Abstract
Background
Inhalation treatment frequently is used in dogs and cats with chronic respiratory disease. Little is known however about the performance of delivery devices and the distribution of aerosolized drugs in the lower airways.
Objective
To assess the performance of 3 delivery devices and the impact of variable durations of inhalation on the pulmonary and extrapulmonary deposition of nebulized 99mtechnetium‐diethylenetriamine‐pentaacetic acid (99mTc‐DTPA).
Animals
Ten university‐owned healthy Beagle dogs.
Methods
Prospective crossover study. Dogs inhaled the radiopharmaceutical for 5 minutes either through the Aerodawg spacer with a custom‐made nose‐muzzle mask, the Aerochamber spacer with the same mask, or the Aerodawg spacer with its original nose mask. In addition, dogs inhaled for 1 and 3 minutes through the second device. Images were obtained by 2‐dimensional planar scintigraphy. Radiopharmaceutical uptake was calculated as an absolute value and as a fraction of the registered dose in the whole body.
Results
Mean (±SD) lung deposition for the 3 devices was 9.2% (±5.0), 11.4% (±4.9), and 9.3% (±4.6), respectively. Differences were not statistically significant. Uptake in pulmonary and extrapulmonary tissues was significantly lower after 1‐minute nebulization, but the mean pulmonary/extrapulmonary deposition ratio (0.38 ± 0.27) was significantly higher than after 5‐minute nebulization (0.16 ± 0.1; P = .03). No significant differences were detected after 3‐ and 5‐minute nebulization.
Conclusion and Clinical Importance
The performance of a pediatric spacer with a custom‐made mask is comparable to that of a veterinary device. One‐minute nebulization provides lower pulmonary uptake but achieves a better pulmonary/extrapulmonary deposition ratio than does 5‐minute nebulization.
Keywords: dog, inhalation, lung, radiopharmaceutical, scintigraphy
Abbreviations
- 99mTc‐DTPA
99mtechnetium‐diethylenetriamine‐pentaacetic acid
- cpm
counts per minute
- GBq
gigabecquerel
- MDI
metered‐dose inhaler
- NM
nose‐muzzle
- ROI
region of interest
1. INTRODUCTION
In human medicine, inhalation treatment for asthma and chronic obstructive pulmonary disease has been used for many years with proven efficacy and few systemic adverse effects. 1 , 2 In the past 2 decades, experimental and clinical studies have reported similar benefits with this treatment modality in dogs and cats with lower airway disease. 3 , 4 , 5 , 6 , 7 However, very little information is available about aerosol distribution in these patients. 8 , 9
Aerosol delivery is influenced by various factors that are patient‐, device‐, and drug‐dependent. 10 , 11 , 12 , 13 It is also considered 1 of the most technically challenging aspects for practitioners treating young children with respiratory disease. 14 Hence, several study groups have focused their research on the performance of multiple spacers, valve‐holding chambers, masks, and delivery methods for inhalation treatment in this particular group of patients. 10 , 11 , 12 , 15 , 16 However, controversy still exists and no consensus has been reached as to which aerosol device is the most appropriate. 14 , 17 Furthermore, device‐independent factors such as availability, cultural background, and financial status also may play a decisive role when choosing a device. 10 , 17 , 18 Many of these factors likely are similar or the same in veterinary medicine.
In infants who are not able to control their breathing pattern, a spacer or valve‐holding chamber attached to a well‐fitting facemask has been considered by many the preferred delivery device when using a metered‐dose inhaler (MDI) to allow drug delivery via tidal breathing and to minimize drug leakage. 10 , 12 , 16 The same seems true for dogs and cats because they share many of the characteristics of infants, such as inability to perform voluntary forced inspirations and breath‐holding, small size of airways, low tidal volumes, and variable levels of cooperation. 10 , 11 , 12 However, although several studies support the use of aerosol treatment in veterinary patients, research comparing the performance of different devices is still scarce and limited to experimental settings. 8
Given the need for further research in this area, our first aim was to compare pulmonary and extrapulmonary deposition of a nebulized radiopharmaceutical agent delivered using 3 inhalation devices in healthy dogs. The second aim was to assess if differences in radiopharmaceutical deposition occur related to different durations of aerosol inhalation.
2. MATERIALS AND METHODS
2.1. Animals and study design
Ten clinically healthy Beagles, property of the University of Veterinary Medicine Vienna, were included in this prospective crossover study, which was approved by the Austrian government as well as the ethics and animal welfare committee of the university (approval number GZ.: BMWFW‐68.205/0230‐WF/V/3b/2016). Body weight ranged between 12 and 18 kg and age between 2 and 4 years. All dogs were current on vaccinations, deworming, and had no history of respiratory disease in the past 4 weeks. Their health status was assessed by clinical examination, CBC and a blood biochemistry panel.
