Abstract
Background
Symmetric dimethylarginine (SDMA) is considered a more sensitive indirect estimate of glomerular filtration rate (GFR) than creatinine (Cr). Symmetric dimethylarginine is not affected by sex or muscle mass in small animals.
Objectives
To validate a commercial SDMA immunoassay (IA) for equine serum; to compare SDMA and Cr in cohorts of draft horse breeds; and to assess effects of age, sex, and breed.
Animals
One hundred and sixty‐five healthy draft horses (0.5‐16 years), including 63 Percherons, 52 Clydesdales, and 50 Belgians.
Methods
Cross‐sectional study. The SDMA IA was validated for equine serum by comparison to liquid chromatography‐mass spectroscopy (LC‐MS) results and other methods. Symmetric dimethylarginine and Cr were compared by analysis of variance and correlation analysis.
Results
Median and 95% confidence intervals (CIs) for LC‐MS (10.0 [9.4, 10.2] μg/dL) and IA (9.7 [9.5, 10.0] μg/dL) SDMA concentrations were strongly correlated (R = .74, P < .001). Symmetric dimethylarginine was lower (P < .01) in Percherons and Belgians, than in Clydesdales. Median values and 95% CI for Cr were 1.3 (1.2, 1.4), 1.4 (1.3, 1.5), and 1.4 (1.3, 1.5) mg/dL (P = .06) for Percherons, Clydesdales, and Belgians, respectively. Symmetric dimethylarginine was correlated to Cr (LC‐MS, R = .60, P < .001; IA, R = .66, P < .001). There were no differences in SDMA or Cr between sexes and there were no correlations between age and SDMA or Cr.
Conclusions and Clinical Importance
Although a significant breed effect on SDMA concentration was found, differences were small and all medians were <14 μg/dL, the cutoff value to support renal dysfunction in dogs and cats.
Keywords: glomerular filtration rate, laboratory assessment, renal disease, renal function, urea
Abbreviations
- AKI
acute kidney injury
- BCS
body condition score
- CI
confidence intervals
- CKD
chronic kidney disease
- Cr
serum creatinine concentration
- GFR
glomerular filtration rate
- IA
immunoassay
- LC‐MS
liquid chromatography‐mass spectroscopy
- SDMA
symmetric dimethylarginine
- UN
urea nitrogen concentration
1. INTRODUCTION
Symmetric dimethylarginine (SDMA) is a stable, continually released end‐product derived from intranuclear methylation of l‐arginine residues on various regulatory proteins that are released into the cytoplasm after proteolysis. It was initially identified in urine in 1970 and further investigation revealed SDMA to be a molecule that could be used to estimate glomerular filtration rate (GFR) due to its small molecular size (MW 202 g/mol) and lack of protein binding, allowing SDMA to be freely filtered across the glomerulus. 1 , 2 Similar to inulin, SDMA is neither reabsorbed nor secreted by the remainder of the nephron. 3 These properties made it likely that SDMA was a potential novel biomarker for indirect estimation of GFR. In support, a meta‐analysis of 18 human studies revealed SDMA to be highly correlated with other estimates of GFR. 4 Finally, SDMA appears to be minimally affected by muscle mass, breed, or sex in dog and cats. 2 , 3 , 5
In contrast, measurement of serum creatinine concentration (Cr), although well accepted by clinicians as an excellent measure of renal function due to similar renal handling as inulin, has limitations. Creatinine is also steadily released into the circulation as a degradation product of creatine. Because creatine is found primarily in skeletal muscle, muscle mass can affect serum Cr concentration. 6 In addition, the Jaffé reaction, long‐used used for determination of Cr in automated chemistry analyzers, measures additional molecules termed non‐Cr chromogens, that could lead to overestimation of Cr by up to 45%, although newer enzymatic methods are now available to reduce this potential error. 6 Finally, Cr is an insensitive measure of a decrease in GFR, as 75% of functional renal mass must typically be lost before the serum concentration can exceed the upper limit of the reference interval. 3 , 6
Increases in serum SDMA concentration were 1st reported in veterinary medicine in cats with spontaneous chronic kidney disease (CKD), in which SDMA was highly correlated with Cr. 7 Several studies support that SDMA is a more sensitive measure of a decrease in GFR, increasing with an average loss of 40% of functional renal mass. 2 , 3 Furthermore, increases in SDMA were documented, on average, 10 and 17 months before an increase in Cr in dogs and cats, respectively, with spontaneous CKD. 8 , 9
A significant limitation of measuring SDMA has been methodology. Liquid chromatography‐mass spectroscopy (LC‐MS), costly, labor intensive, and only available in a research setting, has been the gold standard. Recently, however, IDEXX Laboratories, Inc. (Westbrook, Maine) developed a novel immunoassay (IA) for measurement of SDMA concentration, providing a rapid, commercially available, and economic test. 2 The IA has been validated by comparison to LC‐MS results in both healthy dogs and cats and in patients with elevated SDMA concentrations. 2 , 10 , 11 , 12 Once validated, the SDMA IA was added to an automated multichannel chemistry analyzer allowing high throughput measurement of SDMA on a routine serum chemistry profile. In addition to reporting of SDMA on IDEXX small animal chemistry profiles, measurement of SDMA by IA was recently added to IDEXX equine chemistry profiles. However, to the authors' knowledge, no studies have been published on values for SDMA in either healthy horses or equids with renal dysfunction. Thus, the objectives of this investigation were: (1) to validate a commercial SDMA IA for equine serum; (2) to compare serum SDMA and Cr concentrations in 3 breeds of heavily muscled draft horses; and (3) to examine for differences in SDMA and Cr due to sex and age.
