Abstract
Background
The possible benefits associated with corticosteroid treatment in acute respiratory distress syndrome (ARDS) patients are not fully known. We conducted an updated meta-analysis to assess the effect of corticosteroids in the treatment of patients with ARDS.
Methods
We systematically searched MEDLINE, Embase, and the Cochrane Library from inception to January 2021 via Ovid to identify randomized controlled trials evaluating the efficacy of glucocorticoids in the treatment of patients with ARDS. The primary outcome was hospital mortality. Secondary outcomes included the number of ventilator-free days at day 28, oxygenation improvement (PaO2/FIO2 ratios), and adverse events.
Results
Nine studies with 1371 participants were analyzed. The pooled analysis revealed that glucocorticoid use was associated with reduced mortality [relative risk (RR), 0.83; 95% confidence interval (CI) 0.74–0.93; P < 0.01; I2 = 37], and the statistical power was confirmed by trial sequential analysis. Glucocorticoids might also significantly increase the number of ventilator-free days at day 28 (mean deviation 3.66 days, 95% CI 2.64–4.68; P < 0.01) and improve oxygenation (standardized mean difference 4.17; 95% CI 2.32–6.02; P < 0.01). In addition, glucocorticoid use was not associated with increased risks of new infection (RR 0.84; 95% CI 0.70–1.01; P = 0.07) and hyperglycemia (RR 1.11; 95% CI 0.99–1.23; P = 0.06).
Conclusions
The use of glucocorticoids might result in reduced mortality in patients with ARDS. Glucocorticoids might be recommended as an adjunct to standard care for ARDS; however, the optimal dose and duration of steroid therapy remains unknown and further studies are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03546-0.
Keywords: Acute respiratory distress syndrome, Glucocorticoids, Randomized clinical trial, Meta-analysis
Introduction
The acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by bilateral pulmonary infiltrates on chest imaging and refractory hypoxemia [1]. It is not uncommon in critically ill patients in intensive care units (ICUs) and is associated with considerable mortality [2]. A recent international study found that nearly 10% of ICU patients suffered from ARDS, and the hospital mortality of ARDS patients was about 40% [3]. Despite decades of research, current pharmacological therapies for ARDS are limited [4]. Overwhelming lung inflammation plays a key role in the pathogenesis of ARDS [5]. Therefore, inflammation-directed therapies, such as glucocorticoid treatment, appear to be a reasonable strategy to treat ARDS patients.
Glucocorticoids have anti-inflammatory and anti-fibrosis effects and have been the most investigated immunomodulatory agent for the treatment of ARDS. However, the impact of corticosteroid therapy on clinically relevant outcomes in ARDS patients remains controversial. Clinical trials evaluating corticosteroids in the management of ARDS reported conflicting results [6–8], and previous meta-analyses were underpowered to draw determinate conclusions [9, 10]. Thus, we conducted an updated meta-analysis with trial sequential analysis to assess the effect of corticosteroids in the treatment of patients with ARDS and determine whether the current evidence is reliable.
Methods
Data sources and searches
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [11]. We systematically searched MEDLINE, Embase, and the Cochrane Library from inception to January 2021 via Ovid to identify randomized controlled trials (RCTs), using the following search terms: (“ALI” OR “acute lung injury” OR “ARDS” OR “acute respiratory distress syndrome”) AND (“steroids” OR “corticoid” OR “corticosteroid” OR “glucocorticoids” OR “hydrocortisone” OR “prednisolone” OR “dexamethasone” OR “methylprednisolone”) AND (“randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “randomly” OR “trial”). The detail of the search strategy is shown in Additional file 1. Publication species were limited to humans. Besides, bibliographies of identified studies were also searched manually.
Study selection
The eligibility of each study identified from the literature search was assessed independently in a blinded fashion by two researchers. English-language, peer-reviewed studies meeting the following criteria were included in this meta-analysis: (1) study design: RCTs; (2) participant: adult patients with ARDS; (3) intervention: glucocorticoids versus control; (4) at least one of the following outcomes: all-cause mortality, number of ventilator-free days at day 28, oxygenation improvement (PaO2/FIO2 ratios), and adverse events. Any discrepancies were resolved through discussion.