All dogs participated in 5 ventilation scintigraphy procedures with a washout period of 7 and 122 days between procedures in the first part of the study (comparison between devices). Lung perfusion scintigraphy also was performed immediately after the first ventilation scintigraphy to define lung borders and rule out ventilation‐perfusion mismatches. Dogs were fasted 12 hours in advance and received 0.1 mg/kg butorphanol SC (Alvegesic, Virbac, France) 20 minutes before each study to achieve mild sedation and improve compliance because no training period was provided.
2.2. Delivery of the radiopharmaceutical agent
The inhalator bowl of a commercially available compressor‐driven jet nebulizer (PARI Master with PARI LL nebulizer, GmbH, Starnberg, Germany) was filled with 1.9 to 2.5 GBq 99mtechnetium‐diethylenetriamine‐pentaacetic acid (99mTc‐DTPA, Technescan, BSM Diagnostica GmbH, Vienna, Austria) prepared according to the instructions of the manufacturer (Figure 1). The bowl was attached to the spacer and the inhalation time was set to 5 minutes for the first part of the study (comparison between devices). The aerosol flow rate was 5.2 L/min and the mass median diameter of particles was 3.6 μm in accordance with the manufacturer's information.
FIGURE 1.
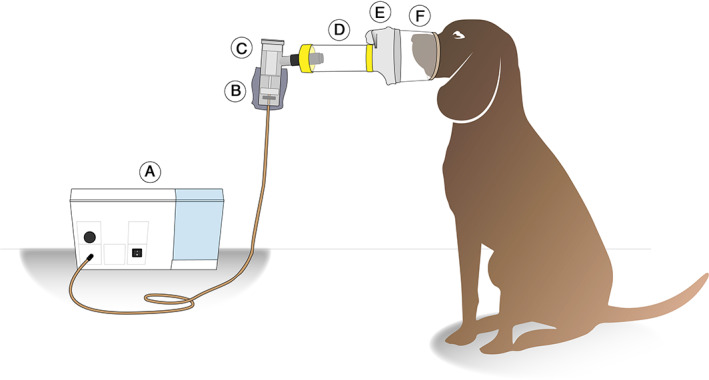
Diagram of the equipment used for the administration of nebulized 99mTc‐DTPA. A, PARI Master compressor; B, lead protective layer; C, PARI LL nebulizer; D, spacer; E, silicon mask; F, custom‐made nose‐muzzle mask. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
Each dog received a dose of nebulized 99mTc‐DTPA through 3 inhalation devices (Figure 2) for 5 minutes: (a) Aerodawg spacer (Trudell Medical International, Ontario, Canada) attached to a pediatric silicone facemask that served as a connector to a custom‐made tightly fitting nose‐muzzle (NM) mask; (b) Aerochamber plus child medium spacer and its facemask (Trudell Medical International) connected to the same custom‐made NM mask; and (c) Aerodawg spacer with its original nose mask. The custom‐made NM mask consisted of the upper half of a hard‐plastic cup, with its widest opening connected to the remainder of the device. For the lung perfusion scintigraphy study, 99mTc‐labeled macroaggregated albumin (Technescan, BSM Diagnostica GmbH, Vienna, Austria) was administered through an IV catheter at a dose ranging from 76 to 89 MBq per dog.
FIGURE 2.

Aerosol devices used for delivery of nebulized 99mTc‐DTPA. Parts are depicted from left to right: A, Custom‐made nose‐muzzle mask + pediatric silicon mask + Aerodawg spacer; B, Custom‐made nose‐muzzle mask + Aerochamber plus facemask and spacer (child‐medium); C, Aerodawg nose mask and spacer. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
2.3. Scintigraphy procedure and calculation of deposition
Immediately after radiopharmaceutical delivery in an adjoining room, dogs were scanned with a Diacam planar scintigraphy gamma camera (MiE GmbH, Seth, Germany) in right lateral, left lateral, and ventrodorsal (sternal) recumbency for 2 minutes each. Lung perfusion scans were obtained in the same manner. For analysis of the ventilation scintigraphy scans, only ventrodorsal scans were considered because superimposition of the esophagus over the lung fields was observed in most lateral scans.
