplementation before measurement of serum cobalamin concentrations and were removed from further cobalamin data analysis. No dogs in the hypoalbuminemic group had received cobalamin supplementation before serum measurement.
Four dogs were suspected to have experienced a thromboembolic event; 2 dogs had acute onset of neurological signs with spontaneous resolution (both hypoalbuminemic), 1 had splenic vein thrombosis identified on CT (hypoalbuminemic), and 1 dog had a filling defect supportive of a pulmonary thromboembolism on CT (normoalbuminemic).
3.2. Hypoalbuminemic vs normoalbuminemic dogs with CE: patient, clinicopathologic, and TEG variables
Summary statistics between the groups are reported in Table 1. The hypoalbuminemic group was significantly older than the normoalbuminemic group (P = .04). The HCT was significantly lower in the hypoalbuminemic group compared with the normoalbuminemic group (P = .01). No significant differences were found between the groups for serum cobalamin concentration, duration of clinical signs before hematostatic analysis, platelet count, or CIBDAI score.
TABLE 1.
Patient and clinicopathological data (age, duration of clinical signs, CIBDAI score, hematocrit, platelet count, and cobalamin) of 19 normoalbuminemic and 19 hypoalbuminemic dogs with chronic enteropathies.
| Variable | Group | Median | Range | P value |
|---|---|---|---|---|
| Age (years) | Low albumin | 7 | 1 to 13 | .04* |
| Normal albumin | 3 | 1 to 10 | ||
| Duration of signs (weeks) | Low albumin | 6 | 2 to 260 | .07 |
| Normal albumin | 12 | 3 to 156 | ||
| CIBDAI score | Low albumin | 5 | 1 to 11 | .30 |
| Normal albumin | 4 | 1 to 11 | ||
| Hematocrit (L/L) | Low albumin | 0.44 | 0.32 to 0.63 | .01* |
| Normal albumin | 0.50 | 0.26 to 0.61 | ||
| Platelet count (×109/L) | Low albumin | 321 | 113 to 1211 | .11 |
| Normal albumin | 270 | 159 to 403 | ||
| Cobalamin (pmol/L) | Low albumin | 250 | 79 to 791 | .22 |
| Normal albumin | 342 | 123 to 776 |
*Significant difference between the groups (P < 0.05).
*Significant difference between the groups (P<0.05).
When comparing TEG variables, the G value was consistent with hypercoagulability (≥10.0 kdyn/cm2) in 11 of 19 dogs in the hypoalbuminemic group (57.9%) and 6 of 19 dogs (31.5%) in the normoalbuminemic group (Figure 1). The overall prevalence of hypercoagulability for all dogs with CE was 44.7%. The mean G value of the hypoalbuminemic group was 11.3 kdyn/cm2 (95% CI, 9.04‐13.6; SD 4.77) and the G value of the normoalbuminemic group was 9.05 kdyn/cm2 (95% CI 7.26‐10.84, SD 3.71; P = .1). No G values were below the lower end of the reference interval in either group. No significant difference was found between groups for other TEG parameters (R, K, α‐angle, MA; P ≥ .08).
FIGURE 1.
