Abstract
Background
Therapeutic plasma exchange (TPE) has been used increasingly to treat immunological diseases in dogs, although data concerning its efficacy are lacking.
Hypothesis/Objectives
To describe the clinic and the outcome of dogs with immune‐mediated hematological disorders (IMHD) treated with membrane filtration TPE.
Animals
One hundred forty‐six dogs diagnosed with IMHD, including 17 dogs treated with and 129 control dogs treated without TPE.
Methods
Retrospective study of cases selected with a search of all medical records for dogs diagnosed with IMHD (2010‐2019). Primary outcomes included the assessment of safety and efficacy of adjunctive TPE compared to medical treatment alone.
Results
The TPE group included 7 dogs with immune‐mediated hemolytic anemia (IMHA), 5 dogs with immune‐mediated thrombocytopenia (IMT), and 5 dogs with combined IMHA‐IMT; the control group included 63 dogs with IMHA, 47 dogs with IMT, and 19 dogs with IMHA‐IMT. Dogs treated with TPE were more often refractory to initial immunosuppression (71%) than controls (43%; P = .04). Complications were observed in 15/43 TPE treatments (35%). The response rate of dogs treated with TPE was 83% in IMHA (controls, 65%; P = .5) and 80% in IMT (controls, 70%; P = .71). Overall, 12/17 dogs (71%) treated with TPE reached complete remission, 2/17 (12%) partial remission, and 3/17 (18%) died or were euthanized. Eighty‐two percent of the dogs treated with TPE survived to discharge (controls, 69%; P = .4).
Conclusions and Clinical Importance
Despite a bias toward dogs refractory to initial immunosuppression, dogs treated with adjunctive TPE had a similar outcome as dogs treated medically.
Keywords: extracorporeal blood purification, plasmapheresis, coagulation, immune‐mediated hemolytic anemia, immune‐mediated thrombocytopenia
Abbreviations
- APPLE
acute patient physiologic and laboratory evaluation
- BW
body weight
- FFP
fresh‐frozen plasma
- HAlb
human albumin
- HES
hydroxyethylstarch
- iCa
ionized calcium
- IMHA
immune‐mediated hemolytic anemia
- IMHD
immune‐mediated hematologic disorder
- IMT
immune‐mediated thrombocytopenia
- IQR
interquartile range
- ISD
immunosuppressive drugs
- mTPE
membrane‐based therapeutic plasma exchange
- niCa
nonionized calcium
- PVE
plasma volume exchange
- RCA
regional citrate anticoagulation
- TCa
total calcium
- TPE
therapeutic plasma exchange
1. INTRODUCTION
Described for the treatment of immunological diseases in dogs in 1983 1 and of immune‐mediated hemolytic anemia (IMHA) in 1985, 2 , 3 the therapeutic removal of plasma, plasmapheresis, has received little attention due mostly to the lack of platforms appropriate for the safe and efficient treatment of small animals. Blood purification techniques have gained new interest with the broader availability of modern multifunctional systems. 4 Large volumes of plasma can now be removed and replaced at least partially with fresh plasma, a procedure more specifically designated as therapeutic plasma exchange (TPE). Centrifugational TPE and membrane filtration TPE (mTPE) provided as continuous‐flow techniques have largely replaced the older intermittent manual centrifugation procedures. Treatment platforms available in extracorporeal blood purification centers have made this technique a clinical reality for the advanced management of small animals. However, most treatment protocols are extrapolated from humans 5 rather than from specific developments in small animals. Although recently described as safe in dogs, 4 these treatments still need to be supported by a stronger scientific evidence to guide clinical decisions, in particular concerning outcome and expectations.
The 2 main immune‐mediated hematological disorders (IMHD), IMHA and immune‐mediated thrombocytopenia (IMT), are among the most commonly diagnosed immunological diseases in small animals. Two recent ACVIM Consensus Statements on the diagnosis 6 and the treatment 7 of IMHA in dogs have clarified some of the diagnostic challenges and have recognized the lack of unanimity among clinicians as to the optimal treatment recommendations. Glucocorticoids are presented as first‐line immunosuppressive agents, followed by azathioprine, cyclosporine, mycophenolate, and leflunomide as second‐line immunosuppressants, and intravenous administration of immunoglobulins for salvage immunomodulation. Most of these treatment recommendations are based on low‐grade evidence. Therapeutic plasmapheresis is mentioned as a potential immunomodulatory treatment. However, further investigations are needed first to document its efficacy and to define its optimal use in IMHA. 7 At this stage, these recommendations can be extrapolated to the treatment of IMT as well, considering the limited literature available. 8
The potential benefits of TPE in IMHD include its ability to rapidly remove plasma anti‐erythrocyte or anti‐platelet autoantibodies, circulating immune complexes, and activated complement components without myelosuppression, and thus to accelerate initial stabilization of severely affected animals. 9 There is indirect modulation of the cellular immunity, including improved T‐suppressor cell function and shift of the T‐helper cell response from a Th2 to a Th1 dominant pattern. 9 , 10 , 11 , 12 There are further effects on the innate immune system, including the restoration of the altered transcriptional profile of monocytes in humans with the severe autoimmune antiphospholipid syndrome. 13 Additional immunomodulation can be expected from the direct removal of soluble inflammatory mediators and adhesion molecules. 14 The nonspecific removal of plasma proteins and their replacement using fresh plasma could also benefit the hemostatic system, replenishing plasma components depleted in dogs with secondary consumptive coagulopathy. 9 , 15
The aims of this retrospective study were therefore to evaluate the safety and the efficacy of mTPE as an adjunct to medical treatment in dogs with IMHD, compared to medical treatment alone.
2. MATERIALS AND METHODS
2.1. Study design, disease definition, and animals
This study was designed as a retrospective evaluation of data collected from the medical records of dogs treated for IMHD between January 2010 and February 2019. Where appropriate for a retrospective study and with the available data, the results are presented following the reporting guidelines recommended by the authors of the ACVIM Consensus Statement on the treatment of IMHA in dogs. 7 Medical and treatment records were analyzed and following data were extracted: signalment, disease, diagnostic basis, specifics of the immunomodulatory treatment, duration of hospitalization, treatment response, outcome, and treatment costs until discharge. Dogs were grouped according to the use of mTPE as part of their treatment protocol.
Immune‐mediated hemolytic anemia was defined according to the diagnostic guidelines established in the ACVIM Consensus Statement on the diagnosis of IMHA in dogs and cats (see Supplementary Information). 6 Immune‐mediated thrombocytopenia was defined based on the presence of relevant thrombocytopenia (platelet count <100 × 109/L) confirmed by the examination of a blood smear. Considering the cutoffs applied in various studies ranging anywhere from 20 × 109/L to 200 × 109/L, our cutoff was chosen arbitrarily to avoid cases with mild and less specific thrombocytopenia without restricting the population to severe cases only. 16 , 17 A coagulation panel including prothrombin time, activated thromboplastin time, and fibrinogen activity was required to rule out disseminated intravascular coagulation. A maximum of 20% deviation from the reference range for any single parameter of this panel was tolerated. The presence of other causes of platelet consumption, decreased production, or sequestration was ruled out by the attending clinicians and reviewed by the authors. Combined IMHA‐IMT was diagnosed in dogs fulfilling both sets of criteria.
