Abstract
Background
Few studies have investigated management and outcome in dogs with acute hemorrhagic diarrhea syndrome (AHDS), and there is a paucity of data on dogs with concurrent signs of sepsis.
Objectives
To report outcome in dogs with suspected AHDS according to disease severity and antimicrobial treatment, and to evaluate effect of fluid resuscitation on clinical criteria.
Animals
Two hundred thirty‐seven dogs hospitalized with suspected AHDS.
Methods
Retrospective study based on medical records. Disease severity was evaluated using AHDS index, systemic inflammatory response syndrome (SIRS) criteria, and serum C‐reactive protein (CRP) according to 3 treatment groups: No, 1, or 2 antimicrobials.
Results
Sixty‐two percent received no antimicrobials, 31% received 1 antimicrobial, predominantly aminopenicillins, and 7% received 2 antimicrobials. At admission, median AHDS index was 13 (interquartile range, 11‐15), which decreased significantly after the first day's hospitalization (P < .001) for all groups. Compared with no antimicrobials (7%), more dogs had ≥2 SIRS criteria in the antimicrobial groups (15% and 36%, respectively). C‐reactive protein (CRP) correlated positively with AHDS index at hospitalization (P < .001). Across treatment groups, rehydration markedly reduced number of clinical SIRS criteria. Survival to discharge was 96%, lower for dogs receiving 2 antimicrobials (77%, P < .05).
Conclusions and Clinical Importance
The majority of dogs hospitalized with suspected AHDS improve rapidly with symptomatic treatment only, despite signs of systemic disease on initial presentation. The often‐used SIRS criteria might be a poor proxy for identifying dogs with AHDS in need of antimicrobial treatment, in particular when hypovolemic. The role of CRP in clinical decision‐making or prognostication warrants further investigation.
Keywords: AHDS, antibiotics, C‐reactive protein, canine, hemorrhagic gastroenteritis
Abbreviations
- AHDS
acute hemorrhagic diarrhea syndrome
- CRP
C‐reactive protein
- IQR
interquartile range
- SIRS
systemic inflammatory response syndrome
1. INTRODUCTION
Acute hemorrhagic diarrhea syndrome (AHDS) is a frequent occurrence in dogs. 1 , 2 The syndrome is characterized by acute onset of hemorrhagic diarrhea due to necrosis of the intestinal mucosal lining, and is often preceded by vomiting with concurrent anorexia. 3 Clostridium perfringens NetE and NetF toxins might play a role in the pathogenesis of AHDS, 4 but to this date the exact underlying etiology remains unknown. Previously, antimicrobials were routinely recommended for all dogs with hemorrhagic diarrhea due to a potentially increased risk of bacterial translocation and subsequent sepsis, 5 and to this date, antimicrobials are frequently administered to dogs presenting with acute diarrhea, with amoxicillin‐clavulanic acid and metronidazole as 2 of the most commonly used active compounds. 6 , 7 , 8 , 9 However, despite the potential bacterial etiology of AHDS, recent findings suggest that antimicrobial treatment is not indicated in all dogs with AHDS. 10 , 11 In a randomized clinical trial investigating the effect of amoxicillin‐clavulanic acid in dogs with AHDS and no concurrent signs of sepsis, no beneficial effect of antimicrobial treatment was found on survival to discharge, days hospitalized, or clinical improvement. 12
Avoidance of unwarranted antimicrobial use reduces the risk of adverse effects, such as prolonged intestinal dysbiosis and selection for antimicrobial resistance. 13 , 14 Consequently, antimicrobial treatment for dogs with AHDS is at present only recommended if the dog is bacteremic, shows signs of sepsis, or there is an inadequate response to symptomatic treatment. 11 , 15 , 16 Signs indicative of sepsis include tachycardia, tachypnea, hypo‐ or hyperthermia, leukocytosis or leukopenia and hypoglycemia, and presence of 2 or more of those abnormalities are regarded as an indication for antimicrobial treatment. 17 However, these abnormalities are not specific for sepsis and some of the clinical signs might be more related to hypovolemia secondary to severe fluid loss and dehydration than actual sepsis. 18
Only few studies have investigated management and outcome in dogs with AHDS, and there is a paucity of data on dogs with AHDS and concurrent signs of sepsis.
The aim of our study was to report disease severity, measured by AHDS index, clinical and hematologic markers of inflammation, including C‐reactive protein (CRP), and outcome according to antimicrobial treatment regime in dogs with suspected AHDS hospitalized at a Danish university hospital. Outcome was investigated as change in AHDS index, days hospitalized, and survival to discharge.
A secondary aim was to investigate prevalence and severity of clinical parameters potentially indicating sepsis, before and after fluid resuscitation.
2. MATERIALS AND METHODS
2.1. Cases
Medical records were retrospectively reviewed for all dogs presenting with vomiting or diarrhea of ≤7 days duration to the Emergency service at the University Hospital for Companion Animals, University of Copenhagen, Denmark, from the 25th of February 2014 to the 31st of May 2019. The initial catchment sample included hospitalized dogs which either had hemorrhagic diarrhea as initial complaint or presented due to vomiting and developed hemorrhagic diarrhea during the subsequent hospitalization. The latter dogs were included, as vomiting often precede diarrhea in dogs with AHDS. 2 , 18 Hemorrhagic diarrhea was defined as watery feces with fresh (bright, red coloring) blood admixed, where the wording “hemorrhagic diarrhea” was used in the medical record. Because of the retrospective nature of the study, it was not possible to evaluate degree of blood admixed. Cases were excluded, if an underlying disease potentially causing acute hemorrhagic diarrhea was identified (Table 1). Dogs with a fecal culture positive for C perfringens remained included, as evidence suggests a link between AHDS and C perfringens. 4 Additionally, cases were excluded if the dog had a history of recurring gastrointestinal disease, in the week before hospitalization had received drugs known as mucosal irritants (nonsteroidal anti‐inflammatory drugs, corticosteroids, or doxycycline), underwent surgery during hospitalization, or received antimicrobials for any other indication than hemorrhagic diarrhea.
TABLE 1.