2. MATERIALS AND METHODS
2.1. Animals and sampling
This study was a convenience sampling of horses competing at the 2015 Michigan Great Lakes International Draft Horse Show and Pull, held at the Michigan State University Pavilion for Agriculture and Livestock Education. All procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University (AUF approval 10/15‐150‐00) and horses were deemed healthy and fit to participate in competition by their owners and/or trainers. After informed consent was obtained, owners/trainers were queried about their horse(s) age, sex, height at withers, and weight. With horses standing quietly in either stalls or cross ties in a shed row, body condition score (BCS) was assessed (1‐9 scale) 13 and blood was collected from the jugular vein into vacutainer tubes without anticoagulant. Blood tubes clotted at ambient temperature for 1 to 2 hours and were subsequently centrifuged (3000 g) for 15 minutes and supernatant serum was transferred to plastic tubes and frozen (−20°C) until shipment within a week of collection to IDEXX Laboratories, Inc., on dry ice.
2.2. Laboratory analyses
Within 3 months of receipt of frozen samples, serum SDMA concentrations were measured in duplicate by LC‐MS by IDEXX Laboratories, as previously described. 8 , 14 , 15 Serum SDMA concentrations were also measured in duplicate by IDEXX Laboratories with a novel, high‐throughput, competitive homogeneous IA using a glucose‐6‐phosphate dehydrogenase conjugate and anti‐SDMA monoclonal antibody to quantify SDMA in serum. 2 , 15 , 16 , 17 Complete serum chemistry profiles, including measurement of serum Cr by an uncompensated Jaffé reaction and urea nitrogen (UN) concentrations by an adaptation of the method of Talke and Schubert, 18 were also performed at IDEXX laboratories using an automated analyzer (Beckman Coulter, Inc, Brea, California).
2.3. Validation of IDEXX SDMA IA in equine serum
Accuracy of the SDMA IA was validated by comparing endogenous SDMA concentrations measured (in duplicate, ranging between 5 and 20 μg/dL) by both LC‐MS and IA in 165 serum samples collected from the draft horses. Liquid chromatography‐mass spectroscopy and IA analyses were performed within 1 week of each other using separate aliquots of frozen sera, thawed on the day of measurement. Remaining sera was pooled and SDMA concentration was measured in duplicate by IA to determine percent recovery in aliquots spiked with exogenous SDMA (NG,NG′‐dimethly‐l‐arginine dihydrochloride, Carbosynth, Compton, Berkshire, UK) to achieve concentrations of 10, 40, and 80 μg/dL greater than the endogenous SDMA concentration of the pooled serum. Next, IA dilutional linearity was evaluated using serial 2‐fold dilutions (1:2 to 1:32) of 5 aliquots of pooled equine serum spiked with exogenous SDMA (to achieve a concentration of ~100 μg/dL), using pooled equine serum with a low SDMA concentration (~10 μg/dL) as the diluent.
Interassay precision for the IA was determined by measuring SDMA concentration in pooled equine serum samples at 3 SDMA concentrations (low [≤10 μg/dL]; medium [10‐20 μg/dL]; and high [≥20 μg/dL]), with 2 replicates performed twice a day for 5 days. The low sample contained endogenous SDMA and medium and high samples were spiked with exogenous SDMA. Mean ± SD values and coefficients of variation (CV) were determined using all 20 replicates. For LCMS precision, low, medium, and high concentration samples were tested on each LCMS run. Last, SDMA stability was evaluated by IA of 10 equine serum samples, in duplicate, after 0, 2, and 7 days of storage at −20, 4, and 25°C.
2.4. Calculations and statistical analysis
Distribution of SDMA, Cr and UN concentrations in the 165 draft horse samples were assessed for normality by the Kolmogorov‐Smirnov test.
Passing‐Bablock regression analysis was performed to compare LC‐MS and IA (means of duplicate results) SDMA concentrations for accuracy. 19 Furthermore, mean bias and 95% limits of agreement between LC‐MS and IA were determined by Bland‐Altman analyses. Next, percent recovery of SDMA from equine serum samples spiked with varying amounts of SDMA, as well as after serial dilution of samples spiked to achieve a SDMA concentration of ~100 μg/dL, were calculated from measured and expected SDMA concentrations and compared by Spearman correlation analysis. Symmetric dimethylarginine stability at days 2 and 7 was assessed by determining bias from day 0 values, with a bias of <3 SDs from day 0 values considered acceptable since SDMA is a new analyte, and a preestablished clinically acceptable change in concentration or total allowable error has not been established. Breed differences were compared by Kruskal‐Wallis analysis of variance (ANOVA) on ranks. Symmetric dimethylarginine, Cr, and UN concentrations were also compared between sexes (stallion, gelding, and mare) by ANOVA on ranks. Comparisons between SDMA, Cr, and UN concentrations and between age, weight, and BCS and these biomarkers of renal function were compared by Spearman correlation analyses. P < .05 was considered significant for all analyses.