Deposition of 99mTc‐DTPA in the head region (upper airways, mouth), lungs and stomach, and whole‐body distribution were recorded and quantified using manual or isocontour region of interest (ROI) analysis in counts per minute (cpm). Preferred isocontour values were 2% for the head region, 10% for the stomach, and 10% for the lungs. Adjustments were made for background radioactivity and radioactive decay. Two independent trained observers verified the measurements (A. C. V. and M. P.).
Pulmonary and extrapulmonary deposition (the latter defined as the sum of head region and stomach) also were calculated as fractions of the registered dose in the whole body. The ratio between pulmonary and extrapulmonary deposition also was calculated. The esophageal radioactivity uptake, if present, was removed for the analysis of the pulmonary uptake, but was incorporated in the whole‐body radioactivity uptake.
Lung perfusion scintigraphy scans were assessed by carefully aligning them to those of the ventilation scans in the 3 views. The lung fields were compared for size and shape and were scanned for regional defects and ventilation‐perfusion mismatches or matches.
To assess the influence of different aerosol delivery time spans in the deposition patterns of nebulized 99mTc‐DTPA, the same analysis was repeated after 1‐ and 3‐minute delivery (washout period between 7 and 35 days). The chosen device for this purpose was the Aerochamber plus spacer with the NM mask.
2.4. Statistical analysis
The performance of the 3 aerosol devices and the influence of different nebulization periods were assessed separately. The following variables were considered for descriptive statistics analysis: radiopharmaceutical uptake in cpm for all ROIs (whole body, extrapulmonary region, lungs), pulmonary and extrapulmonary uptake expressed as a fraction of the registered dose in the whole body, and the pulmonary/extrapulmonary deposition ratio. Conditions were compared using mixed effects models with Sidak's alpha correction procedure. P values <.05 were considered statistically significant. Commercial software (IBM SPSS v24) was used for analysis and GraphPad prism 8.0 and Adobe Illustrator CC were used for figure design.
3. RESULTS
The clinical examination and laboratory variables were unremarkable in all dogs. The inhalation procedure generally was well tolerated. Central and peripheral radiopharmaceutical uptake was detected in all lung scans, regardless of device or duration of inhalation (Figures 3 and 4). Perfusion scintigraphy was normal in all dogs, and no ventilation‐perfusion mismatches or matches were detected.
FIGURE 3.

Ventrodorsal 2D scintigraphic lung scans of dogs 2, 3, 4, and 7 after 5‐minute nebulization of 99mTc‐DTPA through the Aerodawg nose‐muzzle mask (AeroD NM‐mask), Aerochamber plus nosemuzzle mask (AeroC NM‐mask), and Aerodawg nose mask (AeroD nose mask) devices. Esophagus uptake was removed to avoid interference with the pulmonary uptake [blank areas in scans (D), (F), (I), (J), (K), (L)]. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
FIGURE 4.

Ventrodorsal 2D scintigraphic lung scans of dogs 6, 7, 8, and 10 after 1‐, 3‐, and 5‐minute nebulization of 99mTc‐DTPA through the Aerochamber plus nose‐muzzle mask device. Esophagus uptake was removed to avoid interference with the pulmonary uptake [blank areas in scans (B), (E), (F), (H), (K)]. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
3.1. Comparison between inhalation devices
The whole‐body, pulmonary, and combined stomach and head (extrapulmonary) radiopharmaceutical uptake in cpm is shown in Table 1. Differences in the pulmonary uptake were not significant among devices (P > .05). The mean whole‐body uptake with the Aerochamber plus‐NM mask was significantly lower than with the Aerodawg‐NM mask (P = .006) and the Aerodawg‐nose mask (P = .003). The extrapulmonary uptake with the Aerochamber plus‐NM mask also was significantly lower than with the Aerodawg‐NM mask (P = .03) and with the Aerodawg‐nose mask (P = .01).
TABLE 1.
Mean (±SD) whole‐body, lung, and extrapulmonary uptake of nebulized 99mTc‐DTPA in counts per minute for each inhalation device
| ROI | Aerodawg‐NM mask | Aerochamber‐NM mask | Aerodawg‐nose mask |
|---|---|---|---|
| Whole‐body | 544 509 (±215 211) | 287 084 (±135 511) | 463 251 (±88 339) |
| Lung | 45 593 (±32 796) | 32 026 (±22 671) | 39 607 (±12 388) |
| Extrapulm. tissues | 449 953 (±20 531) | 230 315 (±112 212) | 376 497 (±88 501) |
Note: Number of dogs n = 10.
Abbreviations: 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid; NM, nose‐muzzle; ROI, region of interest.