Data abstraction and quality assessment
Using a standardized data collection form, two investigators independently extracted the following data from each eligible study: first author, publication year, number of patients, inclusion criteria, corticosteroid type, corticosteroid dose, therapy duration, and main outcomes. The primary outcome was hospital mortality. If hospital mortality was not reported, we used the closest time point for our analysis. Secondary outcomes included the number of ventilator-free days at day 28, PaO2/FIO2 ratios, and adverse events.
The Cochrane risk-of-bias tool was used to assess the risk of bias of the eligible studies [12]. This tool comprises seven domains, and each domain scores as low, unclear, or high risk of bias: adequate sequence generation, allocation concealment, blinding of participants and personnel to the research protocol, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Any discrepancies were resolved through discussion.
Data synthesis
Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. Weighted mean differences (MDs) or standardized mean differences (SMDs) with their 95% CIs were calculated for continuous data. If only medians and interquartile ranges were available, means and standard deviations (SDs) were estimated according to the methods described by Hozo et al. [13]. Heterogeneity across eligible studies was assessed using the I2 statistic. There was significant heterogeneity if the I2 value > 50% [14]. We analyzed all data using fixed-effects models if the I2 value < 50%; otherwise, random-effects models were used. The risk of publication bias was assessed by a funnel plot. All P values were two-sided, and a P value < 0.05 was recognized as statistically significant. Review Manager Software (version 5.4, The Cochrane Collaboration) was used for all statistical analysis.
Trial sequential analysis (TSA), a method that can correct for the increased risk of type I errors caused by sparse data and repeated significance testing on accumulating data and can determine whether the evidence in a meta-analysis is reliable, was conducted in our study [15, 16]. When the cumulative Z curve crosses the futility boundary or the trial sequential monitoring boundary, there is sufficient evidence to reach a conclusion and no further trials are needed to confirm the results. We conducted the trial sequential analysis to estimate the required information size using a type I error of 5%, a type II error of 20% (power 80%), an anticipated relative risk reduction of 20%, and the control event proportions were calculated from the control group. We used TSA version 0.9.5.10 beta (www.ctu.dk/tsa) for the analyses.
Results
Search results
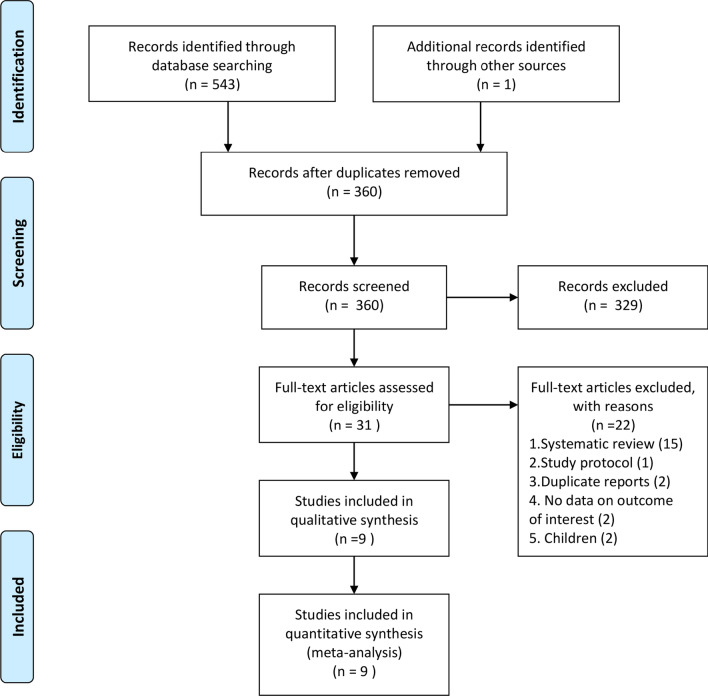
A total of 544 potentially eligible records were identified by a comprehensive literature search. After excluding duplicates and checking the titles and abstracts, thirty-one studies were retrieved. After reviewing the full text, nine studies met all eligibility criteria and were included in the current meta-analysis [17–25]. The detailed flowchart for literature selection is shown in Fig. 1.
Fig. 1.