Excluded dogs and etiology: etiologies in 85 dogs hospitalized with acute hemorrhagic diarrhea excluded from a study investigating disease severity, treatment, and outcome in dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome
| Diagnoses in dogs hospitalized with acute hemorrhagic diarrhea causing exclusion from the study sample | N |
|---|---|
| Parasite infection | 29 |
| Pancreatitis | 21 |
| Mechanical obstruction of the gastrointestinal tract | 9 |
| Neoplasia | 7 |
| Parvoviral enteritis | 5 |
| Intoxication | 4 |
| Coagulopathy | 2 |
| Nephropathy | 2 |
| Pneumonia with secondary multiple organ dysfunction syndrome | 1 |
| Hepatic failure | 1 |
| Diabetes mellitus | 1 |
| Fever of unknown origin | 1 |
| Diarrhea secondary to hypoxic event in anesthesia | 1 |
| Pyometra | 1 |
2.2. Data collection
Data were tabulated in an electronic spreadsheet (Microsoft Excel 365 Proplus, Microsoft Corp., Redmond, Washington). Information collected included age, sex, breed, body weight, duration and severity of clinical signs, physical examination findings, results of clinicopathologic tests and diagnostic imaging, duration of hospitalization, survival to discharge (yes/no), reason for euthanasia where applicable, and treatments received before and during hospitalization. Included dogs were retrospectively allocated to 1 of 3 treatment groups: (a) No antimicrobials, (b) 1 type of antimicrobial, or (c) 2 types of antimicrobials during hospitalization.
2.3. Evaluation of disease severity and outcome
Disease severity on the day of admission was assessed across the 3 treatment groups using (a) the AHDS index 2 (Table 2) with dehydration percent divided into 5 categories based on history and clinical findings (<5% dehydrated, 5%‐6%, 6%‐8%, 8%‐10%, and >10%; for specifications see Table S1) and (b) signs of systemic disease using the systemic inflammatory response syndrome (SIRS) criteria adapted from previous veterinary publications reporting SIRS criteria in dogs (Table 3). 10 , 17 , 19 Using the AHDS index, clinical disease progression after the first day of hospitalization was investigated across the 3 treatment groups for all dogs that survived to discharge. Relation between disease severity and outcome (days hospitalized, survival to discharge) was also assessed.
TABLE 2.
Criteria for assessment of the canine acute hemorrhagic diarrhea syndrome (AHDS) index; total AHDS index 0 to 3: insignificant disease; 4 to 5: mild disease; 6 to 8: moderate disease; ≥9: severe disease. 2
| 0: Normal | 1: Mild | 2: Moderate | 3: Severe | |
|---|---|---|---|---|
| Activity | Normal | Mildly decreased | Moderately decreased | Severely decreased |
| Appetite | Normal | Mildly decreased | Moderately decreased | Severely decreased |
| Stool consistency | Normal | Slightly soft | Very soft | Watery diarrhea |
| Stool frequency (times/day) | 1 | 2‐3 | 4‐5 | >5 |
| Vomiting (times/day) | 0 | 1 | 2‐3 | >3 |
| Dehydration % | 0% | <5% | 5%‐10% | >10% |
TABLE 3.
Systemic inflammatory response syndrome (SIRS) criteria used to evaluate severity of disease in dogs, adapted from Hauptman et al, 17 Okano et al, 19 and Unterer et al. 10
| Clinical SIRS‐criteria | |
| Hypo‐ or hyperthermia (°C) | Small dogs(<15 kg): <37.5 or >39.4 |
| Large dogs (≥15 kg): <37.5 or >39.3 | |
| Tachycardia—heart rate (beats/min) | >140 |
| Tachypnea—respiratory rate (breaths/min) | >40 |
| Laboratory SIRS‐criteria | |
| Leukopenia or leukocytosis (109 cells/L) | <6 or >25 |
| Immature (band) neutrophils (109 cells/L) | >0.3 |
| Hypoglycemia (mg/dL) | <70.2 (<3.9 mmol/L) |
Disease severity according to the SIRS criteria was investigated only in dogs that had a CBC performed on the day of admission. To avoid potential confounding of the SIRS criteria by dehydration, only dogs that were not detectably dehydrated (defined as <5% dehydrated) within the first 24 hours of admission were included in the investigation of SIRS criteria. Physical examination data (body temperature, heart rate, and respiratory rate) were collected from the first physical examination where the dog was not detectably dehydrated. CBCs were performed in either the University hospital's daytime laboratory (ADVIA 2120i, Siemens Healthineers, Erlangen, Germany; 72/180 samples) or in the out‐of‐hours laboratory (IDEXX Procyte Dx, IDEXX Laboratories, Inc, Kornwestheim, Germany; 108/180 samples). Blood glucose‐measurements were available for 177 dogs (ADVIA 1800, Siemens Healthineers or CONTOUR glucometer, Bayer Healthcare, Leverkusen, Germany).
Where available (123/237 dogs), CRP measured on the day of admission was correlated to AHDS index and days hospitalized. CRP analysis was performed either in the University's daytime laboratory (52%; ADVIA 1800, Siemens Healthineers) or in the University hospital's out‐of‐hours laboratory (46%, Eurolyser C‐reactive Protein Assay, Eurolyser Diagnostica, Salzburg, Austria). An in‐house study was performed when the Eurolyser C‐reactive Protein Assay was purchased, to ensure agreement with the analysis method employed in the hospital's daytime laboratory (ADVIA 1800, Siemens Healthineers). Severity of CRP increase was divided into 3 previously defined intervals 20 : Low (10 to <50 mg/L), moderate (≥50 to <100 mg/L), and high (≥100 mg/L).
2.4. Response to fluid therapy
Dogs that were evaluated as ≥5% dehydrated at admission and not detectably dehydrated (moist mucosal membranes and normal skin tenting) before discharge were investigated for prevalence of clinical SIRS criteria (tachycardia, tachypnea, hypo‐, and hyperthermia) at (a) the time of admission and (b) at the first clinical examination where the dog was not detectably dehydrated.
2.5. Statistical analyses
Data management and statistical analyses were performed using statistical software packages (SAS Enterprise Guide 7.15, SAS Institute, Cary, North Carolina). Continuous data were assessed for normality using the Shapiro‐Wilk test. Median and interquartile range (IQR) are presented for non‐normally distributed quantitative variables. The Kruskal‐Wallis test was used to compare quantitative variables between the 3 treatment groups (no antimicrobials, 1 antimicrobial, 2 antimicrobials). If a significance level of P < .05 was identified, the Wilcoxon‐Mann‐Whitney test was used to identify differences between each group. For categorical variables, chi‐squared test or Fisher's exact test was used to test for difference between the 3 treatment groups. Statistical significance was set at P < .05. However, for all multiple comparisons, the Bonferroni correction was applied to adjust significance level. To investigate for development in AHDS index after the first day's hospitalization across the 3 treatment groups, a repeated measures analysis was performed (PROC MIXED).