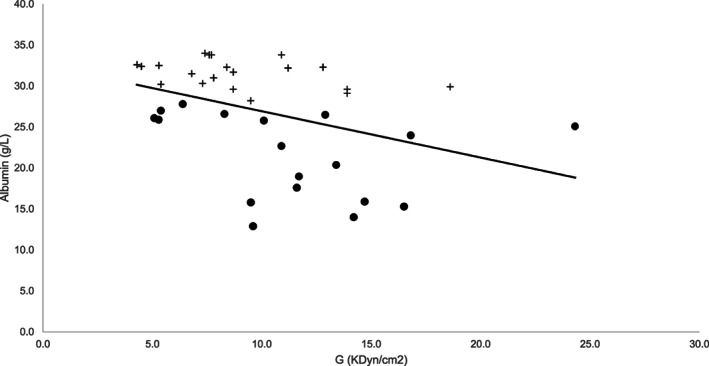
Relationship between serum albumin concentration (g/L) and G (kdyn/cm2) in dogs with chronic enteropathies. Hypoalbuminemic dogs (albumin <28 g/L) are represented by a bullet point (•) and normoalbuminemic dogs are represented by a cross (+). The trend line reflects a negative correlation between serum albumin and increasing G value
3.3. Hypercoagulable vs normocoagulable dogs with CE: clinical and clinicopathologic variables
Age, platelet count, serum cobalamin concentration, and CIBDAI scores were not significantly associated with hypercoagulability in all dogs with CE (P ≥ .07). Duration of clinical signs before diagnosis (P = .01) was significantly shorter in the hypercoagulable group, and serum albumin concentration (P = .02) and HCT (P = .02) were significantly lower in the hypercoagulable group.
3.4. Correlation between G value and various variables
Hematocrit (r = −0.50, P = .001), albumin (r s = −0.49, P = .002), and duration of signs (r s = −0.42, P = .01) were negatively correlated with hypercoagulability (increased G value). Although age was not significantly different between dogs with increased and normal G values, a weak positive correlation was identified between age and G value (r s = 0.37, P = .02).
3.5. Power calculation
Using the population prevalence of hypercoagulability as 57.9% in dogs with hypoalbuminemia and 31.5% in dogs with normoalbuminemia, the sample size required to achieve a power of 80% at a significance of 0.05 is 52 dogs in each group.
4. DISCUSSION
Our results suggest that dogs with CE without hypoalbuminemia can be hypercoagulable as measured by TEG. In this population, no significant difference in the G value was found between dogs with CE that had normal serum albumin concentration vs those that were hypoalbuminemic. However, using the prevalence of hypercoagulability in this population, our study was underpowered to detect differences in hypercoagulability using a serum albumin concentration cutoff of 28 g/L. An increased G value was negatively associated with serum albumin concentration, HCT, and duration of clinical signs before diagnosis in dogs with CE.
Thromboelastography is a validated method to assess the viscoelastic properties of clot formation during hemostasis. In a hypercoagulable state, reaction time (R) decreases, clot formation time (K) decreases, the slope of clot formation (α‐angle) increases, clot strength (maximum amplitude, MA) increases, and the log derivative of MA (G value) increases. 6 Previous studies investigating hypercoagulability in veterinary patients have used various methods to determine hypercoagulability including G, R, and MA results outside of established reference intervals, 7 , 15 significant difference in all parameters from healthy controls, 5 and utilization of a coagulation index. 10 A systematic evaluation on evidence of reporting TEG parameters indicated that a cutoff in ≥ 1 TEG parameters can be used to define hypercoagulability, that maximal clot firmness (G or MA) shows the least variability, and suggested that abnormal coagulability may be defined as >25% above or below the reference intervals for hypercoagulability and hypocoagulability, respectively. 14 In our study, a cutoff of 25% above the reference interval of G was used as a marker of hypercoagulability. However, it is recommended that all TEG variables be reported to allow further systematic evaluation because no clear consensus exists on the definition of hypercoagulability, and this lack of standardization is a limitation of all studies utilizing TEG..