PRISMA 2009 flow diagram of identified studies
Characteristics of studies
The main characteristics of the eligible studies are summarized in Table 1. These studies were published between 1987 and 2020. The number of included participants from each study ranged from 24 to 299 (total 1371). All participants met moderate-to-severe ARDS criteria (PaO2/FIO2 ≤ 200). Corticosteroid regimens varied apparently among studies. Of these, five studies used methylprednisolone, two hydrocortisone, and two dexamethasone. Treatment duration ranged from 1 to 28 days. Corticosteroid dose was also different among studies, ranging from 1 to 120 mg/kg/d of methylprednisolone or equivalent.
Table 1.
The main characteristics of the randomized controlled trials
| Study | No. of patients (steroids/control) | Subjects | Intervention | Treatment duration | Main outcomes |
|---|---|---|---|---|---|
| Bernard/1987 | 50/49 | (1) Patients with PaO2 ≤ 70 mmHg (FiO2 ≥ 40%) or PaO2/PAO2 ≤ 0.3; (2) bilateral diffuse infiltrates on chest radiography; (3) PAWP ≤ 18 mmHg | Methylprednisolone 30 mg/kg IV every 6 h | 24 h | 45-day all-cause mortality |
| Meduri/1998 | 16/8 | (1) patients diagnosed with ARDS by the AECC definition; (2) 7 days of mechanical ventilation with an LIS ≥ 2.5 and less than 1-point reduction from day 1 of ARDS; (3) no evidence of untreated inflection | Methylprednisolone 2 mg/kg bolus followed by 2 mg/kg (day 1–14), 1 mg/kg (day 15–21), 0.5 mg/kg (day 22–28),0.25 mg/day (day 28–32) | 32 days | Improvement in lung function and mortality |
| Annane/2006 | 85/92 | (1) septic shock patients with bilateral infiltrates on chest radiography; (2) PaO2/FIO2 ≤ 200; (3) PAWP ≤ 18 mmHg or no clinical evidence of left atrial hypertension | Hydrocortisone 30 mg IV every 6 h and 9-fludrocortisone 50ug orally once a day | 7 days | 28-day survival |
| Steinberg/2006 | 89/91 | (1) adult patients diagnosed with ARDS by the AECC definition; (2) patients were intubated and mechanically ventilated for 7–28 days after the onset of ARDS | Methylprednisolone 2 mg/kg bolus followed by 2 mg/kg (day 1–14), 1 mg/kg (day 15–21), tapering over (day 22–25) | 25 days | 60-day all-cause mortality |
| Meduri/2007 | 63/28 | Adult intubated patients diagnosed with ARDS by the AECC definition | Methylprednisolone 1 mg/kg bolus followed by 1 mg/kg (day 1–14), 0.5 mg/kg (day 15–21), 0.125 mg/kg (day 22–25), 0.25 mg/day (day 26–28) | 28 days | A 1-point reduction in LIS or successful extubation by day 7 |
| Rezk/2013 | 18/9 | (1) ARDS patients with PaO2/FIO2 < 200, bilateral pulmonary infiltrates, and PAWP < 18 mmHg; (2) patients were mechanically ventilated | Methylprednisolone 1 mg/kg bolus followed by 1 mg/kg (day 1–14), 0.5 mg/kg (day 15–21), 0.125 mg/kg (day 22–25), 0.25 mg/day (day 26–28) | 28 days | Improvements of clinical parameters |
| Tongyoo/2016 | 98/99 | (1) Adult patients with severe sepsis or septic shock receiving mechanical ventilation; (2) patients diagnosed with ARDS by the AECC definition and the Berlin criteria | Hydrocortisone 50 mg IV every 6 h | 7 days | 28-day all-cause mortality |
| Villar/2020 | 139/138 | (1) Adult patients were intubated and mechanically ventilated; (2) patients diagnosed with ARDS by the AECC definition or the Berlin criteria as moderate to severe ARDS | Dexamethasone 20 mg once daily from day 1 to day 5, which was reduced to 10 mg once daily from day 6 to day 10 | 10 days | Ventilator-free days during the first 28 days |
| Tomazini/2020 | 151/148 | (1) Adult patients with confirmed or suspected COVID-19 infection were receiving mechanical ventilation; (2) patients diagnosed with moderate to severe ARDS by the Berlin criteria | Dexamethasone 20 mg daily from day 1 to day 5, followed by 10 mg daily for 5 days or until ICU discharge | 10 days or until ICU discharge | Ventilator-free days during the first 28 days |
ARDS acute respiratory distress syndrome, PaO2 partial pressure of oxygen in arterial blood, PAO2 partial pressure of alveolar oxygen, FiO2 fraction of inspired oxygen, PAWP pulmonary artery wedge pressure, AECC the American-European Consensus Conference criteria, LIS lung injury score, COVID-19 coronavirus disease 2019, ICU intensive care unit, IV intravenous, h hour(s)
Risk of bias
Table 2 illustrates the details of the risk of bias assessment. Overall, seven studies achieved a low overall risk of bias [17–21, 23, 24], and two studies were judged to be at high risk of bias [22, 25]. Among the eligible studies, eight studies generated an adequate randomization sequence and seven studies reported appropriate allocation concealment. Blinding was conducted in most trials except two studies [22, 25].