The IMHD was considered idiopathic when no potential trigger of an immune disease could be identified. The disease was considered possibly secondary when toxic agents were ingested or drugs administered within 7 days of diagnosis, when the dog had been vaccinated within 30 days of diagnosis, or when inflammatory or infectious diseases were identified in these dogs, even if a causal relationship could not be established. The severity for IMHA and IMT was defined based on hematocrit and platelet count, respectively. Additional characteristics of disease severity included the need for transfusion of blood products (IMHA and IMT), the serum bilirubin concentration (IMHA), and, when available, the 5‐variable acute patient physiologic and laboratory evaluation (APPLEfast) score (IMHA and IMT). 18
For most of the study time, mTPE was only offered to dogs that were either considered refractory to medical immunosuppression or affected with severe uncontrolled disease. Refractory IMHD was defined as nonresponse to 7 days of immunosuppression with at least 2 immunosuppressive drugs (ISD) and severe disease, as a high need of blood products because of ongoing hemolysis (IMHA) or bleeding (IMT), rapidly exceeding the hospital resources and therefore the capacity to support the dog until a response could be expected.
2.2. Treatment and outcome
The intensity of immunosuppression was evaluated as the average number of ISD used daily during hospitalization, the peak number of ISD, and the number of ISD at the time of discharge or death. Details of treatments provided after discharge were not available for most of the control dogs whose follow‐up was performed by their primary care providers.
The mTPE treatment was performed as described, using a Prismaflex platform (Baxter Healthcare, Glattpark, Switzerland) (see Supplementary Information). 4 The treatment schedule consisted of 2 treatments on consecutive days followed by additional treatments every other day, as estimated necessary. Standard prescription of treatment intensity was 1.0 to 1.2 plasma volume exchanges (PVE) per treatment. The composition of the replacement fluid consisted of 10% to 30% human albumin (HAlb, Albumin CSL 20%, CSL Behring, Bern, Switzerland; diluted to 5% HAlb), 0% to 10% hydroxyethylstarch (HES, Voluven balanced 6%, 130/0.4, Fresenius Kabi, Oberdorf, Switzerland; diluted to 3% HES), 0% to 50% normal saline, and 35% to 65% fresh‐frozen plasma (FFP), administered sequentially; it was adjusted to the individual needs of the animals, taking into consideration the underlying disease and comorbid disorders, the cardiovascular condition, and the hemostatic status. Anticoagulation consisted of regional citrate anticoagulation (RCA) as described previously, 4 with or without low‐level systemic heparinization, depending on the perceived risk of hemorrhage or thrombosis.
The response to treatment was considered partial with non–transfusion‐related increases in hematocrit to 0.25 to 0.39 L/L and platelet counts to 50 to 149 × 109/L ranges arbitrarily used in our hospital as approximate guidelines to define the need for more intensive treatment, monitoring, and hospitalization. A complete response was defined as the normalization of hematocrit (≥0.40 L/L) or platelets (≥150 × 109/L). A lack of improvement or a failure to reach a hematocrit of 0.25 L/L or a platelet count of 50 × 109/L during the observation period (hospitalization until death or lost to follow‐up) was considered as no response to treatment, although some dogs might have responded later. As most control cases were referred and extensive post‐discharge follow‐up was not available for most of them, treatment response in the control group only included hospitalization time and post‐discharge survival time. Long‐term outcome evaluated survival at 30 and 90 days, as recommended by the ACVIM consensus panel. 7 An overall outcome evaluation for the dogs treated with TPE classified them as nonresponders, responders (partial or complete), or death directly related to IMHD. Dogs deceased from different causes were categorized based on their last known response. The total costs of treatment were recorded for all dogs surviving to discharge.
2.3. Statistical analysis
Considering the limited number of cases treated with mTPE and the retrospective nature of the study, statistical analyses were restricted to descriptive statistics and to comparisons of the main variables between the treatment groups. The clinical data were analyzed separately for dogs with IMHA and for dogs with IMT. Dogs with combined IMHA‐IMT were included in both groups (IMHA and IMT) for the evaluation of their respective specific variables defining disease severity and response to treatment (hematocrit for IMHA and platelet count for IMT). Although the small number of dogs with IMHA‐IMT did not justify a separate analysis, the main characteristics of the treatment response of this group are briefly described separately, as this form of disease is often considered more severe and more difficult to treat.
Because several sets of data were not normally distributed based on Shapiro‐Wilk or on Kolmogorov‐Smirnov testing, numerical data are all reported as median and interquartile range (IQR). Categorical data are presented as proportions (number, percentage). Signalment, disease severity, intensity of treatment, response to treatment, outcome, and treatment costs were compared between the 2 treatment groups (mTPE vs control) using a Mann‐Whitney U test for numerical data and a chi‐square test or a Fisher's exact test for proportions. Physiologic and laboratory variables were compared before and after mTPE treatment using a Wilcoxon signed‐rank test for paired data. Survival was compared between the groups using a Kaplan‐Meier curve and the log‐rank test. Cumulative survival was calculated at 30 and 90 days using the Kaplan‐Meier product limit method. Analyses were performed using a commercial statistical software (NCSS 9.0.15; NCSS, LLC, Kaysville, Utah). A P‐value <.05 was considered significant for all statistical comparisons.
3. RESULTS
3.1. Dogs and diseases
Between January 2010 and May 2019, 167 dogs were identified in the medical record system with a diagnosis of IMHD. Based on the established criteria, 21 dogs were excluded from the study: 3 for insufficient diagnostic workup, 2 for identification of an alternative diagnosis (acute babesiosis and acute leukemia), and 16 for not fulfilling the disease criteria. The remaining 146 dogs were included in the study, with 17 dogs in the mTPE group and 129 dogs in the control group.
Twenty‐five dogs were intact females (17%), 51 spayed females (35%), 34 intact males (23%), and 36 castrated males (25%). Median age was 7.2 years (IQR, 5.0‐9.9; range, 0.3‐16.2) and body weight (BW) 14.9 kg (IQR, 8.4‐27.5; range, 1.5‐60). There was no significant difference in sex (P = .1), neuter status (P = .55), age (P = .05), and BW (P = .6) between the study groups. Twenty dogs (14%) were mixed‐breed dogs and 126 (86%) purebred dogs from 57 different breeds. The main breeds included English Cocker Spaniel (n = 15), Jack Russel Terrier (n = 9), Chihuahua (n = 5), Shih Tzu (n = 5); Bernese Mountain Dog (n = 4), Border Collie (n = 4), Briard (n = 4), Giant Schnauzer (n = 4), and Miniature Poodle (n = 4).