Inflammatory bowel disease (IBD) in humans is associated with a hypercoagulable state and with a 2‐fold to 3‐fold increase in risk of thromboembolic events compared with the general population. 16 , 17 The etiopathogenesis is thought to be complex, and various inflammatory mediators including interleukin 6, interleukin 8, and tumor necrosis factor alpha have been implicated in the development of thromboembolism. 18 In comparison to humans, reports of thromboembolic events in dogs with GI diseases are limited to case reports and case series of dogs with PLE. 4 , 5 , 19 , 20 , 21 Thromboembolic events may be underdetected in veterinary patients because of the often vague‐presenting signs, the limitations of diagnostic imaging in obtaining an antemortem diagnosis, and few cases submitted for necropsy. Thromboelastography therefore could have utility as a surrogate to evaluate predisposition to a hypercoagulable state. An abstract investigating prevalence of a hypercoagulable TEG in 19 surgical human patients with IBD identified alterations in at least 1 TEG parameter consistent with hypercoagulability preoperatively in 74% of patients. 22 However, many of these patients were receiving corticosteroids or biological agents at the time of TEG. Considering the role of systemic inflammation in humans with IBD and a previous study that found lack of association of hypercoagulability with antithrombin in dogs with PLE, 5 it is likely that the hypercoagulability of some patients with CE in our study represents an underlying inflammatory process of variable severity.
Serum albumin concentration has been established as a negative prognostic indicator for dogs with CE. 3 , 23 Correlation of G value with serum albumin concentration and other variables associated with negative outcome in dogs with chronic intestinal disease could validate its utility as a monitoring tool in dogs with CE. Other variables analyzed in our study included some that can influence the TEG curve (HCT and platelet count) and clinical and patient factors that have been associated with a negative prognosis (serum cobalamin concentration and clinical scoring indices). 3 No significant difference was found in cobalamin concentration and CIBDAI between dogs that were hypercoagulable or normocoagulable in our population. This finding may reflect the study being underpowered, or could represent hypercoagulability occurring independently of serum cobalamin concentration and CIBDAI.
In our population, serum albumin concentration was negatively correlated with hypercoagulability as measured by TEG, which is not unexpected given the high prevalence of hypercoagulability reported in dogs with protein‐losing diseases. 5 , 10 Dogs with hypercoagulable TEG had shorter duration of clinical signs before diagnosis, which may support a more severe inflammatory process prompting earlier investigation. Additional studies are required to determine specific risk factors for development of a hypercoagulable state in dogs with CE, including the type of GI inflammation (eg, lymphoplasmacytic, neutrophilic, eosinophilic), the World Small Animal Veterinary Association histopathological standardized score, and whether or not hypercoagulability as measured by TEG is associated with thromboembolic events.
The HCT of dogs in the hypoalbuminemic group was significantly lower than that in the normoalbuminemic group. This finding may reflect more severe intestinal disease resulting in anemia of inflammation, low‐grade GI hemorrhage, or, alternatively, differences in hemoconcentration and hydration status between the groups. In our study, HCT was negatively correlated with G value and was significantly lower in dogs with increased G value, which has been found in previous TEG studies in dogs. 24 , 25 The decreased HCT in the hypoalbuminemic group therefore would be expected to contribute to a hypercoagulable state, rather than mask any potential difference in hypercoagulability when no significant difference in G value was detected between the groups.
The hypoalbuminemic group was also significantly older that the normoalbuminemic group. In our population, increasing age was positively correlated with increased G value, which also would suggest that the older age in the hypoalbuminemic group may contribute to an increased G value rather than mask a potential difference. Previous studies suggest that dogs with food‐responsive enteropathy are younger than those with immunosuppressant‐responsive enteropathies, and dogs with immunosuppressant‐responsive enteropathies have a worse outcome. 23 The positive correlation with G value and increasing age may reflect a more severe underlying inflammatory disease. The effect of age on TEG parameters and the relationship between an abnormal TEG and requiring immunosuppression require further study.
The utility of biomarkers in CE has been an area of interest in the diagnosis and monitoring of patients with CE, but many biomarkers are not commercially available. 26 A previous study showed that dogs with PLE did not show improvement in hypercoagulability after treatment with immunosuppressant drugs, but the time to follow‐up was short and only 9 dogs had repeat TEG. 5 Exogenous corticosteroid administration in healthy beagle dogs has been associated with hypercoagulability and may have been a confounding factor. 8 , 27 Furthermore, the dogs in the PLE study were markedly hypoalbuminemic, with a median serum albumin concentration of 15.6 g/L, compared with the median serum albumin concentration of 24.0 g/L in the dogs categorized as hypoalbuminemic in our study. 5 This difference may represent a more severe disease process that does not permit resolution of hypercoagulability. Additional studies are required to evaluate the utility of TEG for monitoring patients with CE to determine whether hypercoagulability resolves after successful treatment.