3. RESULTS
The study included 165 horses: 63 Percherons, 52 Clydesdales, and 50 Belgians. Sex, age, and physical characteristics (height at withers, estimated weight, and BCS) did not differ between breeds with the exception that weight of Belgian horses was greater than that of Percheron and Clydesdale horses (Table 1). Most horses actively competed in 1 or more classes during the show with the exception of young horses (<3 years) that were exhibited in halter/conformation classes.
TABLE 1.
Ages and physical characteristics of Percheron, Clydesdale, and Belgian horses (BCS = body condition score, 1‐9 scale)
| Breed | Age (years), median (range) | Height (cm), median (range) | Weight (kg), median (range) | BCS (1‐9), median (range) | Sex distribution, mare/gelding/stallion (N) |
|---|---|---|---|---|---|
| Percheron | 6 (0.5‐16) | 180 (137‐190) | 863 (318‐1000) a | 6 (5‐8) | 35/24/4 |
| Clydesdale | 6 (0.5‐18) | 180 (142‐193) | 818 (272‐954) a | 6 (5‐8) | 29/19/4 |
| Belgian | 5 (0.5‐15) | 180 (142‐193) | 920 (455‐1045)b | 6 (5‐8) | 26/19/5 |
| P value | .26 | .93 | .003 | .75 | .68 |
Values with different superscripts within a column are different (P < .05).
3.1. Validation of IDEXX SDMA IA in equine serum
Passing‐Bablock regression analysis of SDMA concentrations in 165 equine serum samples measured by LC‐MS and IA revealed a strong correlation (R = .74, P < .001) (Figure 1). Bland‐Altman analysis yielded a bias of −.028 μg/dL with 95% limits of agreement from −3.26 to 3.21 μg/dL (Figure 2). For 3 aliquots of pooled serum spiked with an additional 10, 40, and 80 μg/dL of SDMA, recoveries ranged from 98% to 127% when measured using the IA (Table S1). Mean percent recovery of SDMA over a range of serial dilutions of 5 spiked pooled serum aliquots, with expected SDMA concentrations of 10 to 100 μg/dL, ranged between 103% and 116% between observed (measured) and expected (calculated) values (Figure S1). Because dilutional recovery of SDMA was within acceptable limits for equine serum with SDMA concentrations from 10 to 100 μg/dL, an analytical measurement range for the IA of 10 to 100 μg/dL was considered valid with a limit of quantification of 3.4 μg/dL (determined in canine and feline sera), similar to the diagnostic range used for dogs and cats. 2
FIGURE 1.
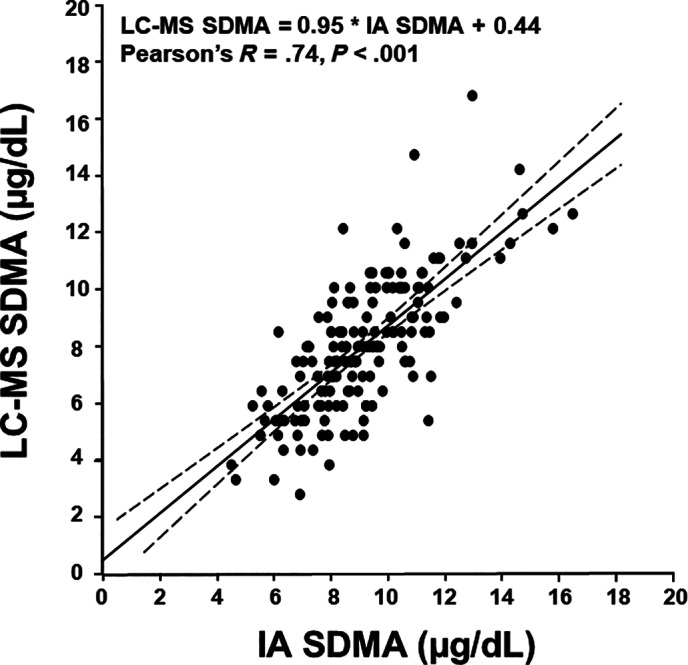
Passing‐Bablock regression analysis (with 95% confidence intervals within dashed lines) between symmetric dimethylarginine (SDMA) concentrations measured by liquid chromatography‐mass spectroscopy (LC‐MS) and immunoassay (IA) in 165 serum samples collected from healthy draft horses
FIGURE 2.