Ninety‐four dogs were diagnosed with IMHA, including 24 dogs with combined IMHA‐IMT. The clinical data were considered diagnostic in 51 dogs (54%), supportive in 34 dogs (36%), and suspicious in 9 dogs (10%). Hematocrit at presentation was 0.13 L/L (IQR, 0.11‐0.17). The anemia was considered regenerative in 70 dogs (74%) and nonregenerative in 24 dogs (26%), with a reticulocyte count of 126 × 109/L (IQR, 58‐229). Additional clinical and laboratory data are presented in Table 1. Dogs diagnosed with IMHA showed a median of 2 signs of immune destruction (IQR, 1‐2) and 2 signs of hemolysis (IQR, 1‐3). A prominent spherocytosis was present in 59/94 dogs (63%), the saline agglutination test was positive in 65/94 dogs (69%) and a Coombs' test was positive in 33/60 dogs (55%); hyperbilirubinemia or bilirubinuria was present in 74/94 dogs (79%), hemoglobinemia in 39/94 dogs (41%), and hemoglobinuria in 39/94 dogs (41%).
TABLE 1.
Main disease characteristics of 146 dogs with immune‐mediated hematological disorders. Treatment included membrane‐based therapeutic plasma exchange (mTPE group) or was limited to immunosuppressive treatment (controls)
| Variable | Unit | mTPE group (n = 17) | Controls (n = 129) | P |
|---|---|---|---|---|
| IMHA (n = 70) | n = 7 | n = 63 | ||
| Hematocrit | L/L | 0.14 (0.13‐0.15) | 0.13 (0.10‐0.18) | .82 |
| Reticulocytes | 109/L | 107 (49‐185) | 137 (59‐238) | .42 |
| Platelets | 109/L | 267 (234‐334) | 208 (148‐284) | .16 |
| Bilirubin | μmol/L | 37.8 (4.1‐58.6) | 20.8 (9.6‐46.6) | .98 |
| APPLEfast score a | 24.0 (20.5‐25.0) | 25.5 (21.5‐29.0) | .24 | |
| Idiopathic IMHA | 5 (71%) | 50 (79%) | .64 | |
| Secondary IMHA | 2 (29%) | 13 (21%) | ||
| IMT (n = 52) | n = 5 | n = 47 | ||
| Hematocrit | L/L | 0.31 (0.26‐0.36) | 0.28 (0.20‐0.39) | .84 |
| Reticulocytes | 109/L | 50 (4‐53) | 86 (65‐183) | .04* |
| Platelets | 109/L | 4 (2‐4) | 6 (5‐18) | .06 |
| APPLEfast score b | 24.0 (21.5‐26.5) | 26.0 (20.0‐30.0) | .31 | |
| Idiopathic IMT | 2 (40%) | 29 (62%) | .38 | |
| Secondary IMT | 3 (60%) | 18 (38%) | ||
| IMHA‐IMT (n = 24) | n = 5 | n = 19 | ||
| Hematocrit | L/L | 0.13 (0.11‐0.14) | 0.13 (0.11‐0.20) | .48 |
| Reticulocytes | 109/L | 18 (2‐31) | 114 (99‐244) | .002* |
| Platelets | 109/L | 71 (35‐83) | 40 (21‐85) | .52 |
| Bilirubin | μmol/L | 30.9 (18.2‐49.9) | 17.1 (7.1‐24.7) | .2 |
| APPLEfast score c | 26.0 (25.3‐28.3) | 27.0 (25.3‐30.0) | .49 | |
| Idiopathic IMHA‐IMT | 3 (60%) | 14 (74%) | .61 | |
| Secondary IMHA‐IMT | 2 (40%) | 5 (26%) |
Note: Numerical data are presented as median (IQR) and proportions as numbers (%).
Abbreviations: APPLE, acute patient physiologic and laboratory evaluation; IMHA, immune‐mediated hemolytic anemia; IMHA‐IMT, immune‐mediated hemolytic anemia and thrombocytopenia; IMT, immune‐mediated thrombocytopenia.
IMHA: the APPLEfast score could be calculated for 5/7 mTPE dogs (71%) and 14/63 control dogs (22%).
IMT: the APPLEfast score could be calculated for 5/5 mTPE dogs (100%) and 11/47 control dogs (23%).
IMHA‐IMT: the APPLEfast score could be calculated for 4/5 mTPE dogs (80%) and 8/19 control dogs (42%).
Indicates a statistically significant difference
Seventy‐six dogs were diagnosed with IMT, including the 24 dogs with combined IMHA‐IMT. Median hematocrit at presentation was 0.22 L/L (IQR, 13‐33) and platelet count was 10 × 109/L (IQR, 5‐36). Additional clinical and laboratory data are presented in Table 1.
The mTPE group included 7 dogs (41%) with IMHA, 5 dogs (29%) with IMT, and 5 dogs (29%) with IMHA‐IMT. Based on the workup available, the IMHD was considered idiopathic in 10 dogs (59%) and possibly secondary in 7 dogs (41%). One dog received its annual SHLPPi vaccine 2 weeks before onset of clinical signs; 1 dog had been treated with ceftiofur and 1 dog with fluralaner 1 week preceding the first signs; 1 dog had multiple ticks removed 1 week before the first signs; 1 dog was diagnosed with dirofilariosis and 1 dog was seropositive for Anaplasma phagocytophilum; and 1 dog had a splenectomy for splenomegaly of unknown etiology 10 days before presentation.
The control group of dogs treated without mTPE included 63 dogs (49%) with IMHA, 47 dogs (36%) with IMT, and 19 dogs (15%) with IMHA‐IMT. In this group, the IMHD was considered idiopathic in 93 dogs (72%) and possibly secondary in 36 dogs (28%). The main diseases possibly associated with the IMHD included infectious diseases in 25 dogs (19%; seropositivity for A phagocytophilum [18], Ehrlichia canis [3], or Borrelia burgdorferi [3], leishmaniasis [2], metropathy [2], diskospondylitis [1], meningitis [1], undetermined urinary tract infection [1], and Staphylococcus pseudointermedius pyoderma [1]), drugs and toxins in 8 dogs (6%; phenylbutazone [2], robenacoxib [2], meloxicam [1], permethrin [1], amoxicillin‐clavulanate [1], trimethoprim‐sulfonamide [1], cefazolin [1], doxycycline [1], multiple drug combination for anesthesia and surgery [2], and nonidentified toxin [1]), vaccine in 4 dogs (3%), and neoplasia in 2 dogs (2%; pulmonary mass [1], splenic mass [1]).
3.2. Medical and mTPE treatment
Dogs from the mTPE group were hospitalized significantly longer (median, 12 days; IQR, 10‐14) than dogs from the control group (median, 7 days; IQR, 5‐9; P < .001). The use of ISD, antithrombotics, and blood products in the 2 study groups is summarized in Table 2. Median treatment costs for the hospitalization period were 5283 USD (IQR, 3825‐6338) for dogs treated with TPE, compared to 2152 USD (IQR, 1714‐2751) for control dogs (P < .001).
TABLE 2.