Table 2.
Assessment of risk of bias using the Cochrane risk-of-bias tool
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Bernard/1987 | Low | Low | Low | Low | Low | Unclear A | Low |
| Meduri/1998 | Low | Low | Low | Low | Low | Low | Low |
| Annane/2006 | Low | Low | Low | Low | Low | Low | Low |
| Steinberg/2006 | Low | Low | Low | Low | Low | Low | Low |
| Meduri/2007 | Low | Low | Low | Low | Low | Low | Low |
| Rezk/2013 | UnclearA | High | High | High | Unclear A | Unclear A | Unclear A |
| Tongyoo/2016 | Low | Low | Low | Low | Low | Low | Low |
| Villar/2020 | Low | Low | Low | Low | Low | Low | Low |
| Tomazini/2020 | Low | High | High | High | Low | Low | Low |
AIndicating insufficient information
Mortality outcomes
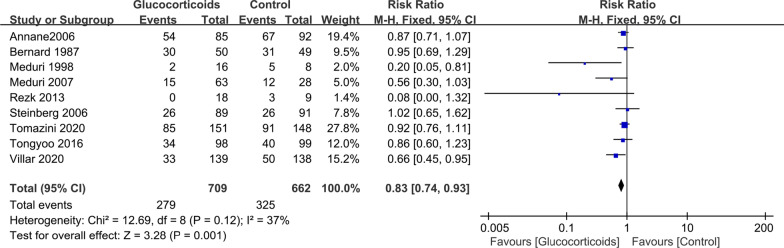
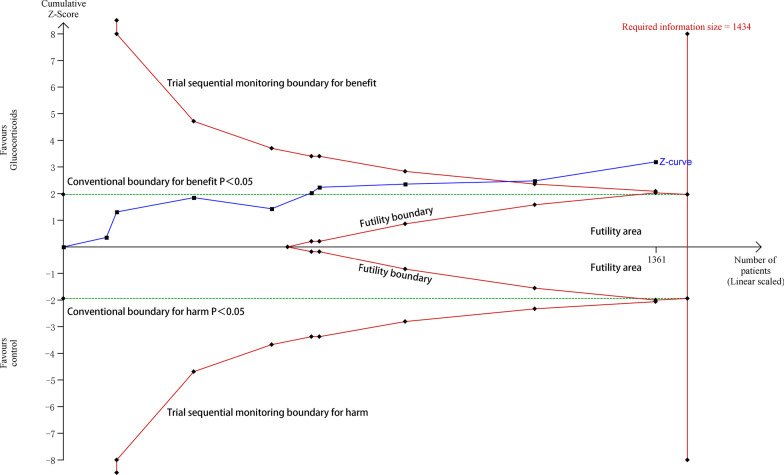
Between 1987 and 2020, nine studies with 1371 participants presented available results on mortality [17–25]. The mortality in the glucocorticoid group and the control group was 39.4% (279 of 709 patients) and 49.1% (325 of 662 patients), respectively. The pooled results showed that glucocorticoids are associated with reduced hospital mortality (RR 0.83; 95% CI 0.74–0.93; P < 0.01), with no significant heterogeneity (I2 = 37, P = 0.12) (Fig. 2). The TSA results showed that the adjusted 95% CI of RR was 0.74–0.94, and the required information size for detecting an intervention effect was 1434 patients. The cumulative Z curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit (Fig. 3), suggesting that current evidence is sufficient and further studies are unlikely to change the current conclusion of benefit with glucocorticoids. There was a potential risk of publication bias in favor of positive findings by inspection of the funnel plot (Additional file 2). After excluding two small studies with low weight [20, 25], glucocorticoids were still associated with reduced hospital mortality (RR 0.85; 95% CI 0.76–0.95; P = 0.006; I2 = 0%).