Mean pulmonary uptake related to the whole‐body uptake with the Aerodawg‐NM mask, Aerochamber plus‐NM mask and Aerodawg‐nose mask was 9.2% (range, 2.1‐16.6; SD ± 5.0%), 11.4% (range, 2.0%‐19.0%; SD ± 4.9%), and 9.3% (range, 4.6%‐19.9%; SD ± 4.6%), respectively (P > .05). Mean extrapulmonary uptake related to the whole‐body uptake was 80.9% (range, 60.0%‐94.2%; SD ± 10.6%), 78.4% (range, 48.0%‐94.2%; SD ± 12.7%), and 81.4% (range, 63.9%‐94.6%; SD ± 9.2%), respectively (P > .05). A low mean pulmonary/extrapulmonary deposition ratio was observed with all devices (0.12, 0.16, and 0.11, respectively). Although aerosol delivery through the Aerochamber plus‐NM mask device showed the highest mean percentage uptake in the lungs and the lowest mean percentage uptake in the extrapulmonary tissues, differences were not significant between devices. The deposition ratios for all devices are shown in Figure 5.
FIGURE 5.

Whisker box plot of nebulized 99mTc‐DTPA uptake expressed as pulmonary/whole‐body (P/WB), extrapulmonary/whole‐body (EP/WB), and pulmonary/extrapulmonary (P/EP) deposition ratios after inhalation through 3 aerosol devices for 5 minutes. N = 10 dogs. Differences were not statistically significant. Error bars represent ranges. *Nose‐muzzle. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
3.2. Comparison among different inhalation time spans
For the second part of the study (comparison of radiopharmaceutical deposition after different durations of inhalation), the Aerochamber plus spacer‐NM mask device was chosen because of its slightly better pulmonary/whole‐body and pulmonary/extrapulmonary deposition ratios compared to the other devices.
The registered whole‐body, pulmonary, and extrapulmonary radiopharmaceutical uptake in cpm after 1‐, 3‐, and 5‐minute nebulization is shown in Figure 6. Radiopharmaceutical uptake was significantly lower in all ROIs, including the lungs, after 1‐minute inhalation than after 3‐ and 5‐minute inhalation (P < .05). No significant differences were found in the whole‐body, pulmonary, and extrapulmonary radiopharmaceutical uptake after 3‐ and 5‐minute nebulization.
FIGURE 6.
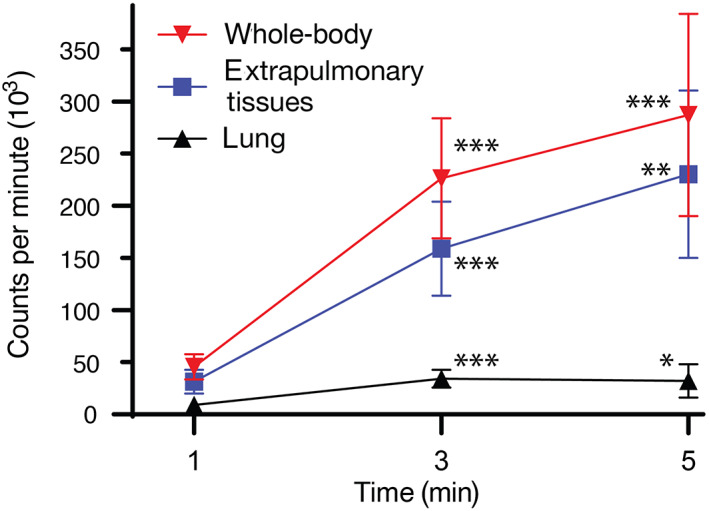
Mean nebulized 99mTc‐DTPA uptake in the whole body, the sum of head region and stomach (extrapulmonary tissues) and the lung expressed in counts per minute after 1‐, 3‐, and 5‐minute nebulization. Used device: Aerochamber plus nose‐muzzle mask. N = 10 dogs. Error bars represent 95% confidence interval. P values related to 1‐minute nebulization are represented as asterisks (*P < .05; **P < .01; ***P < .001). There were no significant differences between 3‐ and 5‐minute nebulization. 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
Mean pulmonary uptake related to the whole‐body uptake was 22.8% (range, 4.7%‐44.1%; SD ± 12.3%), 16.1% (range, 7.6%‐27.8%; SD ± 6.1%), and 11.4% (range, 2.0%‐19.0%; SD ± 4.9%), respectively. Differences were significant between 1‐ and 5‐minute nebulization (P = .02). Mean extrapulmonary uptake related to the whole‐body uptake was 66.8% (range, 49.2%‐82.1%; SD ± 12.1%), 69.6% (range, 61.4%‐81.4%; SD ± 6.3%), and 78.4% (range, 48.0%‐94.2%; SD ± 12.7%), respectively. Differences were evident but still not significant between 1‐ and 5‐minute nebulization (P = .05). The pulmonary/extrapulmonary deposition ratios were 0.38 (range, 0.06‐0.89; SD ± 0.27), 0.23 (range, 0.12‐0.45; SD ± 0.1), and 0.16 (range, 0.02‐0.40; SD ± 0.1), respectively. Again, differences between 1‐ and 5‐minute nebulization were significant (P = .03). Differences in all ratios between 1‐ and 3‐minute nebulization, as well as between 3‐ and 5‐minute nebulization were not significant. The pulmonary/whole‐body and extrapulmonary whole‐body deposition ratios are shown in Figure 7.