Use of immunosuppressive therapies, thromboprophylaxis, and blood products in 146 dogs with immune‐mediated hematological disorders
| mTPE group | Controls | P | |
|---|---|---|---|
| Immunosuppressive treatment | n = 17 | n = 129 | |
| Prednisolone/dexamethasone | 17 (100%) | 128 (99%) | n/a |
| Cyclosporine | 10 (59%) | 77 (60%) | .95 |
| Mycophenolate mofetil | 8 (47%) | 29 (23%) | .03* |
| IVIG | 2 (12%) | 27 (21%) | .53 |
| Leflunomide | 0 (0%) | 1 (1%) | n/a |
| Vincristine | 2 (12%) | 19 (15%) | 1.00 |
| Average number of ISD during hospitalization | 1.9 (1.8‐2.1) | 1.7 (1.0‐2.0) | .009* |
| Peak number of ISD | 3.0 (3.0‐3.0) | 2.0 (2.0‐2.0) | <.001* |
| Number of ISD at discharge or death | 2.0 (2.0‐2.0) | 2.0 (1.0‐2.0) | .06 |
| Thromboprophylaxis | |||
| Aspirin | 1 (6%) | 35 (27%) | .07 |
| Clopidogrel | 10 (59%) | 41 (32%) | .03* |
| Low‐molecular weight heparin | 1 (6%) | 2 (2%) | n/a |
| Rivaroxaban | 1 (6%) | 0 (0%) | n/a |
| Blood products | |||
| Packed red blood cells | 12 dogs (71%), mean 1.65 U/dog | 70 dogs (54%), mean 0.83 U/dog | .2 |
| Whole blood | 2 dogs (12%), mean 0.18 U/dog | 10 dogs (8%), mean 0.10 U/dog | .63 |
| Oxyglobin | 1 dog (6%), mean 0.06 U/dog | 1 dog (6%), mean 0.01 U/dog | n/a |
| Fresh‐frozen plasma | 17 dogs (100%), mean 4.29 U/dog | 1 dog (1%), mean 0.02 U/dog | n/a |
Notes: Numerical data are presented as median (IQR) and proportions as numbers (%). Following immunosuppressive drug (ISD) and antithrombotics doses were used: prednisolone, 2 mg/kg q12‐24h PO; dexamethasone, 0.3 mg/kg q24h IV; cyclosporine, 3‐5 mg/kg q12‐24h PO; mycophenolate mofetil, 8‐10 mg/kg q12h PO; IVIG, 0.3‐1 g/kg IV (single dose); leflunomide, 4 mg/kg q24h PO; vincristine, 0.02 mg/kg IV (single dose); aspirin, 2‐5 mg/kg q24h PO; clopidogrel, 1‐2 mg/kg q24h PO; low‐molecular weight heparin (dalteparin), 150 IU/kg q8h SC; rivaroxaban, 1 mg/kg q24h PO.
*Indicates a statistically significant difference
Forty‐three mTPE treatments were performed in the 17 dogs from the mTPE group, with a median of 3 mTPE treatments per dog (IQR, 2‐3), the first 1 initiated a median of 5 days (IQR, 3‐6) postpresentation. Vascular access was provided with right‐sided jugular central venous catheters of appropriate length and diameter for the size of the dogs (7 Fr, n = 3; 8 Fr, n = 7; 11.5 Fr, n = 2; 12 Fr, n = 4; or 13.5 Fr, n = 1). No clinically relevant hemorrhage was noted after catheter placement in any of the dogs, including those with profound thrombocytopenia.
Treatment prescription was designed to provide 1.0 PVE (IQR, 1.0‐1.2), corresponding to a median exchange of 1117 mL (IQR, 434‐2133) per treatment. Plasma replacement was planned to include FFP (43/43 treatments, 100%), HAlb (36/43 treatments, 84%), HES (11/43 treatments, 26%), and normal saline (33/43 treatments, 77%). The composition of the replacement fluid was designed to contain a median of 38% FFP (IQR, 30%‐55%), 19% HAlb (IQR, 10%‐25%), 0% HES (IQR, 0%‐3%), and 40% normal saline (IQR, 11%‐47%).
Anticoagulation of the circulating blood was performed using RCA, either alone in 6 treatments (14%) or associated with low‐dose systemic heparinization in 37 treatments (86%). Citrate administration (trisodium citrate 3%, 102 mmol/L; Dr Bichsel Pharmacy, Interlaken, Switzerland) was initiated at a rate of 3.8 mmol citrate per liter of blood (IQR, 3.5‐4.0). The citrate infusion rate was increased in 15 treatments (35%) and decreased in 37 treatments (86%), with a total of 100 adjustments (2.3 per treatment). Calcium restitution (calcium chloride 5%, 340 mmol/L; Dr Bichsel Pharmacy) was provided at an initial calcium:citrate molar ratio of 0.33. The calcium infusion rate was increased in 23 treatments (53%) and decreased in 33 treatments (77%), with a total of 84 adjustments (1.95 per treatment).
The data and characteristics of the effectively provided mTPE treatments are summarized in Table 3. The dogs were premedicated with diphenhydramine (1 mg/kg IV) for 20 treatments (47%), and combined diphenhydramine‐dexamethasone (0.2 mg/kg IV) for 1 treatment (2%). Twenty‐two treatments were performed without premedication, before the administration of diphenhydramine became standard protocol at the authors' institution.
TABLE 3.
Data and characteristics of the 43 mTPE treatments provided to 17 dogs with immune‐mediated hematological disorders
| Variable | Unit | Median (IQR) N (%) |
|---|---|---|
| Total blood volume processed | mL/kg | 440 (404‐488) |
| Average blood flow rate | mL/min | 56 (33‐68) |
| Average filtration fraction | % | 15 (13‐18) |
| Replacement volume | mL | 1166 (442‐2135) |
| Plasma volume exchanged | PVE | 1.03 (1.01‐1.18) |
| Treatment duration | min | 150 (111‐198) |
| Filtration intensity | mL/min | 7.5 (4.1‐12.1) |
| Anticoagulation |
RCA: 6 (14%) RCA‐SH: 37 (86%) |
|
| Citrate infusion rate: start; average | mmol/L blood | 3.8 (3.5‐4.0); 3.9 (3.6‐4.0) |
| Heparin dose | U/kg/tx | 56 (40‐73) |
| Composition of the replacement fluid | vol% | |
| HAlb (36/43 tx, 84%) | 18% (9‐25) | |
| HES (10/43 tx, 23%) | 0% (0‐0) | |
| NaCl (29/43 tx, 67%) | 41% (21‐47) | |
| FFP (43/43 tx, 100%) | — |
Notes: Numerical data are presented as median (interquartile range [IQR]) and proportions as number (%). The fluids used as replacement of plasma included human albumin (HAlb, 5%); hydroxyethylstarch (HES, 3%); normal saline (NaCl, 0.9%); and fresh‐frozen plasma (FFP).