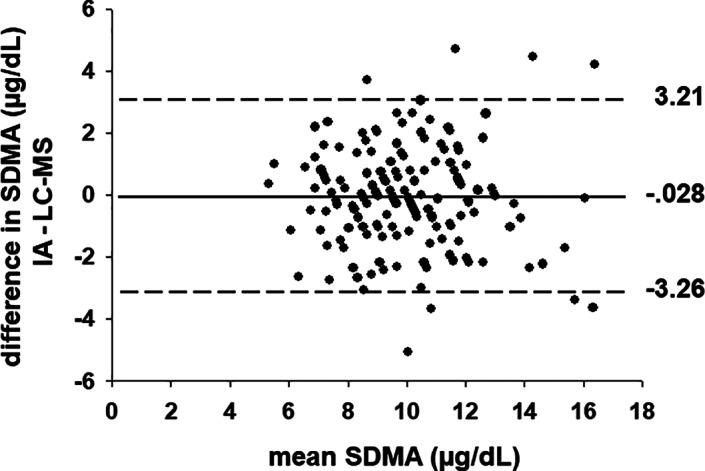
Bland‐Altman scatter plot illustrating bias and 95% limits of agreement for serum symmetric dimethylarginine (SDMA) concentrations measured by liquid chromatography‐mass spectroscopy (LC‐MS) and immunoassay (IA) in 165 serum samples collected from healthy draft horses
Mean and SD concentrations of SDMA measured by IA in 20 replicates of the low, medium, and high pooled sera over 5 days were 9.9 ± 1.3, 17.0 ± 1.9, and 57.8 ± 1.8 μg/dL, respectively, yielding interassay CVs of 13%, 11%, and 3%, respectively. Intraassay CVs for low, medium, and high SDMA samples were all less than 6%. Symmetric dimethylarginine concentrations measured in 10 serum samples were found to be stable after 2 and 7 days of storage with minimum and maximum bias values of −1.7 (−11%) to +0.7 (+4%) μg/dL (−20°C), −2.0 (−12%) to +0.5 (+3%) μg/dL (4°C), and −1.8 (−12%) to +0.7 (+4%) μg/dL (25°C) (Table S2).
3.2. SDMA in draft horses
Median and 95% confidence interval (CIs) for SDMA concentrations were 10.0 (9.4, 10.2; range, 5.0‐18.2) and 9.7 (9.5, 10.0; range, 5.0‐18.5) μg/dL for LC‐MS and IA, respectively. Symmetric dimethylarginine values measured by both LC‐MS and IA were lower in Percherons and Belgians, than in Clydesdales (Figure 3A,B, Table S3). There were no differences in SDMA concentrations between sexes (P = .16) and there were no correlations between age and SDMA concentrations, by both LC‐MS and IA (Figure 4) either for all horses or within breeds (within breed data not shown).
FIGURE 3.
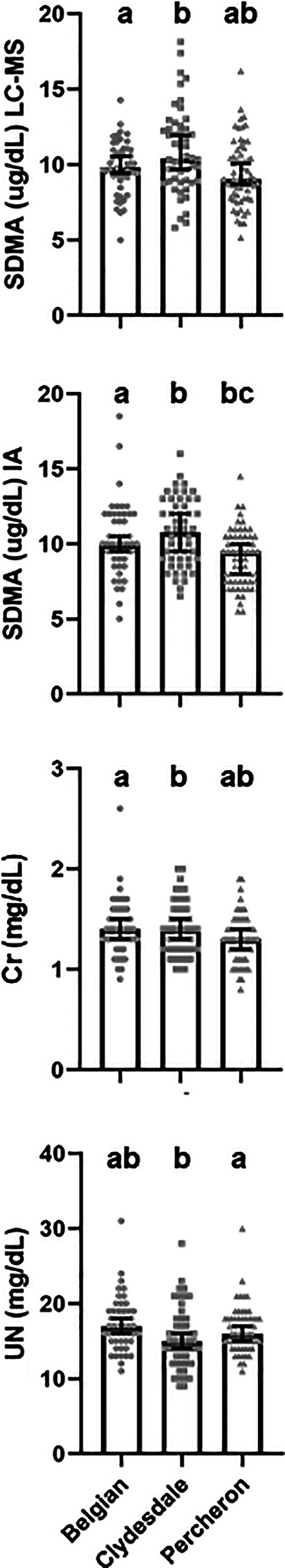
Median and 95% confidence intervals for symmetric dimethylarginine (SDMA), measured by liquid chromatography‐mass spectroscopy (LC‐MS) and immunoassay (IA), creatinine (Cr), and urea nitrogen (UN) concentrations in Percheron, Clydesdale, and Belgian horses. Bars with different superscript letters above them are different (P < .05)
FIGURE 4.