Fig. 2.
The effect of glucocorticoid treatment on mortality. CI confidence interval
Fig. 3.
Trial sequential analysis of nine trials for hospital mortality. The required information size for detecting an intervention effect was 1434 patients. The relative risk was 0.83, and the 95% confidence interval was corrected to 0.74–0.94, from 0.74 to 0.93. The cumulative Z curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit
Number of ventilator-free days at day 28
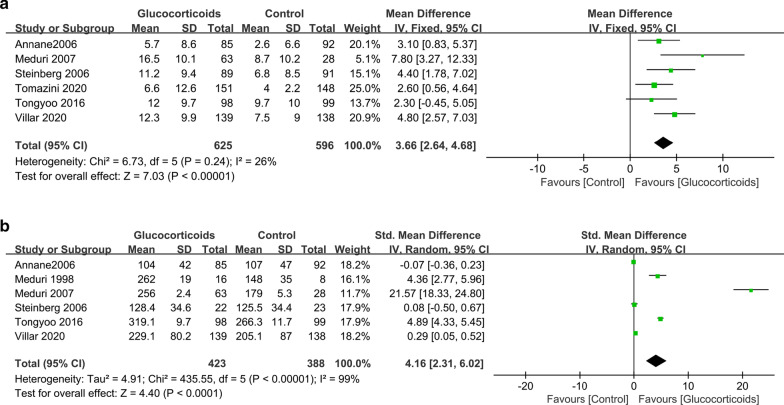
Six trials had data on the number of ventilator-free days at day 28 [17, 19, 21–24]. The pooled result showed that glucocorticoid use was associated with more ventilator-free days at day 28, with a mean difference of 3.66 days (95% CI 2.64–4.68; P < 0.01; I2 = 26%) (Fig. 4a).
Fig. 4.
The effect of glucocorticoid treatment on the number of ventilator-free days at day 28 (a) and PaO2/FIO2 ratios (b). CI confidence interval
PaO2/FIO2 ratios
Six trials investigated oxygenation improvement (PaO2/FIO2 ratios) of the glucocorticoid versus control groups [17, 19–21, 23, 24]. The pooled result showed that glucocorticoid use might significantly improve oxygenation (SMD, 4.16; 95% CI 2.31–6.02; P < 0.01; I2 = 99%) (Fig. 4b).
Adverse events
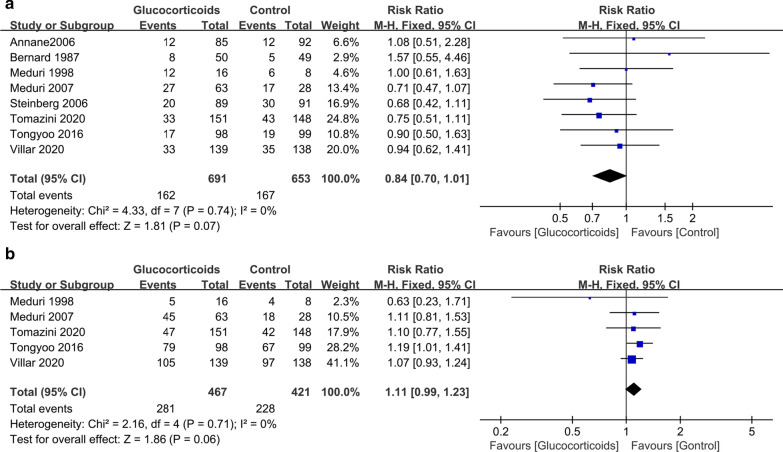
Data on new infection and hyperglycemia were available in eight studies [17–24] and five studies [19, 20, 22–24], respectively. The pooled result showed that glucocorticoid treatment was not associated with a higher incidence of new infection (RR 0.84; 95% CI 0.70–1.01; P = 0.07; I2 = 0) (Fig. 5a). TSA showed that the cumulative Z-curve did not crossed both the conventional boundary and the trial sequential monitoring boundary (Additional file 3), indicating that current evidence is inconclusive and further studies are needed. Glucocorticoid treatment was also not associated with an increased risk of hyperglycemia (RR 1.11; 95% CI 0.99–1.23; P = 0.06; I2 = 0) (Fig. 5b), which was confirmed by TSA (the cumulative Z-curve surpassed the futility boundary) (Additional file 4).