Abbreviations: PVE, plasma volume exchange; RCA, regional citrate anticoagulation; RCA‐SH, regional citrate anticoagulation with systemic heparinization; tx, treatment.
The administration of the individual replacement fluids followed the same order (when used), HAlb – HES – normal saline – FFP, except for 6 treatments where normal saline was administered before HES and 1 treatment where additional normal saline was used last. Physiologic and laboratory changes associated with the mTPE treatments are summarized in Table 4.
TABLE 4.
Physiologic and laboratory variables and their changes associated with 43 mTPE treatments in 17 dogs with immune‐mediated hematological disorders
| Variable | n (before/after) | Before mTPE | After mTPE | P |
|---|---|---|---|---|
| HR (bpm) | 42/42 | 82 (71‐110) | 101 (73‐123) | .12 |
| Temperature (°C) | 41/40 | 38.2 (37.9‐38.6) | 38.2 (37.8‐38.4) | .38 |
| BW (kg) | 37/37 | 16.6 (5.4‐31.3) | 16.8 (5.6‐31.5) | <.001* |
| ΔBW (kg) | 37 | 0.200 (0.060‐0.600) | ||
| SBP (mmHg) | 43/42 | 140 (128‐150) | 140 (130‐157) | .29 |
| Hematocrit (L/L) | 43/43 | 0.19 (0.16‐0.27) | 0.22 (0.19‐0.25) | .01* |
| Albumin (g/dL) | 43/43 | 2.44 (2.22‐2.85) | 2.29 (2.09‐2.45) | <.001* |
| Urea (mg/dL) | 43/43 | 17.9 (13.7‐23.6) | 16.0 (13.5‐21.0) | <.001* |
| Creatinine (mg/dL) | 43/43 | 0.58 (0.42‐0.71) | 0.58 (0.44‐0.71) | .27 |
| Bilirubin (mg/dL) | 18/9 | 1.7 (0.6‐17.4) | 11.1 (1.4‐34.3) | .09 |
| ΔBilirubin (mg/dL) | 9 |
−4.2 (−8.8 to 0) reduction: 15% (0 to 42) |
||
| CRP (mg/dL) | 22/12 | 24.6 (8.7‐44.8) | 7.4 (1.1‐12.3) | .01* |
| pH | 40/39 | 7.42 (7.40‐7.45) | 7.39 (7.37‐7.42) | .008* |
| Bicarbonate (mmol/L) | 40/39 | 21.7 (20.0‐23.1) | 21.7 (20.3‐23.3) | .55 |
Note: Data are presented as median (IQR).
Abbreviations: BW, body weight; CRP, C‐reactive protein; HR, heart rate; SBP, systolic blood pressure.
*Indicates a statistically significant difference
Complications associated with the mTPE treatment were observed in 15 treatments (35%) and included filter clotting (n = 4), hypersensitivity reactions (n = 3), vomiting related timely to hypocalcemia (n = 6), and technical difficulties (self‐test failures associated with low blood flow rates, n = 2). No complication was considered life‐threatening, 1 was potentially severe (beginning laryngeal edema responding quickly to antihistamines and steroid administration), 4 were moderate, necessitating treatment interruption and restart (filter clotting), and all others were mild.
Evidence of citrate accumulation was observed in 34/43 treatments (79%) and it was considered nonrelevant in 5 (12%), mild in 19 (44%), and severe in 10 treatments (23%). The ionized calcium (iCa) decreased >0.2 mmol/L in 16/37 treatments (43%); the nonionized calcium (niCa) increased >1.0 mmol/L in 25/35 treatments (71%); the total calcium (TCa) increased >1.0 mmol/L in 22/41 treatments (54%); and the TCa:iCa ratio increased >1 in 25/35 treatments (71%). Further anticoagulation‐related complications were observed in 4 treatments (9%), in which the mTPE filter clotted and the circuit had to be replaced. No hemorrhagic complication was noticed in any phase of the treatment, including the central venous catheter placement, the extracorporeal circulation, and the intertreatment periods.
3.3. Response to treatment and outcome
The response to treatment and the outcome of the 17 dogs treated with mTPE are summarized in Figures 1 and 2. The IMHD was considered refractory at day 7 of immunosuppression in 12/17 dogs treated with mTPE (71%), compared to 56/129 control dogs (43%; P = .04). At that time, mTPE treatment had not been started yet in 4/17 dogs, and in the other 13 dogs, mTPE treatment had been initiated 3 days (IQR, 2‐5) before. Overall, 12/17 dogs (71%) reached a complete remission, 2/17 (12%) a partial remission, and 3/17 (18%) died or were euthanized because of direct complications of their immune disease or a lack of response to treatment. One dog suffered a cardiac arrest after progressive deterioration of the general condition and multiorgan failure, including liver failure, renal failure, and severe pancreatitis. One dog was euthanized because of a worsening clinical condition with severe pancreatitis, sepsis, and acute respiratory distress syndrome and the other dog was euthanized because of severe hemorrhagic gastrointestinal complications associated with a poor response to 19 days of immunosuppression.
FIGURE 1.
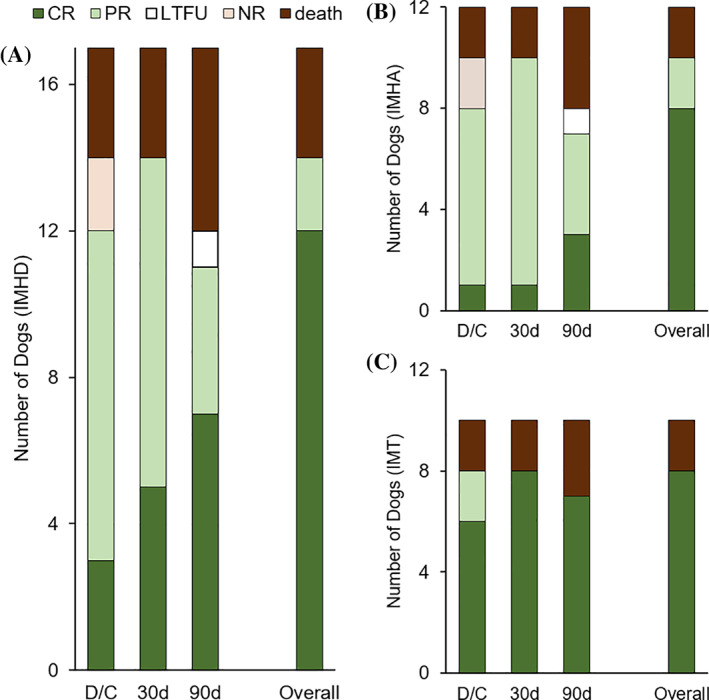
Response to treatment with membrane‐based therapeutic plasma exchange (mTPE) in 17 dogs immune‐mediated hematologic disorder (IMHD) (A), including 12 dogs with immune‐mediated hemolytic anemia (IMHA) (B), and 10 dogs with immune‐mediated thrombocytopenia (IMT) (C). The status is shown for the time of discharge (D/C), and at 30 and 90 days, as complete response (CR, dark green), partial response (PR, clear green), no response (NR, clear pink), death (dark brown), and lost to follow‐up (LTFU, white). The overall status represents the last known response status of the dogs, categorized as complete or partial response, no response, and death directly related to the immune disease or its treatment. Dogs that died from other reasons or that were lost to follow‐up are categorized based on their last known response status
FIGURE 2.