FIGURE 7.
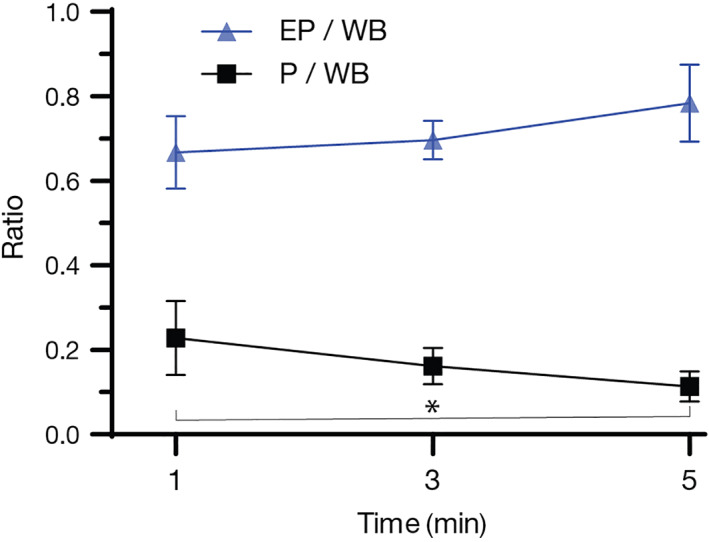
Mean nebulized 99mTc‐DTPA uptake expressed as pulmonary/whole‐body (P/WB) and extrapulmonary/whole‐body (EP/WB) deposition ratios after 1‐, 3‐, and 5‐minute nebulization. Used device: Aerochamber plus nose‐muzzle mask. N = 10 dogs. Error bars represent 95% confidence interval. The difference in the P/WB ratio was statistically significant (*) between 1‐ and 5‐minute nebulization (P = .03). 99mTc‐DTPA, 99mtechnetium‐diethylenetriamine‐pentaacetic acid
4. DISCUSSION
To our knowledge, this study is the first to compare the performance of distinct spacers and masks as well as time‐dependent differences in the pulmonary and extrapulmonary deposition of a nebulized radiopharmaceutical in healthy dogs.
Homogenous radiopharmaceutical uptake was detected in all lung scans independent of device or delivery time, indicating that nebulized aerosol reaches the lower airways of healthy dogs after at least 1‐minute inhalation through a spacer designed for children and a custom‐made NM mask. A homogenous distribution pattern also has been reported in healthy dogs and cats after similar or even shorter nebulization times. 8 , 9 However, this pattern is likely to differ in patients with lower airway disease, necessitating further investigation.
The decreased whole‐body and extrapulmonary radiopharmaceutical uptake observed with the Aerochamber plus‐NM mask compared to the other devices after 5 minutes of nebulization is an unclear finding. Possible explanations could be that more 99mTc‐DTPA leakage occurred through this device or more 99mTc‐DTPA remained in it or both, considering that all dogs inhaled for the same time period. Interestingly, the Aerodawg spacer combined with the same NM‐mask provided higher overall uptake, making differences in mask leakage less likely. In contrast, the mean pulmonary uptake did not differ significantly among devices. Moreover, the decreased extrapulmonary deposition achieved by the Aerochamber plus‐NM mask may even be a desired effect because this fraction may be associated with systemic adverse effects. 16 , 19
A general pattern of low pulmonary and high extrapulmonary radiopharmaceutical uptake was detected with all devices after 5‐minute nebulization, with mean pulmonary uptake ranging between and 9.2% and 11.4% of the registered dose in the whole body. Although our deposition percentages are not directly comparable to those of many studies in children 11 , 12 and a previous veterinary study 8 because of the use of distinct devices, variable inhalation periods, and different study population, the same general pattern of lower pulmonary and higher extrapulmonary deposition compared to that reported in older children and teenagers also was detected in our study. This finding reflects the influence of anatomic differences of the airways, breathing pattern, and patient cooperation on the pulmonary deposition of aerosol drugs.