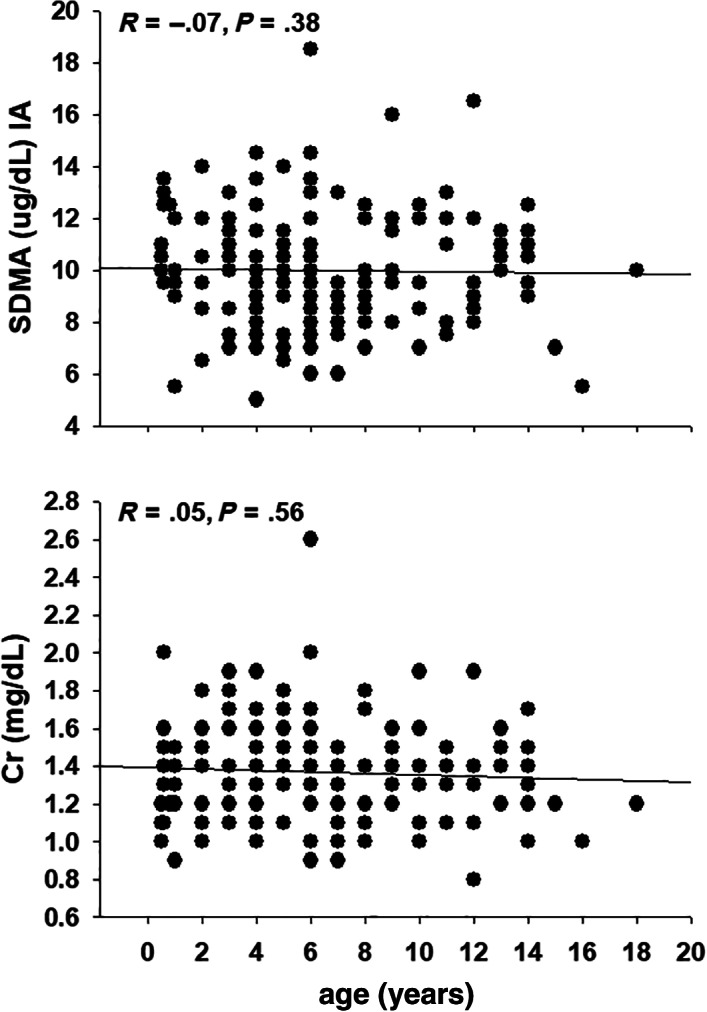
Spearman correlations of serum symmetric dimethylarginine (SDMA) concentrations measured by immunoassay (IA) and creatinine (Cr) and age in 50 Belgian, 52 Clydesdale, and 63 Percheron draft horses
3.3. Comparison of SDMA, Cr, and UN in draft horses
Medians and 95% CIs for Cr and UN concentrations for all 165 horses were 1.3 (0.9, 1.7; range, 0.8‐2.6) and 16 (15, 17; range, 9‐31) mg/dL, respectively. Creatinine was not different between breeds but UN was higher in Percherons as compared to Clydesdales (Figure 3C,D, Table S3) There were no differences (P = .20) in Cr concentrations between sexes but UN was lower (P < .02) in mares than geldings. Creatinine concentrations were correlated with SDMA concentrations, measured in all horses by both LC‐MS (R = .60, P < .001) and IA (R = .66, P < .001) as well as within breeds (Figure 5). In contrast, there were no significant correlations between UN concentrations and SDMA concentrations, measured in all horses by both LC‐MS (R = −.14, P = .07) and IA (R = −.06, P = .42) and findings within breeds were variable (Figure 5). There also were no correlations between Cr and age (Figure 4) or UN and age (R = −.01, P = .95). Finally, there was a significant correlation between weight and BCS (R = .54, P < .001), but there were no correlations between SDMA, measured by either method, and either weight or BCS. In contrast, there was a weak correlation (R = .25, P < .02) between weight and Cr but no correlations between BCS and Cr or between weight or BCS and UN.
FIGURE 5.
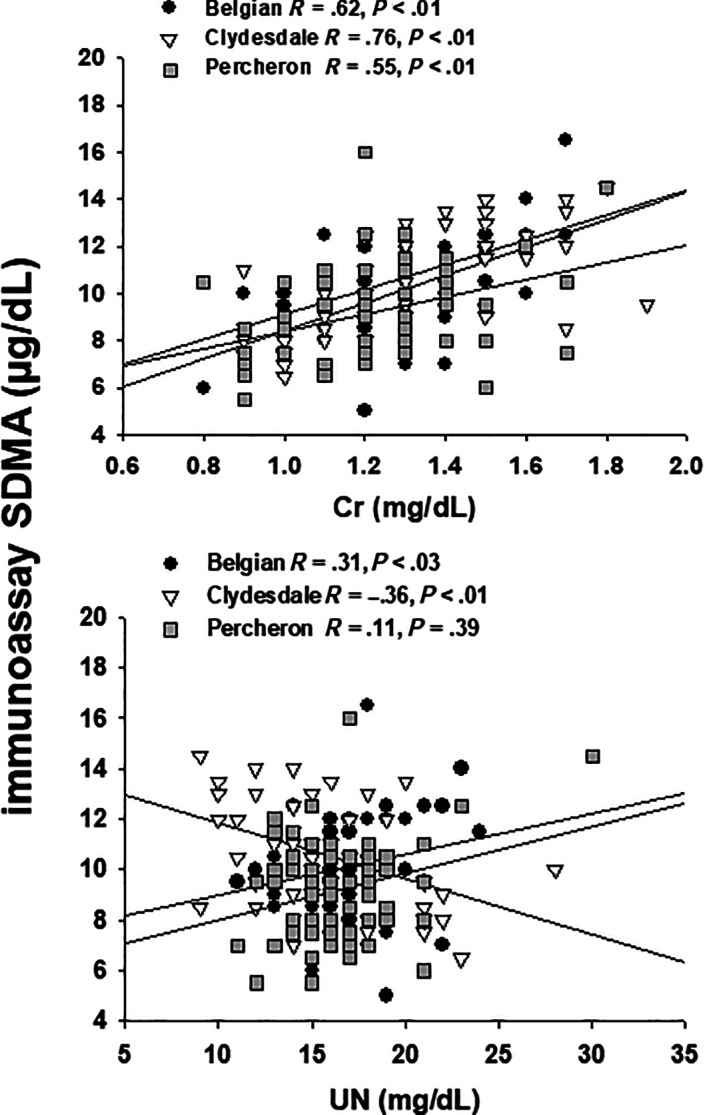
Spearman correlations of serum symmetric dimethylarginine (SDMA) concentrations measured by immunoassay (IA) and creatinine (Cr) or urea nitrogen (UN) concentrations in 50 Belgian, 52 Clydesdale, and 63 Percheron draft horses
4. DISCUSSION
The 1st objective of this study was to validate the IDEXX IA for measurement of SDMA concentration in equine serum samples. Validation was performed by several methods. First, accuracy of the IA was documented by finding a strong correlation between results obtained with the gold standard LC‐MS methodology and the IA. Second, IA results agreed well with expected (calculated) results when equine serum samples were spiked with additional SDMA. Third, linearity of the IA method was demonstrated by dilutional parallelism between measured and expected SDMA concentrations over a wide range of SDMA concentrations (10‐100 μg/dL). These findings are similar to results of previous validation studies of the IDEXX SDMA IA in canine and feline sera. 2 , 10 , 11 , 15 Thus, the IDEXX SDMA IA produces excellent results for measurement of serum SDMA concentration in equine serum over the expected range that could be found in clinical samples. 