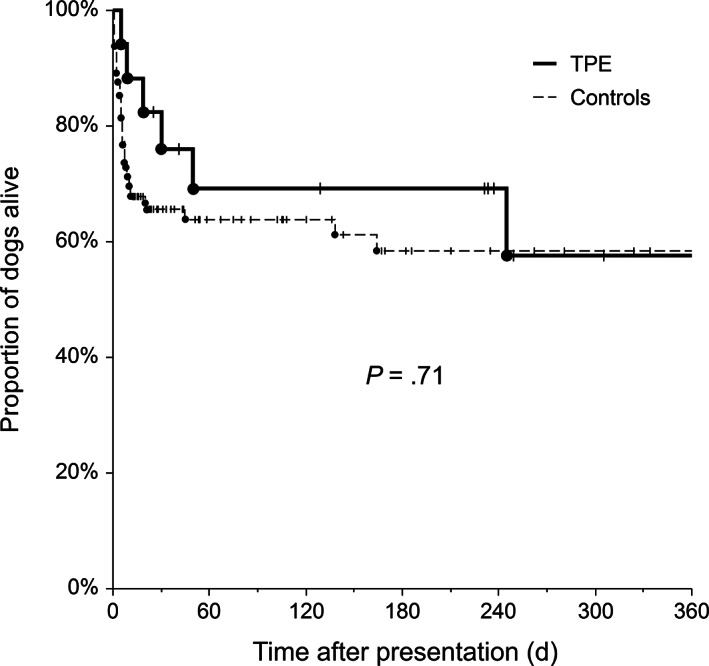
Kaplan‐Meier survival curve for dogs with immune‐mediated hematologic disorder (IMHD), including all causes of death. Cases were categorized based on the 2 treatment groups as membrane‐based therapeutic plasma exchange (mTPE) (thick solid line) or controls (thin dashed line) and they were censored at their last known follow‐up (vertical lines)
For the dogs treated with TPE and discharged from hospital, clinical and laboratory follow‐up was available for a period of 173 months (median, 7.7 months/dog; IQR, 0.8‐8.3). Two dogs showed a recurrence of their IMHD (7 months and 4 years postdischarge, respectively). In 7 dogs (50%), no complication of the disease or of the treatment was noticed; 3 dogs showed mild to moderate complications and 4 dogs severe complications leading to euthanasia. Main complications and comorbidities included gastrointestinal disturbances (7 dogs), pancreatitis (3 dogs), septic peritonitis (1 dog), sepsis (1 dog), chronic hepatopathy with gallbladder rupture (1 dog), pyoderma (2 dogs), and low‐grade protein‐losing nephropathy progressing to a stage 2 chronic kidney disease (1 dog).
The resulting survival rate was 82% (14/17) at the time of discharge for dogs treated with TPE, compared to 69% (89/129) in control dogs (P = .39). Cumulative survival at day 30 was 76% (95% confidence interval, 56‐97%) for dogs treated with TPE and 66% (57%‐73%) for control dogs. At day 90, cumulative survivals were 69% (46%‐92%) and 64% (55%‐73%), respectively. Survival was not significantly different between treatment groups (P = .71; Figure 2).
IMHA group—10/12 dogs (83%) responded at least partially to the treatment including mTPE, compared to 53/82 dogs (64%) in the control group treated only medically (P = .2; Table 5). The median time to partial response after initiation of mTPE treatment was 5 days (IQR, 1.8‐11.3; Figure 3). The mTPE group counted 8/12 dogs (67%) classified as nonresponders on day7 of treatment, of which 7 (88%) responded later. The control group counted 34/82 (41%) nonresponders on day 7, with 20 dogs (59%) responding later. The survival rate was 83% (10/12 dogs) in the mTPE group and 70% (57/82) in the control group (P = .5).
TABLE 5.
Treatment response and outcome of 146 dogs with immune‐mediated hematologic disorder (IMHD), in which the treatment included mTPE (n = 17) or was limited to immunosuppressive treatment (controls, n = 129)
| IMHA (n = 94) | mTPE (n = 12) | Controls (n = 82) | P |
|---|---|---|---|
| No response | 2 (17%) | 29 (35%) | .33 (responders vs nonresponders) |
| Partial response | 5 (42%) | 42 (51%) | |
| Complete response | 5 (42%) | 11 (13%) | |
| Survivors | 10 (83%) | 57 (70%) | .5 (survivors vs nonsurvivors) |
| Nonsurvivors [death/euthanasia] | 2 (17%) [1/1] | 25 (30%) [3/22] |
| IMT (n = 76) | mTPE (n = 10) | Controls (n = 66) | |
|---|---|---|---|
| No response | 2 (20%) | 20 (30%) | .71 (responders vs nonresponders) |
| Partial response | 0 (0%) | 7 (11%) | |
| Complete response | 8 (80%) | 39 (59%) | |
| Survivors | 8 (80%) | 41 (62%) | .48 (survivors vs nonsurvivors) |
| Nonsurvivors [death/euthanasia] | 2 (20%) [1/1] | 25 (38%) [6/19] |
FIGURE 3.
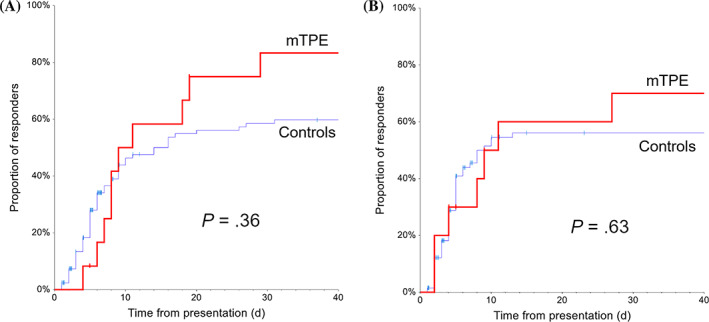
Cumulative incidence of response to treatment in dogs with immune‐mediated hemolytic anemia (IMHA) (A) or immune‐mediated thrombocytopenia (IMT) (B). The population is categorized based on the 2 treatment groups, as membrane‐based therapeutic plasma exchange (mTPE) (red) or controls (blue). Cases were censored at their last known follow‐up (vertical lines)
IMT group—8/10 dogs (80%) responded at least partially to the treatment including mTPE, compared to 46/66 dogs (70%) in the control group treated only medically (P = .5; Table 5). The median time to partial response after initiation of mTPE treatment was 3 days (IQR, 0‐3; Figure 3). The survival rate was 80% (8/10 dogs) in the mTPE group and 62% (41/66) in the control group (P = .48).