Our study had several limitations. First, over half of the dogs were included retrospectively. Only dogs that were seen within the year before commencement of the study were included to ensure that samples were analyzed using the same hospital standardized protocol on the same analyzer. Second, TEG has been associated with between‐center and within‐center variation. 28 The effect of within‐center variation was minimized by using standardized protocol for sample collection, including kaolin activation, and processing to decrease preanalytical and analytical error and using a single analyzer validated for veterinary use. Third, 38 patients were included in our study, with 19 in each group of normal and low serum albumin concentration. A post hoc sample size determined that our study was underpowered (at a power of 80%), and the absence of difference between the hypoalbuminemic and normoalbuminemic dogs may be as a consequence of a type II error. However, the study aim to determine whether dogs with CE can be hypercoagulable with normal serum albumin concentration was achieved. Finally, 6 dogs were diagnosed presumptively with CE without GI biopsy. In these cases, supportive clinical signs, exclusion of other causes, and normal abdominal imaging decreased the likelihood of other comorbidities or differential diagnoses.
In conclusion, our study suggests that dogs with CE can have TEG results indicative of hypercoagulability with a normal serum albumin concentration. Whether hypercoagulable patients with CE should be treated prophylactically with antithrombotic drugs requires a prospective study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. This article was presented as an abstract at the Small Animal Medicine Society (SAMSoc) Autumn 2020 meeting.
Dixon A, Hall EJ, Adamantos S, Kathrani A, McGrath C, Black V. Hypercoagulability in dogs with chronic enteropathy and association with serum albumin concentration. J Vet Intern Med. 2021;35:860–866. 10.1111/jvim.16044
REFERENCES
- 1. Volkmann M, Steiner JM, Fosgate GT, Zentek J, Hartmann S, Kohn B. Chronic diarrhea in dogs—retrospective study in 136 cases. J Vet Intern Med. 2017;31:1043‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakashima K, Hiyoshi S, Ohno K, et al. Prognostic factors in dogs with protein‐losing enteropathy. Vet J. 2015;205:28‐32. [DOI] [PubMed] [Google Scholar]
- 3. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 4. Jacinto AML, Ridyard AE, Aroch I, et al. Thromboembolism in dogs with protein‐losing enteropathy with non‐neoplastic chronic small intestinal disease. J Am Anim Hosp Assoc. 2017;53:185‐192. [DOI] [PubMed] [Google Scholar]
- 5. Goodwin LV, Goggs R, Chan DL, Allenspach K. Hypercoagulability in dogs with protein‐losing enteropathy. J Vet Intern Med. 2011;25:273‐277. [DOI] [PubMed] [Google Scholar]
- 6. Donahue SM, Otto CM. Thromboelastography: a tool for measuring hypercoagulability, hypocoagulability, and fibrinolysis. J Vet Emerg Crit Care. 2005;15:9‐16. [Google Scholar]
- 7. Fry W, Lester C, Etedali NM, Shaw S, DeLaforcade A, Webster CRL. Thromboelastography in dogs with chronic hepatopathies. J Vet Intern Med. 2017;31:419‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park FM, Blois SL, Abrams‐Ogg AC, et al. Hypercoagulability and ACTH‐dependent hyperadrenocorticism in dogs. J Vet Intern Med. 2013;27:1136‐1142. [DOI] [PubMed] [Google Scholar]
- 9. Sinnott VB, Otto CM. Use of thromboelastography in dogs with immune‐mediated hemolytic anemia: 39 cases (2000‐2008). J Vet Emerg Crit Care (San Antonio). 2009;19:484‐488. [DOI] [PubMed] [Google Scholar]