Fig. 5.
The effect of glucocorticoid treatment on new infection (a) and hyperglycemia (b). CI confidence interval
Discussion
This was an updated meta-analysis of RCTs to evaluate the benefits and risks associated with glucocorticoid use in ARDS patients. In our meta-analysis, we found that glucocorticoids might reduce mortality and duration of mechanical ventilation and improve oxygenation in patients with ARDS. Besides, this study also indicated that glucocorticoid treatment was not associated with a higher incidence of new infection and hyperglycemia.
Several systematic reviews and meta-analyses on the topic had been presented [9, 26–29]. Among previous meta-analyses, Zayed, Y.'s study was the most comprehensive one [9]. It included eight RCTs totaling 1091 patients for analysis and found that glucocorticoid use was associated with a significant reduction in-hospital mortality (RR 0.79; 95% CI 0.64–0.98; P = 0.03). Unfortunately, TSA suggested insufficient information size and potentially false-positive results in Zayed, Y.'s study. Although the main outcomes of our meta-analysis were consistent with those reported in Zayed, Y.'s study, there were significant differences between our study and Zayed, Y.'s study. Firstly, our study did not include one RCT which was included in Zayed, Y.'s meta-analysis because this RCT recruited severe pneumonia patients instead of ARDS [30]. Secondly, the present study included two additional RCTs which were published recently, with an added statistical power of about 300 cases [22, 25]. Our study was the most comprehensive meta-analysis and reinforced the earlier results of previous meta-analyses. Thirdly, we used TSA to estimate the effect more conservatively in the present study. Finally, this was the first meta-analysis with sufficient evidence to confirm that glucocorticoids might significantly reduce mortality in patients with ARDS and further studies are unlikely to change the current conclusion.
There were some meta-analyses exploring the effects of corticosteroids on other conditions such as coronavirus disease 2019 (COVID-19) [31], sepsis [32], and community-acquired pneumonia [27]. Glucocorticoid use was found to be associated with significant improvement in clinical outcomes in these conditions. One possible reason for these findings was that critical illness might be associated with an impaired hypothalamic–pituitary–adrenal (HPA) axis response to stress [33]. ARDS is a devastating lung disorder and is associated with a high mortality rate. One study showed that critical illness-related corticosteroid insufficiency (CIRCI) was common in ARDS (about 58%) and stress dose glucocorticoid was associated with prolong survival time [34]. The early use of corticosteroid therapy might alleviate systemic inflammation caused by CIRCI and then was associated with survival benefit.
An anti-inflammatory pharmacologic intervention appears to be a reasonable strategy in ARDS in that dysregulated and excessive pulmonary inflammation is the pathophysiologic hallmarks of ARDS [35]. Among the anti-inflammatory drugs, glucocorticoids are the main immunomodulatory agent for the treatment of ARDS. Early studies demonstrated that glucocorticoid treatment led to rapid improvements in pulmonary and extrapulmonary organ function in ARDS patients, with a significant reduction in bronchoalveolar lavage and plasma levels of proinflammatory mediators and chemokines [36, 37]. Animal experiments also found that corticosteroid treatment could alleviate lung injury and upregulate pulmonary glucocorticoid receptors [38, 39]. Unfortunately, clinical trials reported inconsistent results. Some trials found that glucocorticoids might reduce the risk of death in patients with ARDS [20, 24]. However, there also were some trials that failed to find such benefits [19, 23]. Until today, glucocorticoid use in ARDS remains highly controversial due to unclear benefits and potential side effects [40]. Our meta-analysis pooled the latest data and suggested that glucocorticoids show beneficial effects in patients with ARDS without significant side effects. Therefore, glucocorticoids might be recommended as an adjunct to standard care for ARDS due to the beneficial effects. In addition, there has been significant progress in the management of ARDS in the past 20 years. High-quality clinical trials have confirmed that ventilation with lower tidal volumes and prone positioning can significantly decrease all-cause mortality of ARDS after 2000 [41, 42]. With the progress of standards of care for patients with ARDS, whether glucocorticoid use can reduce the mortality rate of ARDS should be noted. When limiting to trials commenced after 2000 [22–25], we found that glucocorticoid use is also associated with reduced mortality (RR 0.81; 95% CI 0.69–0.95; P = 0.01; I2 = 46), indicating glucocorticoid use is still applicable today.