3. RESULTS
3.1. Study sample overview
Two hundred thirty‐seven dogs were included in the study (Figure 1). Twenty‐three percent (N = 54) had previously been seen as outpatients in either the University Hospital's emergency service or in primary practice. Seventy breeds were represented. The 4 most prevalent breeds were Labrador Retriever (10.9%), small mix breed (8.4%), Miniature Schnauzer (5.5%), and Cavalier King Charles Spaniel (5.0%). All other breeds accounted for <5% each. Compared to the general hospital population, Miniature Schnauzers were significantly overrepresented in the study sample (general hospital population: 0.7%, P < .001). For the remaining 3 breeds, there was no significant difference to the hospital's general population.
FIGURE 1.

Flowchart of the study sample of dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome
3.2. Treatment
Sixty‐two percent of the dogs did not receive antimicrobials during hospitalization (N = 147, Table 4), 31% received 1 antimicrobial (N = 73), and 7% received 2 antimicrobials (N = 17). Among the dogs receiving 1 antimicrobial, 97% were treated only with ampicillin or amoxicillin (71 dogs). The remaining 2 dogs were treated with either amoxicillin/clavulanic acid (N = 1) or metronidazole (N = 1). The dogs receiving 2 antimicrobials were treated with either enrofloxacin and ampicillin or amoxicillin (N = 15) or metronidazole and ampicillin or amoxicillin (N = 2). Antimicrobial treatment was initiated on the day of admission for 71 of the 90 dogs (79%) receiving antimicrobials during hospitalization. Of the remaining dogs, 3 received antimicrobials before hospitalization, 7 were started on the second day, 8 on the third day, and 1 dog on the fourth day of hospitalization.
TABLE 4.
Signalment, acute hemorrhagic diarrhea syndrome (AHDS) index, systemic inflammatory response syndrome (SIRS) criteria, serum C‐reactive protein, outcome, and disease severity on the day of admission in dogs hospitalized at a Danish University hospital with suspected AHDS according to antimicrobial treatment regime
| No antimicrobials | One antimicrobial | Two antimicrobials | |
|---|---|---|---|
| Signalment, AHDS index, and outcome | |||
| N | 147 | 73 | 17 |
| Sex (M/MN/F/FS) | 69/12/46/20 | 28/5/27/13 | 8/3/2/4 |
| Age * (y), median (IQR) | 3.2a (1.2‐6.4) | 5.4b (3.2‐8.1) | 6.8b (3.7‐9.3) |
| Weight (kg), median (IQR) | 10.2 (5.6‐23.7) | 10.3 (6.9‐17.0) | 17.8 (7.1‐31.3) |
| AHDS index at admission * (scale 0‐18), median (IQR) | 13a (11‐15) | 14b,c (12‐15) | 16b,d (15‐18) |
| Days hospitalized * , median (IQR) | 1a (1‐2) | 1 (1‐2) | 2b (2‐3) |
| Survived to discharge * , N (%) | 143 (97%)a | 71 (97%)a | 13 (76%)b |
| Clinical signs at admission | |||
| Heart rate * (beats/min), median (IQR) | 104a (88‐128) | 128b (100‐156) | 140b (120‐164) |
| Respiratory rate * (breaths/min), median (IQR) | 24a (20‐32) | 28a (20‐32) | 40b (32‐44) |
| Temperature * (°C), median (IQR) | 38.2a (37.8‐38.6) | 38.0b (37.4‐38.4) | 38.1 (37.2‐38.3) |
| Dehydration percent * (<5%/5%‐6%/6%‐8%/8%‐10%/>10%) | 45/45/49/7/1a | 20/22/23/8/0a | 1/7/3/3/3b |
| Abdominal pain (no/yes) | 89/58 | 34/39 | 7/10 |
| SIRS‐criteria and C‐reactive protein on day of admission | |||
| ≥2 SIRS‐criteria within the first day * , proportion (%) | 8/112 (7%)a | 9/54 (17%) | 5/14 (36%)b |
| C‐reactive protein (mg/L) N, median (IQR) | 76 dogs, 31.5 (14.2‐59.2)a | 40 dogs, 71.6 (30.9‐154.6)b | 10 dogs, 127.2 (41.0‐165.0)b |
Note: Letters in superscript denote a significant difference between the treatment regimes marked with pairwise letters (a and b; c and d).
Abbreviations: F, female; FN, female neuter; IQR, interquartile range; M, male; MN, male neuter.
Significant difference between any of the 3 treatment regimes.
All dogs received intravenous fluid therapy with Ringer's acetate. Information on exact fluid replacement rate was unavailable. At the University Hospital for Companion Animals, the general recommendations on fluid therapy in dogs with dehydration due to acute diarrhea aim at rehydration within 4 to 8 hours, slower if contraindications are present. Other treatments included antiemetics (maropitant, ondansetron, metoclopramide), gastroprotectants (esomeprazole, omeprazole, sucralfate), analgetics (opioids, acetaminophen), fluid supplements (potassium chloride, glucose, bicarbonate), gastrointestinal diet and probiotic supplements.
Dogs receiving no antimicrobials were significantly younger than dogs receiving 1 (P < .001) or 2 antimicrobials (P = .01, Table 4). No significant difference was found in body weight or sex distribution between the 3 treatment groups (Table 4).
3.3. Clinical signs before and at admission
Ninety percent of the 237 dogs had hemorrhagic diarrhea at presentation (214/237 dogs; median duration of diarrhea before admission: 1 day; IQR 0‐2 days). Among the remaining 23 dogs, 16 dogs developed hemorrhagic diarrhea within the first 24 hours of admission, 4 dogs did not have information registered on time of diarrhea onset, and 3 dogs developed diarrhea >24 hours after admission. Among all 237 dogs, only 4% were reported to either have or develop melena during hospitalization. No differences were identified between the 3 treatment groups in prevalence of hemorrhagic diarrhea at admission, prevalence of melena, duration of diarrhea before admission, or time from admission to development of hemorrhagic diarrhea.
Ninety‐one percent of the dogs (216/237 dogs) presented with vomitus with a median duration of 1 day before admission (IQR 0‐2 days; data on duration missing in 3/216 dogs). Hematemesis was reported in 31% (68/216 dogs). Of the 21 dogs that did not present with vomitus, none developed vomitus during the subsequent hospitalization. No differences in prevalence of vomitus, duration of vomitus before admission, or hematemesis between the 3 treatment groups were identified. Further findings from the physical examination at admission, packed cell volume, and electrolytes for the 3 groups are presented in Table 4 and Supporting Information S1.