2 A further advantage of measuring SDMA as an estimator of GFR is that the molecule appears to remain stable for up to a week at various storage temperatures. Equine serum samples were not tested for interference from lipids, bilirubin, hemoglobin, or SDMA‐related compounds (l‐arginine, asymmetric dimethylarginine, and monomethyl‐l‐arginine). These interfering compounds have been previously examined in pooled dog, cat, and rat serum and SDMA IA results were not affected by mild‐to‐moderate hemolysis, lipemia, or hyperbilirubinemia. 2 , 15 A lack of interfering effects of these compounds would also be expected for equine serum of similar quality.
Serum concentrations of SDMA in healthy draft horses were distributed over a similar range as have been reported for healthy dogs and cats 2 and were unaffected by sex or body weight. Although a significant difference in median values was found between the 3 draft horse breeds, differences were small and were not considered clinically relevant. Thus, SDMA concentrations below 14 μg/dL, the upper limit used in dogs and cats, would seem supportive of adequate renal function in healthy horses. Furthermore, in this cohort of horses, ranging in age from 6 months to 18 years, no effect of age on serum SDMA concentration was found. However, this cohort of horses did not include any aged or geriatric horses and it would not be unexpected for SDMA to increase with advancing age, as has been demonstrated in people. 20 In addition, higher serum SDMA concentrations have recently been reported in healthy neonatal foals up to 30 days of age 21 , 22 ; consequently, using an upper limit of 14 μg/dL might not be appropriate for foals less than 6 months of age.
In the cohort of draft horses in this study, SDMA was greater than 14 μg/dL in 8 (range, 14.3‐18.1 μg/dL) and 5 (range, 14.5‐18.5 μg/dL) horses by LC‐MS and IA measurement, respectively. Of the 8 horses with an elevated SDMA concentration by LC‐MS, 4 had an SDMA concentration >14 μg/dL with both assays while the remaining 4 had an IA SDMA concentration of 13 to 14 μg/dL, near the cutoff value. One the 8 horses with an elevated SDMA concentration by LC‐MS (14.3 μg/dL) was a 6‐year‐old Belgian gelding that had increased values for all measures of renal function (SDMA‐IA 18.5 μg/dL, Cr 2.6 mg/dL, and UN 31 mg/dL). In retrospect, it was suspected that this horse could have been dehydrated by water withholding before weighing in for a pulling competition, although underlying kidney dysfunction could not be excluded. The remaining 7 horses with an elevated SDMA concentration by LC‐MS (14.3‐18.1 μg/dL) included 6 Clydesdales (1 stallion and 5 mares, 0.5‐0.7 years) and one 4‐year‐old Percheron mare. These 7 horses had serum Cr and UN concentrations ranging from 1.4 to 2.0 mg/dL (reference range, 0.8‐1.8 mg/dL) and 9 to 30 mg/dL (reference range, 11‐25 mg/dL), respectively, and there were no correlations between measures of renal function in this group of 8 horses. It would be of interest to follow this group of horses over time to determine if they might develop CKD.