One might expect that glucocorticoid use could cause adverse events such as new infections and hyperglycemia in patients with ARDS. However, we did not find significant differences between the glucocorticoid group and the control group in the incidence of adverse events. The use of low-dose glucocorticoids in the experimental group might be responsible for the unexpected outcomes. Among eligible studies, except for one trial that used high-dose glucocorticoids (methylprednisolone 30 mg/kg every 6 h) for only 24 h, other trials used low-dose glucocorticoids. In addition, TSA indicated that current evidence regarding new infection is inconclusive and further studies are needed.
Several limitations need to be considered in our meta-analysis. First, due to a lack of individual patient data, we were unable to conduct subgroup analyses according to patient baseline characteristics such as the underlying etiology of ARDS. Second, the study population and corticosteroid regimen varied among studies, which might result in clinical heterogeneity. More data are needed to evaluate the impact of corticosteroid regimen on outcomes. Third, two trials were judged to be at high risk of performance and detection bias in that these studies were not blinded to the study protocol, which might compromise the reliability of our results. Finally, there was a potential risk of publication bias in our study. It has been frequently noted that small trials tend to yield more extreme effects than large trials and are particularly susceptible to publication bias. After excluding small studies with low weight, our results did not alter significantly, indicating that our findings were reliable.
Conclusions
Our meta-analysis indicated that glucocorticoid treatment might reduce overall mortality and duration of mechanical ventilation and improve oxygenation in patients with ARDS. Glucocorticoids might be recommended as an adjunct to standard care for ARDS; however, the optimal dose and duration of steroid therapy remains unknown and further studies are needed.
Supplementary Information
Additional file 1. Search strategy terms.
Additional file 2. Test for publication bias for hospital mortality. RR relative risk.
Additional file 3. Trial sequential analysis of eight trials for new infection. The required information size for detecting an intervention effect was 2131 patients. The relative risk was 0.84, and the 95% confidence interval was corrected to 0.66–1.08, from 0.70 to 1.01. TSA showed that the cumulative Z-curve did not cross both the conventional boundary and the trial sequential monitoring.
Additional file 4. Trial sequential analysis of hyperglycemia trials for hyperglycemia. The required information size for detecting an intervention effect was 746 patients. The relative risk was 1.11, and the 95% confidence interval was corrected to 0.98–1.25, from 0.99 to1.23. TSA showed that the cumulative Z-curve crossed the futility boundary.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care unit
- RCT
Randomized controlled trial
- RR
Risk ratio
- CI
Confidence interval
- MD
Mean difference
- SMD
Standardized mean difference
- SD
Standard deviation
- TSA
Trial sequential analysis
- HPA
Hypothalamic–pituitary–adrenal
- CIRCI
Critical illness-related corticosteroid insufficiency
Authors' contributions
PL contributed to study concept and design, literature search and study selection, data analysis, and drafting the article. YZ and XL contributed to quality assessment, and acquisition of data, and data analysis. FJ contributed to study concept and design, literature search and study selection, and critical revision. ZL contributed to study concept and design, quality assessment, critical revision, and submitted the report for publication. All authors read and approved the final manuscript.
Funding
This study was funded by the National Key Research and Development Program of China (Grant 2016YFC1304303).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Faming Jiang, Email: jiangfaming@wchscu.cn.
Zongan Liang, Email: liangza@scu.edu.cn, Email: 2020324025198@stu.scu.edu.cn.