3.4. AHDS index
At admission, the median AHDS index was 13 (IQR 11‐15) across the entire study sample (Table 4). Ninety percent of the dogs had severe AHDS (AHDS index ≥ 9), with dogs receiving 2 antimicrobials having the significantly highest AHDS index (P < .01) followed by dogs receiving 1 antimicrobial, which also had a significantly higher AHDS index compared to dogs receiving no antimicrobials (P < .01) (Table 4). There was no significant difference in AHDS index at admission between survivors (13; IQR 11‐15) and nonsurvivors (16; IQR 12‐17). Distribution according to treatment group and severity of each AHDS index parameter is presented in Table S2.
All dogs that survived to discharge had a significant decrease in AHDS index after the first day of hospitalization regardless of antimicrobial treatment regime with a median AHDS index of 6 (IQR 3‐8) after 1 day's hospitalization (P < .001) with no significant differences between treatment groups.
3.5. SIRS criteria
Of the 237 dogs, 180 dogs (76%) had CBC data and were evaluated as not clinically dehydrated within the initial 24 hours after admission. For these dogs, the median time to reach <5% dehydration was 12 hours (IQR 6.5‐13.0 hours). Despite not being clinically dehydrated when the physical examination data were collected, 1 or more SIRS criteria was met in 70 of 180 dogs with tachycardia as the most prevalent clinical finding and left‐shift as the most frequent laboratory finding (Table 5).
TABLE 5.
Clinical and laboratory systemic inflammatory response syndrome (SIRS) criteria within the first 24 hours of hospitalization (proportion and range) in relation to antimicrobial treatment regime in 180 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome
| No antimicrobials, N = 112 | One antimicrobial, N = 54 | Two antimicrobials, N = 14 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Median | IQR | N | % | Median | IQR | N | % | Median | IQR | |
| Clinical SIRS‐criteria at first exam where <5% dehydrated | ||||||||||||
| Tachycardia, > 140 beats/minute a | 8/105 | 7.6 | 157 | 144‐184 | 5/51 | 9.8 | 156 | 144‐160 | 3/14 | 21.4 | 156 | 144‐160 |
| Tachypneic, > 40 breaths/minute a | 7/86 | 8.1 | 52 | 50‐60 | 4/42 | 9.5 | 49 | 45‐51 | 1/9 | 11.1 | 48 | — |
| Hypothermia T < 37.5—all dogs a | 5/82 | 6.1 | 37.1 | 36.9‐37.3 | 3/40 | 7.5 | 36.6 | 36.1‐37.2 | 1/11 | 9.1 | 37.4 | — |
| Hyperthermia T > 39.4—small dogs a | 0/82 | 0.0 | — | — | 0/40 | 0 | — | — | 0/11 | 0.0 | — | — |
| Hyperthermia T > 39.3—large dogs a | 2/82 | 2.4 | 39.7 | 39.7 | 1/40 | 2.5 | 40.6 | — | 0/11 | 0.0 | — | — |
| Laboratory SIRS‐criteria | ||||||||||||
| Leukocytosis, white blood cell count > 25 × 109 cells/L | 1/112 | 0.9 | 30.0 | — | 2/54 | 3.7 | 49.15 | 34.6‐63.7 | 0/14 | — | — | — |
| Leukopenia, white blood cell count <6 × 109 cells/L | 1/112 | 0.9 | 5.9 | — | 3/54 | 5.6 | 4.8 | 3.1‐4.9 | 3/14 | 21.4 | 4.2 | 3.1‐5.9 |
| Left‐shift >0.3 × 109 cells/L | 18/112 | 16.1 | — | — | 11/54 | 20.4 | — | — | 4/14 | 28.6 | — | — |
| Hypoglycemia <70.2 mg/dL a | 6/110 | 5.5 | 3.55 | 2.4‐3.8 | 6/53 | 11.3 | 2.7 | 2.4‐3.7 | 5/14 | 35.7 | 2.9 | 1.9‐3.4 |
Abbreviation: IQR, interquartile range.
Because of the retrospective nature of this study, missing data points were present.
Overall, 12% of the 180 dogs met ≥2 SIRS criteria (21/180 dogs). When comparing the proportion of dogs meeting ≥2 SIRS criteria between the 3 treatment groups (Table 6), significantly fewer of the dogs receiving no antimicrobials met ≥2 SIRS criteria (7%, 8/112 dogs) compared to dogs receiving 2 antimicrobials (36%, 5/14 dogs, P < .001). There was no significant difference between the other treatment groups. No significant relation between number of SIRS criteria and survival to discharge was found.
TABLE 6.
Distribution of number of systemic inflammatory response syndrome (SIRS) criteria met within the first 24 hours of hospitalization in relation to antimicrobial treatment regime in 180 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome
| No antimicrobials, N = 112 | One antimicrobial, N = 54 | Two antimicrobials, N = 14 | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| SIRS criteria met | |||
| 0 | 74 (66%) | 33 (61%) | 3 (21%) |
| 1 | 30 (27%) | 13 (24%) | 6 (43%) |
| 2 | 8 (7%) | 4 (7%) | 4 (29%) |
| 3 | 0 (0%) | 3 (6%) | 1 (7%) |
| 4 | 0 (0%) | 1 (2%) | 0 (0%) |
In dogs which did not meet any SIRS criteria (N = 110), no significant difference was found in days hospitalized or percentual decrease in AHDS index after the first day of hospitalization between dogs receiving no antimicrobials (N = 74) and dogs receiving 1 antimicrobial (N = 33). However, dogs receiving 2 antimicrobials that also met no SIRS criteria (N = 3) had both a significantly longer hospitalization with a median of 3 days hospitalized (IQR 2‐4; P = .01) and a lesser decrease in AHDS index after the first day of hospitalization compared to dogs which met no SIRS criteria in the other treatment groups (percentual decrease in AHDS index over the first day of hospitalization: no antimicrobials: median 57% decrease, IQR 38%‐75%; 1 antimicrobial: median 57% decrease, IQR 40%‐75%; 2 antimicrobials: median 31% decrease, IQR 0%‐60%; P < .01). When investigating days hospitalized and decrease in AHDS index after the first day of hospitalization, irrespective of treatment groups, no significant difference was found when comparing dogs with 0, 1, and ≥2 SIRS criteria.