None of the calculated deposition ratios (pulmonary/whole‐body, extrapulmonary/whole‐body, pulmonary/extrapulmonary) differed significantly among devices. This finding could be of particular interest for 2 reasons. Custom‐made devices in combination with pediatric spacers may be the only option in areas where devices specifically designed for dogs are not available, therefore making this treatment modality accessible for a wider range of patients. In addition, “noncanine” devices may be a valid option when financial issues exist, considering that the Aerodawg device is significantly more expensive than the Aerochamber plus device. Interestingly, a study of children from South Africa reported higher pulmonary uptake after radiopharmaceutical delivery though a modified cold‐drink bottle used as a spacer compared to 2 commercial spacers, showing that device availability and affordability also is an issue that needs to be addressed in developing countries. 10
One explanation for the similar deposition ratios observed among devices could be that both spacers used in this study are manufactured by the same company and are similar in size and design, possibly showing comparable characteristics such as capturing large droplets of aerosol on the walls while delivering smaller droplets into the lungs. 10 , 12 , 16 , 17 , 20 In addition, both the canine nose mask and the custom‐made NM mask were intended to be tight‐fitting on the dog's face to minimize leakage. Because two‐thirds of the delivered dose from the nebulizer may be lost during expiration, 21 a tightly fitting mask is crucial to optimize pulmonary delivery and decrease drug deposition on the skin and eyes. 12 , 16 , 17
Another contributing factor for similar deposition ratios could be the acceptable level of cooperation the dogs showed with both type of masks. In small children, acceptance of the mask is essential for successful treatment and some freedom of movement is desired to increase compliance because children may become impatient and move during aerosol administration, especially with rigid devices. 12 , 17 However, in our study, dogs received a low dose of butorphanol which possibly contributed to this behavior. Therefore, acclimatization sessions are recommended in patients that are about to start inhalation treatment to improve device acceptance. 5 , 22 , 23
A significantly lower radiopharmaceutical uptake was detected in all body regions, including the lungs, after 1‐minute nebulization compared to 3‐ and 5‐minutes nebulization. This was an expected finding because a shorter nebulization period implies a lower delivered dose. Although a lower pulmonary uptake could raise concern about the therapeutic effects of aerosol drugs, it is important to note the concurrent lower extrapulmonary uptake, which has no therapeutic value. Interestingly, 1‐minute nebulization provided significantly higher pulmonary/whole‐body and significantly lower extrapulmonary/whole‐body deposition ratios compared to 5‐minute nebulization, which are actually desired patterns for aerosol treatment. 2 , 15 , 16 These results might reflect why short inhalation periods, for example, after drug delivery with an MDI and a spacer, do have therapeutic effects with few systemic adverse effects. 5 , 22 , 23 , 24
Surprisingly, the pulmonary and extrapulmonary uptake after 3‐ and 5‐minute nebulization did not differ significantly in our study, suggesting that inhalation times beyond 3 minutes do not result in increased aerosol uptake. This is a relevant finding given the fact that prolonged nebulization periods negatively affect patient compliance. 25 A possible explanation could be the nebulization process itself because changes in temperature, viscosity, and output of pharmaceuticals using jet nebulizers may occur over time, leading to changes in droplet size and increased precipitation of the drug in the delivery system. 21 , 26 , 27 , 28 , 29 However, this finding may not apply for all types of nebulizers or aerosol drugs and requires further investigation. Another possibility (to our knowledge not investigated so far) could be that prolonged delivery of nebulized 99mTc‐DTPA might lead to saturation of the alveoli and subsequent exhalation of the remaining amount, considering that this radiopharmaceutical is intended to be slowly absorbed because of its hydrophilic characteristics 30 (half‐time of pulmonary clearance of 24.6 ± 5.3 minutes in dogs). 31
Our study had several limitations. It included a small group of healthy young Beagle dogs living under the same housing conditions. This factor possibly resulted in a more precise assessment of device performance and the effect of different delivery time intervals with minimal influence of patient‐dependent factors. These results, however, may not apply to other breeds, other housing conditions, older dogs, and patients with respiratory disease.