3.4. Dogs with combined IMHA‐IMT
Five dogs with combined IMHA‐IMT were treated with mTPE and 19 dogs were included in the controls. Laboratory data are presented in Table 1.
Of the dogs treated with mTPE, 4/5 (80%) responded to treatment (IMHA: 2 complete and 2 partial responses; IMT: 4 complete responses), reaching a hematocrit of 0.25 L/L within 13.5 days (range, 8‐29) and a platelet count of 50 × 109/L within 2 days (range, 0‐9). The complete responses of the IMHA (2 dogs) were reached on day 48 and 55 and of IMT (4 dogs) on day 3 to 9. Four dogs (80%) survived and 1 dog (20%) died.
In the control dogs, the anemia responded to treatment in 11/19 dogs (58%; 4 complete responses and 7 partial responses) and the thrombocytopenia in 15/19 dogs (79%; 11 complete and 4 partial responses). The hematocrit of 0.25 L/L was reached within 5 days (IQR, 5‐7) and the platelet count of 50 × 109/L within 1 day (IQR, 0‐4). Nine dogs survived (47%), 8 dogs were euthanized (42%), and 2 dogs died (11%).
4. DISCUSSION
Adjunctive TPE appeared to be a safe procedure in dogs with IMHD. Dogs treated with TPC had similar response rates and outcomes compared to dogs treated medically.
Based on the current study with 43 treatments and on a previously published wider retrospective study including 64 treatments, 4 this form of treatment appears safe, when administered according to current empirical recommendations. The main complication observed, laryngeal edema, was associated timely with the administration of FFP as replacement. It was suspected to be a reaction to plasma and it responded rapidly to the administration of antihistamines and steroids. However, despite a small number of serious complications, the potential for profound cardiovascular, hemostatic, and metabolic alterations resulting from this extracorporeal blood purification technique in severely sick dogs should encourage the development of evidence‐based species‐specific treatment protocols and guidelines. Historically, such guidelines have led the evolution from an empirical technique to a solid evidence‐based discipline in human medicine. 5 Ideally, the proof of efficacy of TPE should be based on prospective and randomized studies for specific diseases, but considering the treatment costs and the resources involved, this will be difficult to reach in the near future. Retrospective analyses of treatment data provided in single or multiple institutions are therefore essential to provide more objective treatment recommendations to the clinicians confronted to diseases with a potential TPE indication.
In the present study, we decided to include both IMHA and IMT, 2 different diseases, because they share a similar immunological basis and they are often treated very similarly concerning the mode and the intensity of immunosuppression. Additionally, both diseases occur often concomitantly in affected dogs, presenting sometimes a diagnostic challenge. In some dogs, the differentiation between a hemorrhagic cause of anemia in IMT, a secondary thrombocytopenia in IMHA, or a true IMHD affecting both the erythrocytic and thrombocytic lines (Evans syndrome) is not easily possible. Considering the information available retrospectively, this differentiation was not attempted and dogs were just classified according to their fulfilment of the separate criteria defined for each disease. Furthermore, the data were analyzed separately for IMHA and IMT, the dogs diagnosed with combined IMHA‐IMT being included in both groups with their respective treatment response criteria. Dogs with combined IMHA‐IMT are often considered clinically affected with a more severe disease and this sometimes justifies a more intensive therapeutic approach. 19 , 20 The higher case fatality rate observed in the control arm of the study possibly reflects this fact. Considering the small number of these dogs, only the main data concerning treatment response and outcome were presented separately, without statistical analysis.
The efficacy of TPE when added to medical immunosuppressive treatment in dogs with IMHD was not established with this small‐scale retrospective study. The case selection for mTPE treatment is certainly highly biased with variables reflecting disease severity and financial commitment. For most of the study time, until shown safe for use in dogs with severe IMHD, mTPE was only offered to dogs that were either considered refractory to medical immunosuppression or affected with severe uncontrolled disease. The higher rate of nonresponders at day 7 in the mTPE group (71% of the dogs treated with TPE compared to 43% of the control dogs, P = .04) possibly reflects this preferential selection of dogs with refractory disease in the treatment group. However, no difference in the variables used to assess disease severity was observed between the treatment groups at initial presentation. The data suggest a decrease of the nonresponse rate and of the case fatality rate with a magnitude of approximately 50%, although these rates were not statistically different between the treatment groups. This should not be overinterpreted but provides data with which to design adequately powered future prospective studies. Such studies could possibly focus on intermediate endpoints other than survival, including time to response, need for ISD, or transfusion requirements. To facilitate their use in this regard, the data are presented according to the guidelines suggested in the ACVIM Consensus statement on the treatment of IMHA in dogs. 7 The demonstration of a safe treatment, at least noninferior to medical treatment, should also facilitate a wider recruitment not restricted to the severe and refractory cases. In the meantime, the demonstrated safety of the procedure and the outcome results from the present study place TPE at a comparable level of evidence as other widely used immunosuppressive and immunomodulatory therapies such as cyclosporine, mycophenolate mofetil, leflunomide, or immunoglobulins. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
The 2 main modalities of TPE, centrifugational TPE and mTPE, differ mostly in the technique used. In recent years, mTPE has gained a stronger interest with the development and the wider availability of multifunctional platforms providing various modalities of blood purification including TPE. The centrifugation technique allows a higher plasma extraction fraction than the filtration technique and thus it necessitates a lower access blood flow rate, shorter procedure times, and a lower risk of citrate accumulation when used for regional anticoagulation. As demonstrated previously however, RCA can be used in dogs as the sole mode of anticoagulation in mTPE when necessary, with only minimal to moderate citrate accumulation that has no negative clinical consequences for the animal. 4
The main limitations of this study are related to the small number of mTPE cases and the retrospective nature of the study, including a strong selection bias guided mostly by individual preferences and a perceived belief of efficacy for specific forms and severity of the disease. The development of disease‐specific illness severity scores would be useful to identify earlier animals with delayed or more severe course of disease and therefore better allocate treatment resources. The costs of the procedure and of the replacement fluids were likely to play a significant role in the treatment decision process, especially in large breed dogs. The absence of a significant difference in BW between the treatment groups however does not rule out a body size bias in the results. Another serious limitation is the lack of data and of follow‐up after discharge for the control dogs, especially for diseases known for long treatment durations and for high rates of recurrences and drug adverse effects.
In summary and conclusion, the data presented in this retrospective study indicate that despite a bias of selection toward dogs refractory to initial immunosuppressive treatment, the response to additional TPE and the outcome of the dogs treated with this procedure was at least as good as in dogs treated with medical immunosuppression alone.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information.
Supplementary Figure S1 Cumulative incidence of response to mTPE therapy in dogs with IMHD. The time is counted from the initial presentation (A) or from the day the first mTPE treatment was performed (B). The population is categorized based on the disease, as IMHA (blue) or IMT (green). Cases were censored at their last known follow‐up (vertical lines).
ACKNOWLEDGMENT
Funding provided by Robmar Foundation for Research and Promotion of the Human‐Animal Bond.