3.6. C‐reactive protein
C‐reactive protein was measured on the day of admission for 123 of the 237 dogs (Table 7) with a median of 52 mg/L (IQR 16.4‐93.3). There was a significant positive correlation between AHDS index and CRP at hospitalization (P < .001). C‐reactive protein was not significantly correlated with days hospitalized. Dogs receiving antimicrobials had a significantly higher CRP compared to dogs receiving no antimicrobials (P < .001). No significant difference in CRP was found between dogs receiving 1 antimicrobial and dogs receiving 2 antimicrobials. However, 7% of the dogs that received no antimicrobials (5/75 dogs) had a CRP ≥ 100 mg/L and 22% of the dogs receiving 2 antimicrobials had a CRP < 50 on the day of admission. Of the dogs receiving 2 antimicrobials, 1 was severely lethargic, hypothermic (T: 37.1), tachycardic (200 bpm) and had pale, sticky mucosal membranes at the time of initial presentation, despite having a CRP of only 35.2 mg/L.
TABLE 7.
Serum C‐reactive protein (CRP) levels (mg/L) on day of admission for 123 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome, distributed by treatment groups and CRP‐intervals as stated by Hindenberg et al. 20
| No antimicrobials | One antimicrobial | Two antimicrobials | ||||
|---|---|---|---|---|---|---|
| N (%) | Median (IQR) | N (%) | Median (IQR) | N (%) | Median (IQR) | |
| Total | 75 (100%) | 31.7 (14.4‐59.5) | 39 (100%) | 68.1 (28.8‐143.8) | 9 (100%) | 140.8 (95.4‐164.9) |
| Low (0 to <50) | 48/75 (64%) | 16.4 (11.2‐30.9) | 11/39 (28%) | 15.5 (10.0‐22.3) | 2/9 (22%) | 37.9 (35.2‐41.0) |
| Moderate (≥50 to <100) | 22/75 (29%) | 61.1 (57.6‐76.3) | 11/39 (28%) | 65.6 (55.0‐68.1) | 1/9 (11%) | 95.4 (−) |
| High (≥100) | 5/75 (7%) | 124.9 (120.8‐141.0) | 17/39 (44%) | 165.4 (124.5‐230.0) | 6/9 (66%) | 157.4 (140.8‐190.8) |
Abbreviation: IQR, interquartile range.
3.7. Response to fluid therapy
Of the 237 dogs, 73% were evaluated as ≥5% dehydrated at admission, received fluid therapy during hospitalization, and were evaluated as not detectably dehydrated at a clinical examination before discharge (174/237 dogs). The median time until rehydration was 12 hours (IQR 12‐24 hours).
Across all 3 treatment groups, rehydration markedly reduced the number of clinical SIRS criteria (Figure 2; Table 8) with only 1 dog meeting ≥2 clinical SIRS criteria following rehydration. The dog had tachycardia (180 beats/min) and tachypnea (52 breaths/min), received no antimicrobials during hospitalization and survived to discharge. The most prevalent finding both before and after rehydration was tachycardia (Table 8). There was no significant relationship between days hospitalized and number of clinical SIRS criteria after rehydration.
FIGURE 2.
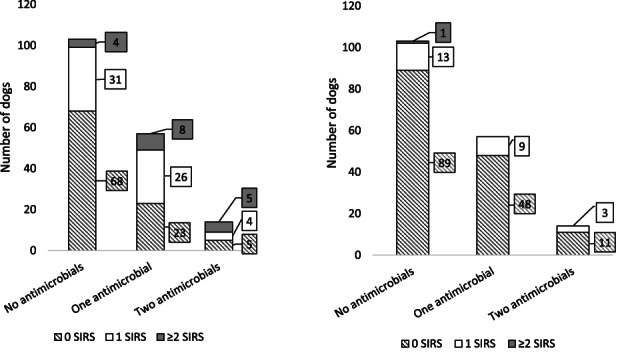
Number of clinical criteria met for systemic inflammatory response syndrome (SIRS), ie, tachycardia, tachypnea, hypo‐, and hyperthermia, distributed according to antimicrobial treatment groups in 174 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome at admission (left) and at first clinical examination with normal mucosal membranes and skin tenting (right)
TABLE 8.
Clinical signs of systemic inflammatory response syndrome before and after rehydration (proportion and range) in 174 dogs evaluated to be ≥5% dehydrated at admission and hospitalized with suspected acute hemorrhagic diarrhea syndrome
| At admission, ≥5% dehydrated | After rehydration, <5% dehydrated | |||||
|---|---|---|---|---|---|---|
| N | Median | IQR | N | Median | IQR | |
| Tachycardia, > 140 beats/minute a | 44/170 (26%) | 160 | 158‐180 | 12/162 (7%) | 160 | 144‐180 |
| Tachypnea, > 40 breaths/minute a | 17/141 (12%) | 48 | 44‐56 | 8/133 (6%) | 51 | 46‐56 |
| Hypothermia T < 37.5 a | 36/167 (22%) | 37.1 | 36.4‐37.3 | 7/121 (6%) | 37.1 | 36.6‐37.3 |
| Hyperthermia T > 39.3 a | 0/167 (0%) | — | — | 0/121 (0%) | — | — |
Abbreviation: IQR, interquartile range.
Because of the retrospective nature of this study, missing data points were present.
3.8. Days hospitalized and survival to discharge
The majority of dogs were discharged within the first 48 hours (1 day 56%; 2 days 27%; 3 days 8%; ≥4 days 9%) with a median hospitalization time of 1 day (range, 1‐8; IQR 1‐2). Dogs receiving 2 antimicrobials were hospitalized significantly longer compared to dogs receiving no antimicrobials (P = .005) (Table 4). No significant difference in days hospitalized was found between any of the other treatment groups. All, except for 1 dog, were discharged according to the attending clinician's recommendation.
Overall, 96% survived to discharge (227/237 dogs, Table 4). A significantly higher proportion of dogs which received 2 antimicrobials were euthanized or died (4/17 dogs) compared to dogs receiving no antimicrobials (4/173 dogs, P = .004) and 1 antimicrobial (2/73 dogs, P = .01). There was no significant difference in survival to discharge between dogs receiving no antimicrobials and 1 antimicrobial. All dogs that had antimicrobial treatment initiated later than 24 hours after admission survived to discharge. Of the 10 dogs that did not survive to discharge, 3 died within the first 24 hours after admission (1 dog within each treatment group) and 7 were euthanized during hospitalization at the owner's request due to financial constraints and/or advanced age of the dog. Among the 3 dogs that died, necropsy was performed on the dog receiving no antimicrobials and the dog receiving 1 antimicrobial. Both dogs had diffuse acute enterocolitis.