- 10. Lennon EM, Hanel RM, Walker JM, Vaden SL. Hypercoagulability in dogs with protein‐losing nephropathy as assessed by thromboelastography. J Vet Intern Med. 2013;27:462‐468. [DOI] [PubMed] [Google Scholar]
- 11. Bovens C, Tennant K, Reeve J, Murphy KF. Basal serum cortisol concentration as a screening test for hypoadrenocorticism in dogs. J Vet Intern Med. 2014;28:1541‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291‐297. [DOI] [PubMed] [Google Scholar]
- 13. Goggs R, Brainard B, de Laforcade AM, et al. Partnership on Rotational ViscoElastic Test Standardization (PROVETS): evidence‐based guidelines on rotational viscoelastic assays in veterinary medicine. J Vet Emerg Crit Care (San Antonio). 2014;24:1‐22. [DOI] [PubMed] [Google Scholar]
- 14. Hanel RM, Chan DL, Conner B, et al. Systematic evaluation of evidence on veterinary viscoelastic testing part 4: definitions and data reporting. J Vet Emerg Crit Care (San Antonio). 2014;24:47‐56. [DOI] [PubMed] [Google Scholar]
- 15. Kelley D, Lester C, Shaw S, Laforcade A, Webster CRL. Thromboelastographic evaluation of dogs with acute liver disease. J Vet Intern Med. 2015;29:1053‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papa A, Gerardi V, Marzo M, Felice C, Rapaccini GL, Gasbarrini A. Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol. 2014;20:3173‐3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giannotta M, Tapete G, Emmi G, Silvestri E, Milla M. Thrombosis in inflammatory bowel diseases: what's the link? Thromb J. 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simmerson SM, Armstrong PJ, Wunschmann A, et al. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in Yorkshire terrier dogs. J Vet Intern Med. 2014;28:331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laurenson MP, Hopper K, Herrera MA, Johnson EG. Concurrent diseases and conditions in dogs with splenic vein thrombosis. J Vet Intern Med. 2010;24:1298‐1304. [DOI] [PubMed] [Google Scholar]
- 21. Finco DR, Duncan JR, Schall WD, Hooper BE, Chandler FW, Keating KA. Chronic enteric disease and hypoproteinemia in 9 dogs. J Am Vet Med Assoc. 1973;163:262‐271. [PubMed] [Google Scholar]
- 22. Holubar S, Lee Chun HA, Feinburg A, et al. P085 Hypercoagulability in patients undergoing abdominopelvic surgery for inflammatory bowel disease. Am J Gastroenterol. 2019;114:S23. [Google Scholar]
- 23. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178:368. [DOI] [PubMed] [Google Scholar]
- 24. McMichael MA, Smith SA, Galligan A, Swanson KS. In vitro hypercoagulability on whole blood thromboelastometry associated with in vivo reduction of circulating red cell mass in dogs. Vet Clin Pathol. 2014;43:154‐163. [DOI] [PubMed] [Google Scholar]
- 25. Marschner CB, Wiinberg B, Tarnow I, et al. The influence of inflammation and hematocrit on clot strength in canine thromboelastographic hypercoagulability. J Vet Emerg Crit Care (San Antonio). 2018;28:20‐30. [DOI] [PubMed] [Google Scholar]
- 26. Heilmann RM, Steiner JM. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J Vet Intern Med. 2018;32:1495‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose LJ, Dunn ME, Allegret V, Bédard C. Effect of prednisone administration on coagulation variables in healthy beagle dogs. Vet Clin Pathol. 2011;40:426‐434. [DOI] [PubMed] [Google Scholar]
- 28. Goggs R, Borrelli A, Brainard BM, et al. Multicenter in vitro thromboelastography and thromboelastometry standardization. J Vet Emerg Crit Care (San Antonio). 2018;28:201‐212. [DOI] [PubMed] [Google Scholar]