References
- 1.Mac Sweeney R, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 5.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 7.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 8.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zayed Y, Barbarawi M, Ismail E, Samji V, Kerbage J, Rizk F, et al. Use of glucocorticoids in patients with acute respiratory distress syndrome: a meta-analysis and trial sequential analysis. J Intensive Care. 2020;8:43. doi: 10.1186/s40560-020-00464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mammen MJ, Aryal K, Alhazzani W, Alexander PE. Corticosteroids for patients with acute respiratory distress syndrome: a systematic review and meta-analysis of randomized trials. Pol Arch Intern Med. 2020;130(4):276–286. doi: 10.20452/pamw.15239. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61:763–769. doi: 10.1016/j.jclinepi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA) Copenhagen: Copenhagen Trial Unit Centre for Clinical Intervention Research; 2011. [Google Scholar]
- 17.Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. 2006;34:22–30. doi: 10.1097/01.CCM.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 18.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 19.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ards: Results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 20.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 22.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MV, Baldassare FP. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: Results of a randomized controlled trial. Crit Care. 2016;20:329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 25.Abdelsalam Rezk N, Mohamed IA. Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc. 2013;62:167–172. doi: 10.1016/j.ejcdt.2013.02.013. [DOI] [Google Scholar]
- 26.Sun S, Liu D, Zhang H, Zhang X, Wan B. Effect of different doses and time-courses of corticosteroid treatment in patients with acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2019;18:4637–4644. doi: 10.3892/etm.2019.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209–219. doi: 10.1378/chest.15-1733. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZG, Lei XL, Li XL. Early application of low-dose glucocorticoid improves acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Exp Ther Med. 2017;13:1215–1224. doi: 10.3892/etm.2017.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Q, Shi JX, Hu R, Li Q, Zhang CY, Li JS. Effect of glucocorticoids on mortality in patients with acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2019;18:4913–4920. doi: 10.3892/etm.2019.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 31.The WHOREAfC-TWG Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med. 2019;179:213–223. doi: 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arabi YM, Chrousos GP, Meduri GU. The ten reasons why corticosteroid therapy reduces mortality in severe COVID-19. Intensive Care Med. 2020;46:2067–2070. doi: 10.1007/s00134-020-06223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Li J, Huang Y-z, Liu S-q, Yang C-s, Guo F-m, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi. 2012;51:599–603. [PubMed] [Google Scholar]
- 35.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 36.Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 37.Meduri GU, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. 1995;108:1315–1325. doi: 10.1378/chest.108.5.1315. [DOI] [PubMed] [Google Scholar]
- 38.Rocco PRM, Souza AB, Faffe DS, Pássaro CP, Santos FB, Negri EM, et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med. 2003;168:677–684. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- 39.Wang XQ, Zhou X, Zhou Y, Rong L, Gao L, Xu W. Low-dose dexamethasone alleviates lipopolysaccharide-induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology. 2008;13:772–780. doi: 10.1111/j.1440-1843.2008.01344.x. [DOI] [PubMed] [Google Scholar]
- 40.Arulkumaran N, Snow TAC, Longobardo A, Brealey D, Singer M. Steroids in ARDS: more light is being shed. Intensive Care Med. 2020;46:2108–2110. doi: 10.1007/s00134-020-06230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 42.Laffey JG, Kavanagh BP. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343:812. doi: 10.1056/NEJM200009143431113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Search strategy terms.
Additional file 2. Test for publication bias for hospital mortality. RR relative risk.
Additional file 3. Trial sequential analysis of eight trials for new infection. The required information size for detecting an intervention effect was 2131 patients. The relative risk was 0.84, and the 95% confidence interval was corrected to 0.66–1.08, from 0.70 to 1.01. TSA showed that the cumulative Z-curve did not cross both the conventional boundary and the trial sequential monitoring.
Additional file 4. Trial sequential analysis of hyperglycemia trials for hyperglycemia. The required information size for detecting an intervention effect was 746 patients. The relative risk was 1.11, and the 95% confidence interval was corrected to 0.98–1.25, from 0.99 to1.23. TSA showed that the cumulative Z-curve crossed the futility boundary.
Data Availability Statement
Not applicable.