4. DISCUSSION
The results of our study indicate that a large proportion of dogs with suspected AHDS, including dogs presenting with ≥2 SIRS criteria, improve markedly solely with supportive treatment and survive to discharge after a short hospitalization period. Clinical SIRS criteria are significantly affected by degree of dehydration, making it difficult for the clinician to distinguish between dehydration and severity of inflammation at initial presentation. C‐reactive protein correlated with AHDS index, but did not correlate with days hospitalized and it is questionable whether it can be used to guide decision on antimicrobial treatment.
The majority of dogs administered antimicrobials received ampicillin only. The short median hospitalization time and high survival rate were similar to the group receiving no antimicrobials. Likewise, percent decrease in AHDS index did not differ between the 2 groups. Some dogs receiving ampicillin only, exhibited signs of severe disease such as hypoglycemia (11%) and leukopenia (6%) but still improved without additional antimicrobials, suggesting that aminopenicillins alone might offer adequate treatment in most cases. Guidelines on management of dogs with AHDS, including use of antimicrobials, are available at the University Hospital of Companion Animals, and are based on the Danish antimicrobial use guidelines for companion animal practice 16 which recommends to only initiate antimicrobial treatment in dogs with severe hemorrhagic diarrhea and concurrent signs of sepsis. It is possible that dogs that met 1 or more SIRS criteria would have benefitted from a more aggressive antimicrobial treatment protocol. However, both dogs receiving no antimicrobials and dogs receiving 1 antimicrobial had a high survival rate, a median hospitalization duration of 1 day, and no significant difference in AHDS index decrease over the first 24 hours of hospitalization. Benefits of more narrow‐spectrum aminopenicillins over potentiated penicillins or metronidazole include the potential for less selection for antimicrobial resistance 14 as well as avoiding the potential risk of secondary dysbiosis. 21 , 22
When investigating days hospitalized or improvement in AHDS index for dogs that met no SIRS criteria, no difference was found between dogs receiving no antimicrobials and dogs receiving 1 antimicrobial. This is in concurrence with previous studies, which indicate that antimicrobials are not needed in dogs with acute diarrhea and no signs of sepsis. 11 , 12 , 13 , 14 , 21 A study from 2015 also found that the prevalence of bacteremia in dogs with AHDS is similar to healthy dogs, 10 suggesting that acute hemorrhagic diarrhea might not be tantamount to bacterial translocation from the gut lumen to the blood stream. Additionally, 7% of dogs receiving no antimicrobials met ≥2 SIRS criteria with no relation between number of SIRS criteria met and survival to discharge. This might indicate that the presence of 1 of more SIRS criteria based on the current definitions does not accurately identify dogs in need of antimicrobial treatment.
Clinical signs of systemic disease, such as marked changes in temperature, heart rate, and respiratory rate combined with leukocyte changes and hypoglycemia, can be viewed collectively under the term SIRS, providing a method to identify dogs at risk of developing sepsis, if the dog meets 2 or more of the defined criteria. 17 However, the SIRS criteria as a proxy for sepsis is unfortunately neither very sensitive or specific 17 and it has been argued that the current SIRS criteria are overly simplistic when attempting to identify dogs with sepsis. 23 , 24 Furthermore, a consensus on threshold values for the individual parameters has yet to be reached, leading to studies utilizing differing values, with, for example, tachycardia defined as both a heart rate above 120 beats, 17 140 beats, 25 and 160 beats/min. 19 Also, it is difficult to distinguish whether the inflammatory response is due to an infectious or noninfectious insult. Despite these limitations, the SIRS criteria remain the mainstay for many veterinary practitioners to identify dogs in need of antimicrobial treatment, as both the clinical and laboratory values are easily accessible. A crucial limitation to the use of the clinical SIRS criteria, that is, tachycardia, tachypnea, hypo‐, and hyperthermia, is that they are also affected by other factors, such as pain, stress, and dehydration. One of the main reasons for hospitalization of dogs with AHDS is the need for supportive treatment, with fluid therapy as an essential cornerstone in the treatment plan. 26 Approximately 3‐quarters of the dogs in the current study were evaluated as ≥5% dehydrated at admission, and when investigating number of clinical SIRS criteria before and after rehydration, a marked decrease in the proportion of dogs with clinical SIRS criteria was seen following rehydration. Because of the retrospective nature of this study's design, it is not possible to know to which degree the intravenous fluid resuscitation vs other initiated treatments, each contributed to the dogs' improvement. However, due to the relatively short time interval to clinical rehydration (median of 12 hours), degree of dehydration must be considered as a central contributing factor. The marked reduction in number and severity of individual clinical SIRS criteria following rehydration underlines the importance of not relying solely on tachycardia, tachypnea, and hypo‐ or hyperthermia before fluid resuscitation when assessing whether to initiate antimicrobial treatment.
To the authors' knowledge, this is the first study investigating CRP in dogs with suspected AHDS. A significant positive correlation between AHDS index and CRP was identified. As for the AHDS index, CRP did not correlate with survival. C‐reactive protein has previously been identified as a potential copredictor when evaluating prognosis in parvoviral enteritis in puppies 27 and acute pancreatitis in dogs. 28 However, the results of our study do not suggest CRP alone as an outcome predictor in dogs with AHDS. C‐reactive protein was higher in dogs receiving antimicrobials compared to dogs not receiving antimicrobials. However, this result should be interpreted with caution as the causal relationship between CRP and antimicrobial treatment cannot be established in this retrospective study. It is possible that the attending clinicians have utilized CRP in the clinical decision‐making process and initiated antimicrobials based on a high CRP. The correlation between CRP and antimicrobial treatment might therefore merely be a result of CRP being used as an indicator for antimicrobial treatment rather than proof of its usefulness as an indicator for when to initiate antimicrobial treatment. Also, being treated with antimicrobials does not equal benefit of antimicrobial treatment, and it is likely that some dogs in our study would have experienced the same positive outcome without antimicrobial treatment. The potential usefulness of CRP as a discriminator between dogs with AHDS that will or will not benefit from antimicrobial treatment should therefore be investigated in a prospective study.
The general perception that AHDS carries a good prognosis was confirmed by the findings of our study, where 96% of the dogs survived to discharge despite most dogs presenting with severe AHDS and 1 or more SIRS criteria. Generally, there was a marked improvement in clinical disease after the first day of hospitalization, and the majority were discharged within the first 2 days following admission (83%). These findings are in concordance with Mortier et al who also reported a high proportion of dogs hospitalized with AHDS surviving to discharge and a rapid clinical improvement. 2 It may be speculated that the 7 dogs that were euthanized in the current study might have survived to discharge, if the owners had elected to continue treatment. Four of the 7 dogs had notations in the medical file of clinical improvement. Regardless, the owners elected euthanasia due to either financial constraints or advanced age of the dog. These findings underscore a frequent stumbling block in veterinary studies, as these dogs might have recovered to discharge if the owners had pursued further treatment.