We did not consider the esophageal radiopharmaceutical uptake in the calculation of the extrapulmonary uptake because of difficulties in precisely defining esophageal borders. This inevitably may have led to an underestimation of the extrapulmonary uptake in some dogs. However, this decision was made for all dogs, resulting in a similar degree of underestimation in all cases. In addition, the uptake in the whole‐body included the esophageal deposition, meaning that the pulmonary/whole‐body deposition ratio was a reliable parameter of aerosol distribution in the dogs.
A 5‐minute nebulization time was chosen for the first part of the study based on previous recommendations. 32 Nonetheless, this interval may not reflect the performance of devices after shorter nebulization times or when using an MDI. Considering that aerosol treatment is mainly delivered using an MDI in small animals, further scintigraphic studies comparing the performance of aerosol devices using shorter nebulization times or with radiolabeled drugs provided by an MDI 8 , 33 would be valuable.
In conclusion, we showed that a spacer designed for children attached to a tightly fitting custom‐made NM mask performs similarly to an inhalation device specifically designed for dogs after 5‐minute nebulization, suggesting that this combined device may be a valid option when financial limitations or unavailability of canine‐specific devices are an issue. Additionally, 1‐minute nebulization using this device reliably reached the lung and provided a more favorable pulmonary/extrapulmonary deposition ratio compared to 5‐minute nebulization.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Austrian government as well as the ethics and animal welfare committee of the University of Veterinary Medicine, Vienna (approval number GZ.: BMWFW‐68.205/0230‐WF/V/3b/2016).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. This study was presented as an abstract at the 28th ECVIM‐CA Annual Congress in Rotterdam, The Netherlands. The authors acknowledge Siegfried Kosik for the support with the scintigraphic procedure.
Carranza Valencia A, Hirt R, Kampner D, et al. Comparison of pulmonary deposition of nebulized 99mtechnetium‐diethylenetriamine‐pentaacetic acid through 3 inhalation devices in healthy dogs. J Vet Intern Med. 2021;35:1080–1087. 10.1111/jvim.16064
REFERENCES
- 1. Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10‐19. [DOI] [PubMed] [Google Scholar]
- 2. Ibrahim M, Verma R, Garcia‐Contreras L. Inhalation drug delivery devices: technology update. Med Devices. 2015;8:131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leemans J, Kirschvink N, Clercx C, Snaps F, Gustin P. Effect of short‐term oral and inhaled corticosteroids on airway inflammation and responsiveness in a feline acute asthma model. Vet J. 2012;192(1):41‐48. [DOI] [PubMed] [Google Scholar]
- 4. Cohn LA, DeClue AE, Cohen RL, et al. Effects of fluticasone propionate dosage in an experimental model of feline asthma. J Feline Med Surg. 2010;12(2):91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bexfield NH, Foale RD, Davison LJ, Watson PJ, Skelly BJ, Herrtage ME. Management of 13 cases of canine respiratory disease using inhaled corticosteroids. J Small Anim Pract. 2006;47(7):377‐382. [DOI] [PubMed] [Google Scholar]
- 6. Hirt R, Haderer A, Bilek A. Effectiveness of inhaled glucocorticoids in canine chronic inflammatory respiratory tract disease. Wien Tierarztl Monatsschr. 2008;95(1‐2):45‐51. [Google Scholar]
- 7. Canonne AM, Bolen G, Peeters D, Billen F, Clercx C. Long‐term follow‐up in dogs with idiopathic eosinophilic bronchopneumopathy treated with inhaled steroid therapy. J Small Anim Pract. 2016;57(10):537‐542. [DOI] [PubMed] [Google Scholar]
- 8. Chow KE, Tyrrell D, Yang M, Abraham LA, Anderson GA, Mansfield CS. Scintigraphic assessment of deposition of radiolabeled fluticasone delivered from a nebulizer and metered dose inhaler in 10 healthy dogs. J Vet Intern Med. 2017;31(6):1849‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulman RL, Crochik SS, Kneller SK, McKiernan BC, Schaeffer DJ, Marks SL. Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats. Am J Vet Res. 2004;65(6):806‐809. [DOI] [PubMed] [Google Scholar]