Francey T, Etter M, Schweighauser A. Evaluation of membrane‐based therapeutic plasma exchange as adjunctive treatment for immune‐mediated hematologic disorders in dogs. J Vet Intern Med. 2021;35:925–935. 10.1111/jvim.16049
REFERENCES
- 1. Matus RE, Scott RC, Saal S, et al. Plasmapheresis‐immunoadsorption for treatment of systemic lupus erythematosus in a dog. J Am Vet Med Assoc 1983;182(5):499–502. [PubMed] [Google Scholar]
- 2. Matus RE, Gordon BR, Leifer CE, et al. Plasmapheresis in five dogs with systemic immune‐mediated disease. J Am Vet Med Assoc 1985;187(6):595–9. [PubMed] [Google Scholar]
- 3. Matus RE, Schrader LA, Leifer CE, et al. Plasmapheresis as adjuvant therapy for autoimmune hemolytic anemia in two dogs. J Am Vet Med Assoc 1985;186(7):691–3. [PubMed] [Google Scholar]
- 4. Francey T, Schweighauser A. Membrane‐based therapeutic plasma exchange in dogs: prescription, anticoagulation, and metabolic response. J Vet Intern Med 2019;33(4):1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice‐evidence‐based approach from the Writing Committee of the American Society for Apheresis: the Seventh Special Issue. J Clin Apher 2016;31(3):149–338. [DOI] [PubMed] [Google Scholar]
- 6. Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immune‐mediated hemolytic anemia in dogs and cats. J Vet Intern Med 2019;33(2):313–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swann JW, Garden OA, Fellman CL, et al. ACVIM consensus statement on the treatment of immune‐mediated hemolytic anemia in dogs. J Vet Intern Med 2019;33(3):1141–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kopecny L, Palm CA, Naylor S, et al. Application of therapeutic plasma exchange in dogs with immune‐mediated thrombocytopenia. J Vet Intern Med 2020;34(4):1576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanatta E, Cozzi M, Marson P, et al. The role of plasma exchange in the management of autoimmune disorders. Br J Haematol 2019;186(2):207–219. [DOI] [PubMed] [Google Scholar]
- 10. De Luca G, Lugaresi A, Iarlori C, et al. Prednisone and plasma exchange improve suppressor cell function in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol 1999;95(1–2):190–4. [DOI] [PubMed] [Google Scholar]
- 11. Goto H, Matsuo H, Nakane S, et al. Plasmapheresis affects T helper type‐1/T helper type‐2 balance of circulating peripheral lymphocytes. Ther Apher 2001;5(6):494–6. [DOI] [PubMed] [Google Scholar]
- 12. Kambara C, Matsuo H, Fukudome T, et al. Miller Fisher syndrome and plasmapheresis. Ther Apher 2002;6(6):450–3. [DOI] [PubMed] [Google Scholar]
- 13. Martirosyan A, Petrek M, Kishore A, et al. Immunomodulatory effects of therapeutic plasma exchange on monocytes in antiphospholipid syndrome. Exp Ther Med 2016;12(2):1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsui M, Okuma Y, Yamanaka J, et al. Kawasaki disease refractory to standard treatments that responds to a combination of pulsed methylprednisolone and plasma exchange: cytokine profiling and literature review. Cytokine 2015;74(2):339–42. [DOI] [PubMed] [Google Scholar]
- 15. McLeod BC. Plasma and plasma derivatives in therapeutic plasmapheresis. Transfusion 2012;52(Suppl 1):38S‐44S. [DOI] [PubMed] [Google Scholar]
- 16. Balog K, Huang AA, Sum SO, et al. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med 2013;27(3):536–41. [DOI] [PubMed] [Google Scholar]
- 17. Jans HE, Armstrong PJ, Price GS. Therapy of immune mediated thrombocytopenia. A retrospective study of 15 dogs. J Vet Intern Med 1990;4(1):4–7. [DOI] [PubMed] [Google Scholar]
- 18. Hayes G, Mathews K, Doig G, et al. The acute patient physiologic and laboratory evaluation (APPLE) score: a severity of illness stratification system for hospitalized dogs. J Vet Intern Med 2010;24(5):1034–47. [DOI] [PubMed] [Google Scholar]
- 19. Ishihara M, Fujino Y, Setoguchi A, et al. Evaluation of prognostic factors and establishment of a prognostic scoring system for canine primary immune‐mediated hemolytic anemia. J Vet Med Sci 2010;72(4):465–70. [DOI] [PubMed] [Google Scholar]
- 20. Goggs R, Boag AK, Chan DL. Concurrent immune‐mediated haemolytic anaemia and severe thrombocytopenia in 21 dogs. Vet Rec 2008;163(11):323–7. [DOI] [PubMed] [Google Scholar]
- 21. Grundy SA, Barton C. Influence of drug treatment on survival of dogs with immune‐mediated hemolytic anemia: 88 cases (1989‐1999). J Am Vet Med Assoc 2001;218(4):543–6. [DOI] [PubMed] [Google Scholar]
- 22. Swann JW, Skelly BJ. Evaluation of immunosuppressive regimens for immune‐mediated haemolytic anaemia: a retrospective study of 42 dogs. J Small Anim Pract 2011;52(7):353–8. [DOI] [PubMed] [Google Scholar]
- 23. Husbands B, Polzin D, Armstrong PJ, et al. Prednisone and cyclosporine vs prednisone alone for treatment of canine immune‐mediated hemolytic anemia (IMHA). J Vet Intern Med 2004;18(3):389. [Google Scholar]
- 24. Wang A, Smith JR, Creevy KE. Treatment of canine idiopathic immune‐mediated haemolytic anaemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007 to 2011). J Small Anim Pract 2013;54(8):399–404. [DOI] [PubMed] [Google Scholar]
- 25. Sato M, Veir JK, Legare M, et al. A retrospective study on the safety and efficacy of leflunomide in dogs. J Vet Intern Med 2017;31(5):1502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerber B, Steger A, Hässig M, et al. Anwendung von humanem Immunglobulin bei Hunden mit primärer immunbedingter hämolytischer Anämie. Schweiz Arch Tierheilkd 2002;144(4):180–5. [DOI] [PubMed] [Google Scholar]
- 27. Whelan MF, O'Toole TE, Chan DL, et al. Use of human immunoglobulin in addition to glucocorticoids for the initial treatment of dogs with immune‐mediated hemolytic anemia: original Study. J Vet Emerg Crit Care 2009;19(2):158–64. [DOI] [PubMed] [Google Scholar]
- 28. Spurlock NK, Prittie JE. A review of current indications, adverse effects, and administration recommendations for intravenous immunoglobulin. J Vet Emerg Crit Care 2011;21: 471–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Supplementary Figure S1 Cumulative incidence of response to mTPE therapy in dogs with IMHD. The time is counted from the initial presentation (A) or from the day the first mTPE treatment was performed (B). The population is categorized based on the disease, as IMHA (blue) or IMT (green). Cases were censored at their last known follow‐up (vertical lines).