Miniature Schnauzers were significantly overrepresented compared to the hospital's general population. Miniature Schnauzers have been identified as a breed predisposed to AHDS 2 and to acute diarrhea in general. 8 Dogs receiving no antimicrobials were significantly younger than both dogs receiving 1 or 2 antimicrobials. The younger age of dogs receiving no antimicrobials might be due to several factors, among which are the possibility that younger dogs have a less severe disease course, differences in underlying etiology, or a potential tendency among clinicians to prescribe antimicrobials more readily if the dog is older.
To the authors' knowledge, the AHDS index, previously referred to as the hemorrhagic gastroenteritis activity index, 12 has yet to be validated. Based on experience from our hospital and current research projects, large observer variations (both intra‐ and interobserver) occur—especially in the subjective variable “activity level,” necessitating in‐depth uniform guidelines to increase observer agreement. However, despite the lack of validation, the AHDS index constitutes a method to quantify degree of clinical disease, which is both easily understood and applied. 2 , 11 , 12 , 14 , 29 , 30 When interpreting results reported using the AHDS index, it is important to recognize that the term “severe disease” denotes an index of ≥9. Consequently, the term covers a wide span of disease severity, as a dog with a normal activity level but with a slightly decreased appetite, watery diarrhea, and both defecation and vomiting 4 times daily (total AHDS index = 9) is allocated to the same category as an inappetent dog in lateral recumbency with continuous, profuse, hemorrhagic diarrhea, frequent hematemesis, and severe dehydration (total AHDS index = 18). Additional studies are warranted to validate the AHDS index.
Disease severity at the time of admission and hospitalization time are not necessarily directly correlated. Some dogs in our study had evident signs of hypovolemia with severely decreased activity, tachycardia, hypothermia, and dehydration but improved rapidly following fluid resuscitation and additional supportive care. Had these dogs not received the necessary care, the outcome would most likely not have been favorable. Furthermore, the decision on when to discharge, that is, duration of hospitalization, might also be affected by in‐house policies and attending veterinarian. At the University Hospital for Companion Animals, owners will often be offered the possibility of an open hospitalization if the attending veterinarian deems this to be a feasible and safe possibility. This entails discharge of the dog but with the opportunity of readmittance free of charge if the dog's condition deteriorates or fails to improve when returned to its home environment. Consequently, the majority of dogs are discharged before normalization of fecal consistency, and some dogs are discharged before normalization of appetite. If practically feasible, we recommend that a set of criteria is made for discharge in prospective studies where days hospitalized is investigated.
Dogs were included if the wording “hemorrhagic diarrhea” was used in the medical record. However, because of the retrospective nature of this study, it is not possible to ascertain potential discrepancies between individual clinicians on when the wording “hemorrhagic diarrhea” was used. Consequently, the degree of fresh blood admixed in the feces might have varied between the included dogs. Another limitation of our study is the lack of full work‐up of all dogs, as all diagnostics were performed at the discretion of the attending veterinarian. It is therefore not possible to rule out the possibility that some dogs might have had hemorrhagic diarrhea secondary to unidentified causes. However, we believe that the current study sample reflect the population attended in most first opinion practices and in referral hospitals where the clients' financial restrictions limit the possibility of in‐depth work‐up. Follow‐up after discharge was not available for all dogs included in our study. It is therefore possible that dogs later relapsed and were hospitalized or initiated on antimicrobials at another veterinary practice. However, only 1 dog was discharged against advice.
The time interval between clinical signs registered in the medical file and the time at which blood was sampled is unknown. According to the hospital's standard procedure, blood is sampled for analysis when the IV catheter is first placed in relation to admission to the hospital's fluid ward, unless a known contraindication for withdrawal of blood is known or it is not possible because of practical reasons (eg, marked hypotension/aggressive dog). Consequently, the majority of samples were collected in close relation to the initial clinical examination on admission. However, for a few dogs, blood samples might have been collected at a slightly later time point. To avoid marked differences in time, only blood samples analyzed within 1 day of hospitalization was used in our study.
In conclusion, our study indicates that the majority of dogs hospitalized with suspected AHDS do not need antimicrobial treatment despite showing signs of systemic disease on initial presentation. The often‐used SIRS criteria might be a poor proxy for identifying dogs presenting with AHDS in need of antimicrobial treatment, in particular when they are hypovolemic. Furthermore, antimicrobial treatment with ampicillin might be sufficient in the majority of dogs in need of antimicrobial treatment. Overall, AHDS carries a good prognosis with a rapid recovery after initiation of symptomatic treatment. C‐reactive protein was found to correlate with AHDS index. However, whether and how CRP can contribute to the clinical decision‐making or prognostication is yet to be determined. The term severe AHDS in the AHDS index currently denotes a wide range of severity in clinical disease. Future studies might benefit from a qualitative categorization with more tiers.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information.
Supplemental Table S1 Percent dehydration according to clinical findings in dogs.
Supplemental Table S2 Distribution of 237 dogs hospitalized with suspected Acute Hemorrhagic Diarrhea Syndrome (AHDS) according to antimicrobial treatment regime, disease severity score (0‐3) for each parameter of the AHDS index and total AHDS index, where the total AHDS index is divided into four categories according to disease severity: 0‐3 clinically insignificant disease, 4‐5 mild AHDS, 6‐8 moderate AHDS and ≥ 9 severe AHDS.
ACKNOWLEDGMENT
No funding was received for this study.