Whether or not SDMA will become a more sensitive or earlier biomarker than Cr or UN for assessment of GFR in horses remains uncertain. In small animal patients, SDMA appears to be more useful than Cr for detecting a decline in renal function, as it has been found to increase, above the cutoff value of 14 μg/dL, months before Cr become elevated in the course of CKD. 8 , 9 In contrast, measurement of SDMA for assessment of acute kidney injury (AKI) 23 or as a prognostic indicator in critically ill dogs 24 has not been shown to have an advantage over Cr. However, comparison of SDMA to Cr could prove useful as the SDMA/Cr ratio tended to be greater in dogs with CKD, as compared to AKI, in a recent study. 25 Furthermore, because CKD appears to be less common in horses than in small animal patients, applicability of this novel indirect measure of GFR and renal function might prove less useful in horses. Nevertheless, further evaluation of SDMA in light breed horses and horses with both AKI and CKD is warranted.
Limitations of this study include that sampling was limited to more heavily muscled draft breed horses and that horses could have been in varying physiological states (eg, rest, posttransport, postprandial, or postexercise) at the time of sample collection. Unfortunately, as a convenience sampling study, it was not possible to standardize whether blood samples were collected before or after exercise. Nevertheless, more than 95% of horses had serum SDMA concentrations below 14 μg/dL. Next, because none of the horses had a substantially elevated SDMA concentration, stability of high SDMA concentrations in equine sera could not be assessed in this study. Another limitation was that validation of SDMA concentration as an indirect estimate of GFR was not compared to a direct measure of GFR (eg, inulin or endogenous Cr clearance); however, timed urine collections were not feasible in this convenience sampling study.
In conclusion, the IDEXX IA appears to be an accurate test for determination of SDMA concentration in equine serum. At least 95% of healthy draft horses that are at least 6 months of age should have a serum concentration below 14 μg/dL, the cutoff value used for dogs and cats. Further work is needed to determine if a similar cutoff value will be applicable for light breed horses and what normal SDMA concentrations might be in foals from birth to 6 months of age. Finally, whether an increase in SDMA will prove to be a more sensitive or earlier indicator of a decline in GFR, as compared to a change in Cr, remains to be determined through prospective evaluation of cohorts of horses with various medical problems, AKI, or CKD.
CONFLICT OF INTEREST DECLARATION
Michael Coyne, Rachel Murphy, Julie Cross, Marilyn Strong‐Townsend, Maha Yerramilli, Jun Li have an affiliation to the commercial funders of this research, as current or former employees of IDEXX Laboratories, Inc. (https://www.idexx.com/en/about-idexx/).
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval from Michigan State University IACUC.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed.
Supporting information
Figure S1 Dilutional linearity between symmetric dimethylarginine (SDMA) concentrations measured by immunoassay and expected (calculated) SDMA concentrations in 5 aliquots of equine serum spiked with ~100 μg/dL SDMA that were serially diluted (1:2 to 1:32) with pooled equine serum with a low SDMA concentration (~10 μg/dL)
Table S1 Mean recovery of symmetric dimethylarginine (SDMA) from duplicate immunoassay measurement of SDMA concentration in 3 equine serum samples spiked with an additional 10, 40, or 80 μg/dL of SDMA
Table S2 Stability of symmetric dimethylarginine (SDMA), measured in duplicate by immunoassay, in 10 equine serum samples stored at −10, 4, or 25oC for 2 and 7 days
Table S3 Median and 95% confidence intervals for symmetric dimethylarginine (SDMA), measured by liquid chromatography‐mass spectroscopy (LC‐MS) and immunoassay, creatinine (Cr), and urea nitrogen (UN) concentrations in Percheron, Clydesdale, and Belgian horses
ACKNOWLEDGMENTS
Funding provided by the Michigan State University College of Veterinary Medicine Endowed Research Funds and IDEXX Laboratories, Inc. The data were presented at the 2018 American College of Veterinary Internal Medicine Forum, Seattle, WA. The authors acknowledge the efforts of the organizers and competitors of the 2015 Michigan Great Lakes International Draft Horse Show and Pull. We are also grateful to S. Wismer LVT for technical assistance and for the help of Michigan State University preveterinary and veterinary students with data collection and record keeping.