- 10. Zar HJ, Weinberg EG, Binns HJ, Gallie F, Mann MD. Lung deposition of aerosol ‐ a comparison of different spacers. Arch Dis Child. 2000;82(6):495‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devadason SG, Huang T, Walker S, Troedson R, le Souëf PN. Distribution of technetium‐99m‐labelled QVAR™ delivered using an Autohaler™ device in children. Eur Respir J. 2003;21(6):1007‐1011. [DOI] [PubMed] [Google Scholar]
- 12. Kwok PCL, Chan HK. Delivery of inhalation drugs to children for asthma and other respiratory diseases. Adv Drug Deliv Rev. 2014;73:83‐88. [DOI] [PubMed] [Google Scholar]
- 13. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiBlasi RM. Clinical controversies in aerosol therapy for infants and children. Respir Care. 2015;60(6):894‐914. [DOI] [PubMed] [Google Scholar]
- 15. Roller CM, Zhang G, Troedson RG, Leach CL, le Souëf PN, Devadason SG. Spacer inhalation technique and deposition of extrafine aerosol asthmatic children. Eur Respir J. 2007;29(2):299‐306. [DOI] [PubMed] [Google Scholar]
- 16. Ditcham W, Murdzoska J, Zhang G, et al. Lung deposition of 99mTc‐radiolabeled albuterol delivered through a pressurized metered dose inhaler and spacer with facemask or mouthpiece in children with asthma. J Aerosol Med Pulm Drug Deliv. 2014;27(suppl 1):63‐75. [DOI] [PubMed] [Google Scholar]
- 17. Amirav I, Newhouse MT. Review of optimal characteristics of face‐masks for valved‐holding chambers (VHCs). Pediatr Pulmonol. 2008;43:260‐274. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan A, Price D. Matching inhaler devices with patients: the role of the primary care physician. Can Respir J. 2018;2018:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skoner DP. Balancing safety and efficacy in pediatric asthma management. Pediatrics. 2002;109(2 suppl):381‐392. [PubMed] [Google Scholar]
- 20. Nikander K, Nicholls C, Denyer J, et al. The evolution of spacers and valved holding chambers. J Aerosol Med Pulm Drug Deliv. 2014;27(suppl 1):4‐23. [DOI] [PubMed] [Google Scholar]
- 21. O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52(suppl 2):31‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nafe LA. Respiratory and inhalant therapy. In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine. 8th ed. St. Louis, MO: Elsevier; 2017:371‐374. [Google Scholar]
- 23. Rozanski E. Canine chronic bronchitis. Vet Clin North Am Small Anim Pract. 2014;44(1):107‐116. [DOI] [PubMed] [Google Scholar]
- 24. Fok TF, Monkman S, Dolovich M, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21(5):301‐309. [DOI] [PubMed] [Google Scholar]
- 25. Sidler‐Moix AL, Di Paolo ER, Dolci U, et al. Physicochemical aspects and efficiency of albuterol nebulization: comparison of three aerosol types in an in vitro pediatric model. Respir Care. 2015;60(1):38‐46. [DOI] [PubMed] [Google Scholar]
- 26. McCallion ONM, Taylor KMG, Bridges PA, et al. Jet nebulisers for pulmonary drug delivery. Int J Pharm. 1996;130(1):1‐11. [Google Scholar]
- 27. Phipps PR, Gonda I. Droplets produced by medical nebulizers. Some factors affecting their size and solute concentration. Chest. 1990;97(6):1327‐1332. [DOI] [PubMed] [Google Scholar]
- 28. Steckel H, Eskandar F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur J Pharm Sci. 2003;19(5):443‐455. [DOI] [PubMed] [Google Scholar]
- 29. Cockcroft DW, Hurst TS, Gore BP. Importance of evaporative water losses during standardized nebulized inhalation provocation tests. Chest. 1989;96(3):505‐508. [DOI] [PubMed] [Google Scholar]
- 30. O'Doherty MJ, Peters AM. Pulmonary technetium‐99m diethylene triamine penta‐acetic acid aerosol clearance as an index of lung injury. Eur J Nucl Med. 1997;24(1):81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizk NW, Luce JM, Hoeffel JM, et al. Site of deposition and factors affecting clearance of aerosolized solute from canine lungs. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1984;56:723‐729. [DOI] [PubMed] [Google Scholar]
- 32. Daniel GB, Berry CR. Pulmonary and mucociliary scintigraphy. In: Daniel GB, Berry CR, eds. Textbook of Veterinary Nuclear Medicine. 2nd ed. Chapell Hill, NC: American College of Veterinary Radiology; 2006:303‐328. [Google Scholar]
- 33. Newman SP, Pitcairn GR, Hirst PH, Rankin L. Radionuclide imaging technologies and their use in evaluating asthma drug deposition in the lungs. Adv Drug Deliv Rev. 2003;55(7):851‐867. [DOI] [PubMed] [Google Scholar]