Dupont N, Jessen LR, Moberg F, Zyskind N, Lorentzen C, Bjørnvad CR. A retrospective study of 237 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome: Disease severity, treatment, and outcome. J Vet Intern Med. 2021;35:867–877. 10.1111/jvim.16084
REFERENCES
- 1. Burrows C. Canine hemorrhagic gastroenteritis. J Am Anim Hosp Assoc. 1977;13:451‐458. [Google Scholar]
- 2. Mortier F, Strohmeyer K, Hartmann K, Unterer S. Acute haemorrhagic diarrhoea syndrome in dogs: 108 cases. Vet Rec. 2015;176(24):627. [DOI] [PubMed] [Google Scholar]
- 3. Unterer S, Busch K, Leipig M, et al. Endoscopically visualized lesions, histologic findings, and bacterial invasion in the gastrointestinal mucosa of dogs with acute hemorrhagic diarrhea syndrome. J Vet Intern Med. 2014;28(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sindern N, Suchodolski JS, Leutenegger CM, et al. Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J Vet Intern Med. 2019;33(1):100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gatt M, Reddy B, MacFie J. Bacterial translocation in the critically ill—evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25(7):741‐757. [DOI] [PubMed] [Google Scholar]
- 6. Hughes LA, Williams N, Clegg P, et al. Cross‐sectional survey of antimicrobial prescribing patterns in UK small animal veterinary practice. Prev Vet Med. 2012;104(3‐4):309‐316. [DOI] [PubMed] [Google Scholar]
- 7. De Briyne N, Atkinson J, Pokludová L, et al. Antibiotics used most commonly to treat animals in Europe. Vet Rec. 2014;175(13):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones P, Dawson S, Gaskell R, et al. Surveillance of diarrhoea in small animal practice through the Small Animal Veterinary Surveillance Network (SAVSNET). Vet J. 2014;201(3):412‐418. [DOI] [PubMed] [Google Scholar]
- 9. Singleton DA, Noble P, Sánchez‐Vizcaíno F, et al. Pharmaceutical prescription in canine acute diarrhoea: a longitudinal electronic health record analysis of first opinion veterinary practices. Front Vet Sci. 2019;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unterer S, Lechner E, Mueller RS, et al. Prospective study of bacteraemia in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec. 2015;176(12):309. [DOI] [PubMed] [Google Scholar]
- 11. Ziese A‐L, Suchodolski JS, Hartmann K, et al. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS One. 2018;13(9):e0204691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unterer S, Strohmeyer K, Kruse B, et al. Treatment of aseptic dogs with hemorrhagic gastroenteritis with amoxicillin/clavulanic acid: a prospective blinded study. J Vet Intern Med. 2011;25(5):973‐979. [DOI] [PubMed] [Google Scholar]
- 13. Chaitman J, Ziese A‐L, Pilla R, et al. Fecal microbial and metabolic profiles in dogs with acute diarrhea receiving either fecal microbiota transplantation or oral metronidazole. Front Vet Sci. 2020;7:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werner M, Suchodolski JS, Straubinger RK, et al. Effect of amoxicillin‐clavulanic acid on clinical scores, intestinal microbiome, and amoxicillin‐resistant Escherichia coli in dogs with uncomplicated acute diarrhea. J Vet Intern Med. 2020;34(3):1166‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall JD, Day MJ. Acute small intestinal disease. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 8th ed. Philadelphia, PA: Saunders; 2017:1538‐1539. [Google Scholar]
- 16. Jessen LR, Damborg P, Spohr A, et al. Antibiotic Use Guidelines for Companion Animal Practice. 2nd ed. Frederiksberg, Denmark: Companion Animal Group, Danish Veterinary Association; 2018. [Google Scholar]
- 17. Hauptman J, Walshaw R, Olivier N. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet Surg. 1997;26(5):393‐397. [DOI] [PubMed] [Google Scholar]
- 18. Dunowska M. What is causing acute haemorrhagic diarrhoea syndrome in dogs? Vet Rec. 2017;180:539‐541. [DOI] [PubMed] [Google Scholar]
- 19. Okano S, Yoshida M, Fukushima U, Higuchi S, Takase K, Hagio M. Usefulness of systemic inflammatory response syndrome criteria as an index for prognosis judgement. Vet Rec. 2002;150:245‐246. [DOI] [PubMed] [Google Scholar]
- 20. Hindenberg S, Keßler M, Zielinsky S, Langenstein J, Moritz A, Bauer N. Evaluation of a novel quantitative canine species‐specific point‐of‐care assay for C‐reactive protein. BMC Vet Res. 2018;14(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shmalberg JW, Montalbano C, Morelli G, Buckley GJ. A randomized double blinded placebo‐controlled clinical trial of a probiotic or metronidazole for acute canine diarrhea. Front Vet Sci. 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Espinosa‐Gongora C, Jessen LR, Kieler IN, et al. Impact of oral amoxicillin and amoxicillin/clavulanic acid treatment on bacterial diversity and β‐lactam resistance in the canine faecal microbiota. J Antimicrob Chemother. 2020;75(2):351‐361. [DOI] [PubMed] [Google Scholar]
- 23. Balk RAJV. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5(1):20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otto CM. Clinical trials in spontaneous disease in dogs: a new paradigm for investigations of sepsis. J Vet Emerg Crit Care. 2007;17(4):359‐367. [Google Scholar]
- 25. Mantione NL, Otto CM. Characterization of the use of antiemetic agents in dogs with parvoviral enteritis treated at a veterinary teaching hospital: 77 cases (1997–2000). J Am Vet Med Assoc. 2005;227(11):1787‐1793. [DOI] [PubMed] [Google Scholar]
- 26. Tello L, Perez‐Freytes R. Fluid and electrolyte therapy during vomiting and diarrhea. Vet Clin North Am Small Anim Pract. 2017;47(2):505‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClure V, Van Schoor M, Thompson PN, et al. Evaluation of the use of serum C‐reactive protein concentration to predict outcome in puppies infected with canine parvovirus. J Am Vet Med Assoc. 2013;243(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 28. Sato T, Ohno K, Tamamoto T, et al. Assesment of severity and changes in C‐reactive protein concentration and various biomarkers in dogs with pancreatitis. J Vet Med Sci. 2017;79(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Busch K, Suchodolski J, Kühner K, et al. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec. 2015;176(10):253. [DOI] [PubMed] [Google Scholar]
- 30. Ortiz V, Klein L, Channell S, et al. Evaluating the effect of metronidazole plus amoxicillin‐clavulanate versus amoxicillin‐clavulanate alone in canine haemorrhagic diarrhoea: a randomised controlled trial in primary care practice. J Small Anim Pract. 2018;59(7):398‐403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Supplemental Table S1 Percent dehydration according to clinical findings in dogs.
Supplemental Table S2 Distribution of 237 dogs hospitalized with suspected Acute Hemorrhagic Diarrhea Syndrome (AHDS) according to antimicrobial treatment regime, disease severity score (0‐3) for each parameter of the AHDS index and total AHDS index, where the total AHDS index is divided into four categories according to disease severity: 0‐3 clinically insignificant disease, 4‐5 mild AHDS, 6‐8 moderate AHDS and ≥ 9 severe AHDS.