Schott HC II, Gallant LR, Coyne M, et al. Symmetric dimethylarginine and creatinine concentrations in serum of healthy draft horses. J Vet Intern Med. 2021;35:1147–1154. 10.1111/jvim.16042
Funding information IDEXX Laboratories, Inc; Michigan State University College of Veterinary Medicine Endowed Research Funds
REFERENCES
- 1. Kakimoto Y, Akazawa S. Isolation and identification of N‐G,N‐G‐ and N‐G,N'‐G‐dimethyl‐arginine, N‐epsilon‐mono‐, di‐, and trimethyllysine, and glucosylgalactosyl‐and galactosyl‐delta‐hydroxylysine from human urine. J Biol Chem. 1970;245:5751‐5758. [PubMed] [Google Scholar]
- 2. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin Small Anim. 2016;46:941‐960. [DOI] [PubMed] [Google Scholar]
- 3. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol. 2016;45:28‐56. [DOI] [PubMed] [Google Scholar]
- 4. Kielstein JT, Salpeter SR, Bode‐Böger SM, et al. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta analysis. Nephrol Dial Transplant. 2006;21:2445‐2451. [DOI] [PubMed] [Google Scholar]
- 5. Szlosek C, Robertson J, Quimby J, et al. A retrospective evaluation of the relationship between symmetric dimethylarginine, creatinine and body weight in hyperthyroid cats. PloS One. 2020;15(1):e0227964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun JP, Lefebvre HP, Watson ADJ. Creatinine in the dog: a review. Vet Clin Pathol. 2003;32:162‐179. [DOI] [PubMed] [Google Scholar]
- 7. Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l‐arginine and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med. 2008;22:317‐324. [DOI] [PubMed] [Google Scholar]
- 8. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28:1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall JA, Yerramilli M, Obare E, Yerramilli M, Almes K, Jewell DE. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med. 2016;30:794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ernst R, Ogeer J, McCrann D, et al. Comparative performance of IDEXX SDMA test and the DLD SDMA ELISA for the measurement of SDMA in canine and feline serum. Plos One. 2018;13:e0205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rentko V, Nabity M, Yerramilli M, et al. Determination of serum symmetric dimethylarginine reference limit in clinically healthy dogs. J Vet Intern Med. 2013;27:750. [Google Scholar]
- 12. Bilbrough GE, Evert B, Hathaway K, et al. Validation of an point‐of‐care immunoassay for measurement of symmetric dimethylarginine in feline serum. J Vet Intern Med. 2018;32:118. [Google Scholar]
- 13. Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;5:371‐372. [DOI] [PubMed] [Google Scholar]
- 14. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29:808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29:1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prusevich P, Patch D, Obare E, et al. Validation of a novel high throughput immunoassay for the quantitation of symmetric dimethylarginine (SDMA). Clin Chem. 2015;16:S135. [Google Scholar]
- 17. Patch D, Obare E, Prusevich P, et al. High throughput immunoassay for kidney function biomarker symmetric dimethylarginine (SDMA). Clin Chem. 2015;16:S135. [Google Scholar]
- 18. Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in Warburg optical test. Klin Wochenschr. 1965;43:174‐175. [DOI] [PubMed] [Google Scholar]
- 19. Haeckel R, Wosniok W, Klauke R. Comparison of ordinary linear regression, orthogonal regression, standardized principal component analysis, Deming and Passing‐Bablok approach for method validation in laboratory medicine. J Lab Med. 2013;37:147‐163. [Google Scholar]
- 20. Schwedhelm E, Xanthakis V, Maas R, et al. Plasma symmetric dimethylarginine reference limits from the Framingham Offspring Cohort. Clin Chem Lab Med. 2011;49:1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozorgmanesh R, Coyne M, Murphy R, et al. Equine neonatal symmetric dimethylarginine (SDMA): results of two pilot studies. J Vet Intern Med. 2019;33:2440‐2441. [Google Scholar]
- 22. Bozorgmanesh R, Magdesian G, Offer K, et al. Equine neonatal symmetric dimethylarginine in sick neonates with hypercreatininemia. J Vet Emerg Crit Care. 2019;29:S30. [Google Scholar]
- 23. Yerramilli M, Farace G, Quinn J, et al. Kidney disease and the nexus of chronic kidney disease and acute kidney injury: the role of novel biomarkers as early and accurate diagnostics. Vet Clin Small Anim. 2016;46:961‐993. [DOI] [PubMed] [Google Scholar]
- 24. Köster LS, Peda A, Fraites T, Sithole F. A preliminary investigation into the prognostic relevance of symmetric dimethylarginine in critically ill dogs. J Vet Emerg Crit Care. 2018;28:527‐531. [DOI] [PubMed] [Google Scholar]
- 25. Dahlem DP, Neiger R, Schweighauser A, et al. Plasma symmetric dimethylarginine concentration in dogs with acute kidney injury and chronic kidney disease. J Vet Intern Med. 2017;31:799‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Dilutional linearity between symmetric dimethylarginine (SDMA) concentrations measured by immunoassay and expected (calculated) SDMA concentrations in 5 aliquots of equine serum spiked with ~100 μg/dL SDMA that were serially diluted (1:2 to 1:32) with pooled equine serum with a low SDMA concentration (~10 μg/dL)
Table S1 Mean recovery of symmetric dimethylarginine (SDMA) from duplicate immunoassay measurement of SDMA concentration in 3 equine serum samples spiked with an additional 10, 40, or 80 μg/dL of SDMA
Table S2 Stability of symmetric dimethylarginine (SDMA), measured in duplicate by immunoassay, in 10 equine serum samples stored at −10, 4, or 25oC for 2 and 7 days
Table S3 Median and 95% confidence intervals for symmetric dimethylarginine (SDMA), measured by liquid chromatography‐mass spectroscopy (LC‐MS) and immunoassay, creatinine (Cr), and urea nitrogen (UN) concentrations in Percheron, Clydesdale, and Belgian horses


